Abstract
Autism spectrum disorders (ASD) are complex, polygenic and heterogenous neurodevelopmental conditions. The severity of autism-associated variants is influenced by environmental factors, particularly social experiences during the critical neurodevelopmental period. While early behavioral interventions have shown efficacy in some children with autism, pharmacological support for core features — impairments in social interaction and communication, and stereotyped or restricted behaviors — is currently lacking. In this study, we examined how the social environment influences both wild-type (WT) and Shank3 knockout (KO) mice, a model reflecting core autism-like traits. Our findings revealed that chronic social isolation enhanced social interaction and olfactory neuron responses in WT animals. Furthermore, it restored impairments in social novelty preference and olfactory function, as well as self-grooming in Shank3 KO mice. Conversely, an enriched social environment heightened social interest toward novel conspecifics in WT mice, but elicited the opposite effect in Shank3 KO mice. Notably, Shank3 KO mice displayed distinct social responses when exposed to WT or Shank3 KO mice. These results offer novel insights that could favor the implementation of behavioral interventions and inclusive classroom programs for children with ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) represents a complex neurodevelopmental condition. Diagnosis is primarily based on two core behavioral characteristics: impairments in social interaction and communication and repetitive, restrained and stereotyped behaviors or interests [1]. Often, ASD is accompanied with co-occurring traits, such as anxiety, epilepsy, motor and cognitive deficits and sleep perturbations [1]. Over the past decade, the prevalence of ASD has reached 1% of the global population [2,3,4]. Genome-wide association studies have identified over a thousand susceptibility genes, with SHANK3 standing out as a high confidence gene (SFARIgene). Located within the 22q13 chromosomal region, which is deleted in the syndromic Phelan-McDermid syndrome [5], the SHANK3 gene has been linked to ASD through 142 reports, with more than 340 mutations or rare variants (S). Consequently, Shank3 knockout (KO) mice serve as a valuable model for studying ASD, effectively mirroring core features of the condition. The phenotype includes social novelty preference impairments (e.g., reduced interaction with a novel mouse compared to a familiar one), along with exacerbated motor stereotypies [6]. With half of ASD cases remaining idiopathic, the etiology is influenced by environmental factors and gene dosage [7]. Indeed, genetic variants alone cannot explain the variability in ASD severity among twins or siblings. Thus, ASD stands as a complex, polygenic, and heterogeneous condition.
So far, no pharmacological support addresses social interaction impairments and/or stereotyped behaviors in individuals with ASD. Atypical antipsychotics, such as risperidone and aripiprazole, as well as antidepressants, target co-occurring irritability, self-injury, rituals, anxiety, and depression [8,9,10]. However, they have limited efficacies and come with side effects. Numerous compounds targeting receptors or signaling pathways have been extensively tested [11], but they have failed in clinical trials. Their lack of efficacy can be attributed to the large placebo effect and the inherent diversity among individuals with ASD [12,13,14]. Targeting the oxytocin receptor, one of G protein-coupled receptors that control dysregulated signaling pathways in ASD, offers promise for developing new and personalized medicine for subgroups of individuals with ASD [11].
Applied Behavior Analysis (ABA)-based strategies for children with ASD have demonstrated some efficacy in addressing core social and cognitive impairments compared to standard child care services [15,16,17]. However, these interventions are expensive and highly demanding, requiring 35 h per week of intensive training for both children and their caregivers [15]. It is recommended that ABA interventions start as early as possible, ideally, before 18 months [16], which aligns with the challenges associated with early access to diagnosis. Moreover, these interventions improve only the targeted behavior [17]. Notably, the translation of ABA to Oprm1 KO young adult mice specifically ameliorated the targeted social interaction impairments, without normalizing other behaviors [18]. Despite these findings, the outcomes of ABA strategies in children with ASD remain uncertain, preventing broader implementation across all ASD children and their families [19, 20]. Alternatives may involve combining professionally adapted interventions with integrating ASD and neurotypical children in inclusive classrooms. While positive outcomes have been associated with neurodiversity among classroom participants and teacher engagement compared to autism-specific classrooms [21,22,23], the implementation and sustainability of such approaches remains challenging. Indeed, inappropriate implementation within inclusive school programs results in significant behavioral difficulties for children with ASD, often leading them to discontinue those programs and join specialized schools [24, 25].
During the COVID pandemic, children and adolescents have experienced chronic social isolation, ranging from separation from their peers to complete loneliness, particularly during a crucial period for the establishment of social relationships. Reports indicate increased aggression and depression during or shortly after chronic social isolation (reviewed in [26]), underscoring the critical impact of such unprecedented global isolation, including its implications for individuals with ASD. Studies in rodents reveal that acute (1 day) or chronic isolation can lead to opposing effects on sociability, either enhancing social seeking for a conspecific or leading to social impairments and aggression, respectively (reviewed in [26]). Recent findings in rats demonstrate that acute (1 day) or short-term (7 days) social isolation rapidly induces social memory impairment [27]. Although behaviors are restored upon the reunion of isolated animals, dysregulation in neural plasticity gene and protein expressions (e.g., Bdnf, Egr1, Arc) persists in the medial amygdala [27]. These observations highlight the significant impact of the duration of social isolation on sociability. However, the effects of social isolation on ASD mouse models or early social environment on social skills in wild-type (WT) animals have yet to be explored. Therefore, this study aims to determine the impact of early social environment on WT and Shank3 KO mice, a mouse model useful for the study of ASD-like core features.
Materials and methods
Animals
All mouse breeding, care and experimental procedures adhered to the European and French Directives and were approved by the local ethical committee CEEA Val de Loire N°19 and the French ministry of teaching, research and innovation (APAFIS #18035-2018121213436249). Sexually naive 2-month-old Shank3 KO (JAX stock #017688) [6] and control wild-type males and females, bred on a mixed 50% C57BL/6 J - 50% 129S2 background, were included in the study. Detailed sample sizes and sex ratio are provided in the figure legends. To mitigate potential early social environment effects, after heterozygous breeding of the parental animals, 3-4 WT and Shank3 KO F1 couples were bred separately, and the F2 generation was tested. All WT and Shank3 KO mice were raised in groups of 2 to 4 animals, unless exposed to 1- or 4-week chronic isolation prior to behavioral phenotyping. Cages of WT and Shank3 KO mice were randomly allocated to the different housing conditions. All animals were housed in the same experimental room on a 12-h regular light/dark cycle, with food and water ad libitum with controlled temperature (±21 °C) and humidity (±50%) in conventional health housing status.
Behaviors
All behavioral tests were conducted in the mornings under dim lighting conditions in standardized behavioral equipment designed to minimize anxious-like behaviors. Detailed timelines, procedures, and scoring methods are provided in the supplementary methods. Social interaction was assessed over a 10-min period using both the reciprocal social interaction and the three-chambered tests (habituation, followed by sociability and social novelty phases). Scoring was performed manually for both tests. Reciprocal social interaction in a distinct social environment (over a 10-min period) was evaluated across three consecutive trials in the Live Mouse Tracker [28], which automatically detects and scores various simultaneous behavioral parameters of social interactions among four sex- and age-matched animals. In the experiment involving only WT mice (Fig. 3 and S3A–I), the consecutive trials consisted of three interactions on separate days with four unknown sex- and age-matched animals placed in the open field of the Live Mouse Tracker. In the experiment with both WT and KO animals (Fig. 4 and S3J–L), the trials included interaction with cage mates (Trial 1), followed by interaction with a mix of unknown sex- and age-matched WT and KO (Trial 2 ‘WT + KO’), and, finally, interaction with unknown WT or KO separately (Trial 3 ‘WT or KO’). Cognitive inflexibility and stereotyped and repetitive behaviors were evaluated using the spatial Y-maze or motor stereotypy tests, respectively. Scoring was conducted automatically in the Y-maze or manually for motor stereotypies. Locomotion and anxious-like behaviors were automatically measured in the open field. Olfaction was quantified manually, using an olfactory two-choice preference test. In the motor stereotypy and olfactory preference tests, 4 to 6 mice were simultaneously recorded, enabling the quantification of synchronized grooming behavior. All manual scoring was performed by a trained experimenter who was blinded to the genotypes and housing conditions. All aspects of animal experiments have been reported according to ARRIVE guidelines [29].
Quantitative PCR
Total RNAs from main olfactory epithelium (MOE), vomeronasal organ (VNO) and olfactory bulb (OB) were extracted according to the manufacturer’s instructions (Zymo Research Corporation Kit Direct-zol RNA Microprep) and quantified using a Nanodrop (Thermo Fisher Scientific, Waltham, MA). MOE, VNO and OB cDNAs were generated from 450 ng, 320 ng and 115 ng of total RNAs, respectively, using the SuperScript III kit (Invitrogen). All quantitative PCRs were performed in triplicates in 384 well plates using 1 µL of cDNA (dilution 1/50 for MOE and VNO and 1/25 dilution for OB) and 1 µM of validated primers for Actb, Gapdh, Oxt and Oxtr genes (Table S1), according to the manufacturer protocol (2X Ozyme ONEGreen® Fast qPCR premix). Reactions were carried out with an initial incubation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 60 °C for 15 s, and elongation at 60 °C for 30 s.
Plasma and urine oxytocin concentration
Plasma or urine oxytocin concentrations were determined by Enzyme ImmunoAssay (Enzo Life Sciences, ADI-901-153) after solid-phase extraction using Sep-Pack C18 cartridges (Waters, WAT054945). 120 µL of plasma or 150 µL of urine samples were extracted and reconstituted in 120 µL or 150 µL assay buffer. Measurements were performed in duplicate and normalized by the number of animals in each sample. The assay sensitivity was 7.8 pg/mL.
RNAscope In situ hybridization
Mice were anesthetized with a mixture of 100 mg/kg ketamine and 5 mg/kg xylazine, perfused transcardially with 1X cold phosphate buffer saline, followed by 4% paraformaldehyde (PFA). VNO tissue was dissected, postfixed overnight in 4% PFA and cryoprotected in 30% sucrose. Samples were embedded in Tissue-Tek O.C.T. compound, snap-frozen in cold isopentane, sectioned in 16 μm thick coronal slices on a Leica CM3050S cryostat and mounted on SuperFrost Plus glass slides (Thermo Scientific). Fluorescent in situ hybridization (FISH) for Oxtr mRNA was performed according to the RNAscope Fluorescent Multiplex V2 labeling kit (ACDBio 323110) coupled to tyramide signal amplification signal development using the mm-Oxtr probe (ACDBio 412171). Procedures are fully detailed in supplementary methods. Briefly, slices were incubated with HRP RNAscope reagent, washed, incubated with Biotin-conjugated Tyramide 1:50, washed, incubated with Cy2-labeled streptavidin 1:500, washed, counterstained with Hoechst 33258 and mounted with antifade fluorescent mounting medium (Dako). Fluorescent images were acquired using laser scanning confocal microscopy (Zeiss LSM-780). Quantification was performed using the “Subcellular detection” feature of QuPath software [30].
Calcium imaging
Ca2+ imaging of freshly dissociated vomeronasal sensory neurons (VSNs) was performed as previously described [31, 32] and in supplementary methods. Cells from 5 to 6 males for each genotype and housing conditions were stimulated successively, in random order, using a bath application with control HBSS-Hepes buffer, 1 µM oxytocin (Sigma-Aldrich, O3251), and urine from at least three different adult male mice diluted to 1:100.
Statistical analyses
Data and statistical analyses were performed using R software (version 4.4.0). Code is available upon request. Linear models were fitted for behavioral and Live Mouse Tracker data, encompassing variables such as mouse line, housing condition, number of mice per cage, trial and their interactions to identify significant differences between mouse lines among variables detailed in Tables S2–S4. A stepwise model selection procedure based on the Akaike Information criterion (AIC) was used to select the variables added to the model. Post-hoc tests, based on estimated marginal means with Tukey p-value correction, were conducted using the emmeans package [33] (R package version 1.8.5) to identify differences between the factors of the significant variables (for example, differences between mouse lines). The assumptions of the linear model are verified with the DHARMa package [34]. For quantitative PCR data, gene expression ratio was computed using Pfaffl method, which accounts for primer efficiencies [35], then analysis was performed using Kruskal-Wallis tests followed by Dunn’s post-hoc tests with the rstatix package [36]. Prior to this analysis, one animal outlier was removed according to the median absolute deviation (MAD) from the median, which is a more robust version of the empirical rule (+/- 3 MAD). For calcium imaging, statistical analyses were performed using the GraphPad Prism software 10.0.2 and the Fisher’s exact test, with probability of error level (alpha) at 0.05. Raw data, mean ± standard deviation (sd) and statistics are presented in Tables S2–S4.
Results
Chronic social isolation enhances social interactions in both WT and Shank3 KO mice
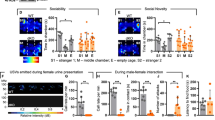
Initially, we hypothesized that exposing WT mice to social isolation might result in social interaction impairments. However, 4-week chronic social isolation actually led to increased social interaction in WT mice, as evidenced by an increase in nose contacts during the reciprocal test, with no effect on huddling behavior, an indicator of social comfort and bonds [37], or social preference in the three-chambered test (Fig. 1A–D, Table S2). Notably, this effect was consistent across different backgrounds and sexes of the mice (Fig. S1A–F). As expected [6], Shank3 KO mice exhibited impairment only in social novelty preference in the three-chambered test (Fig. 1A–D). Given this phenotype, we hypothesized that chronic isolation of Shank3 KO mice might exacerbate their social phenotype. Surprisingly, social isolation led to heightened social interaction in Shank3 KO mice, accompanied by exacerbated social novelty preference and the appearance of huddling behavior (Fig. 1, Table S2). Using the Live Mouse Tracker [28], which allows simultaneous and automatic assessment of multiple parameters of social interaction and mixing four unknown animals from each genotype and housing condition, we observed that isolated WT and Shank3 KO animals tended to engage in longer interactions (and together) compared to those raised in groups over a 30-min period (Fig. S1G–I). Furthermore, 1-week social isolation induced an intermediate phenotype in Shank3 KO mice compared to 4-week isolation (Fig. S2A–C), with enhanced social interaction in the reciprocal test, but no effect on social novelty. In conclusion, chronic social isolation enhanced social interactions in both WT and Shank3 KO mice, with Shank3 KO mice displaying a more pronounced increase and a distinct behavioral pattern.
In the reciprocal social interaction test, WT isol (burgundy; n = 26, 13 males and 13 females) and KO isol (purple; n = 18, 8 males and 10 females) spent more time in nose contacts (A) with a sex-, housing- and genotype-matched conspecific compared to WT group (gray; n = 62, 32 males and 30 females) and KO group (turquoise; n = 16, 8 males and 8 females). KO isol spent significantly more time huddling (B) than the other conditions. In the three-chambered test, KO isol spent more time in nose contacts with the mouse in the sociability phase (C) and with the novel mouse in the social novelty phase (D) compared to WT group and WT isol. All conditions, except KO group, preferred the novel mouse over the familiar one. Data are presented as mean ± sd (Table S2, sex effect in Fig. S1). Statistical analysis was performed using a linear model followed by pairwise comparisons using the estimated marginal means, with asterisks indicating housing effects and hash symbols indicating preference effects (p = P adjusted in all 10-min tests). * or #: p < 0.05, ** or ##: p < 0.01, *** or ###: p < 0.001. KO group, Shank3 KO raised in groups; KO isol, Shank3 KO exposed to 4-week chronic social isolation; WT group, WT raised in groups; WT isol, WT exposed to 4-week chronic social isolation.
Chronic social isolation normalizes self-grooming in Shank3 KO mice
In WT mice, chronic social isolation did not induce any effects on motor stereotypies (Fig. 2A–C). Shank3 KO mice exhibited exacerbated self-grooming, along with an increased number of head shakes and reduced time spent digging (Fig. 2A–C). Interestingly, social isolation normalized certain motor stereotypies in Shank3 KO mice, including the time spent, number and mean duration of self-grooming, as well as partially the time spent digging, but not head shakes (Fig. 2A–C, Table S2). This effect was also found in 1-week isolated animals (Fig. S2D–F). Notably, this effect was consistent across both WT and Shank3 KO males and females, irrespective of housing conditions (Fig. S3A–C).
In the motor stereotypy test, KO group (turquoise; n = 16, 8 males and 8 females) exhibited increased time spent self-grooming (A), more head shakes (B), decreased time spent digging (C), and increased time in synchronized grooming (D) compared to WT isol (burgundy; n = 26, 13 males and 13 females) and WT group (gray; n = 62, 32 males and 30 females). Self- and synchronized grooming were normalized in KO isol (purple; n = 18, 8 males and 10 females), while digging was partially restored and head shakes remained increased. Representative Gantt charts (E) illustrate the pattern of synchronized grooming in WT group and KO group from females raised in the same cage and isolated animals over a 10-min period. Data are presented as mean ± sd (Table S2, sex effect in Fig. S3). Statistical analysis was performed using a linear model followed by pairwise comparisons using the estimated marginal means. *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001. KO group, Shank3 KO raised in groups; KO isol, Shank3 KO exposed to 4-week chronic social isolation; WT group, WT raised in groups; WT isol, WT exposed to 4-week chronic social isolation.
Remarkably, we observed that shortly after one Shank3 KO mouse initiated self-grooming, other KO mice in separate and adjacent cages also began exhibiting grooming behavior. We named this new behavior, synchronized self-grooming. It was present in both males and females, although to a much lesser extent in WT mice and nearly absent in both isolated WT and Shank3 KO animals (Fig. 2D, E, S2D). These findings suggest that the heightened self-grooming phenotype observed in Shank3 KO mice raised in social groups results from exposure to conspecifics. To our knowledge, this is the first report of synchronized self-grooming and a behavior induced by social housing in a mouse model of ASD.
Additionally, chronic social isolation did not induce any effects on cognitive flexibility, locomotion and anxious-like behaviors in WT mice (Fig. S4A, B). In contrast, Shank3 KO mice exhibited more spontaneous alternations in the Y-maze and spent more time in the periphery of the open field, which was restored by chronic social isolation (Fig. S4A, B). In addition, social isolation enhanced locomotor activity in Shank3 KO mice, as evidenced by the number of alternations and traveled distance in the Y-maze and open field (Fig. S4C, D). Chronic isolation did not induce aggression in WT and Shank3 KO mice (Table S2). In conclusion, chronic social isolation normalized socially-induced motor stereotypies in Shank3 KO mice.
Distinct social environments differentially impact WT and Shank3 KO mice
Building upon the effects of chronic social isolation and that the social environment can influence social skills in mice (for review [26]), we investigated whether varied social environments affect WT and Shank3 KO mice differently. Using the Live Mouse Tracker to automatically detect various social interaction parameters among four animals [28], we examined the impact of low, moderate, and rich early social environments — housing animals in groups of 2, 3, and 4 individuals per cage — on social skills in WT and Shank3 KO mice (Figs. 3–4, S5, Table S3).
In the Live Mouse Tracker, WT male and female mice raised in groups of 4 animals (dark gray; n = 46, 19 males and 27 females) displayed constant time in nose contacts (A), huddling (B), social approach (C), ‘move in contact’ (D) and isolated (E) across trials. In contrast, animals raised in groups of 2 (light gray; n = 18, 9 males and 9 females) and 3 (middle gray; n = 33, 27 males and 6 females) spent less time in these parameters over consecutive trials. F Regardless of housing conditions, mice spent more time in the periphery as the number of trials increased. Data are presented as mean ± sd (Table S3). Robust linear model followed by pairwise comparisons using the estimated marginal means, with stars as trial effect and hash as housing effect (p = P adjusted in all tests). * or #: p < 0.05, ** or ##: p < 0.01, *** or ###: p < 0.001, **** or ####: p < 0.0001.
In the Live Mouse Tracker, WT mice (gray; n = 21, 13 males and 8 females) and Shank3 KO mice (turquoise; n = 26, 15 males and 11 females) spent less time in nose contacts (A, D), huddling (B, E) and social approach (C, F) with their cage mates (Trial 1; lighter color) than with a mix of 2 unknown KO and 2 unknown WT mice (Trial2 WT + KO), or with 4 unknown KO or WT mice separately another day (Trial3 WT or KO; darker color). Shank3 KO mice showed increased nose contacts (A) and huddling (B) when interacting only with Shank3 KO mice in Trial 3, compared to Trial 2, while WT exhibited increased nose contacts only. Both Shank3 KO and WT mice spent comparable amounts of time in social approach, regardless of the trials (C). In Trial 3, Shank3 KO mice raised in groups of 2 (light turquoise; n = 6 females) showed increased nose contacts (D) and huddling (E) behavior compared to WT mice raised in groups of 2. Conversely, Shank3 KO mice raised in groups of 4 (dark turquoise; n = 6 males) exhibited reduced nose contacts and social approach (F) than their WT counterparts. Social exploration in Shank3 KO mice differed between groups of 2 and 4, but not in groups of 3 (turquoise; n = 4, 2 males and 2 females). Data are presented as mean ± sd (Table S3, sex effect in Fig. S5). Statistical analysis was performed using a linear model followed by pairwise comparisons using the estimated marginal means, with asterisks indicating genotype effects and hash symbols indicating trial (A–C) or housing (D–F) effects (p = P adjusted in all tests). * or #: p < 0.05, ** or ##: p < 0.01, *** or ###: p < 0.001, **** or ####: p < 0.0001.
When interacting with unknown sex-matched animals, WT mice raised in groups of 4 displayed sustained social motivation (social approach and ‘move in contact’) and exploration (nose contact, less isolated) and enhanced huddling behavior across three subsequent trials (Fig. 3A–E). In contrast, mice in lower social environments showed a reduction in these social parameters over the trials. While the time spent in the periphery and stretch-attend posture (SAP) generally increased across trials, none of the housing conditions affected these anxious-like behaviors or other parameters, such as time spent in ‘get away’, an index of social avoidance (Fig. 3F, Table S3). No differences were observed between WT animals raised in groups of 2 and 3 during the third trial, likely due to increased social interaction in WT males raised in groups of 2 (Fig. S5A–I). Overall, WT females exhibited enhanced social skills compared to WT males in Trials 2 and 3 (Fig. S5A–I).
Next, we investigated the effect of distinct genotypes, interactions and housing conditions on WT and Shank3 KO social skills using the Live Mouse Tracker (Fig. 4, Table S3). After a trial with their cage mates (Trial 1), mice were exposed to a mix of unknown WT and KO mice (Trial 2 WT + KO), followed by an interaction with unknown WT or KO mice separately (Trial 3 WT or KO). As expected, all parameters of interaction with unknown animals were higher compared to interactions with cage mates (Fig. 4A–C). However, Shank3 KO mice spent less time in nose contact with their cage mates compared to WT mice. While WT mice were less affected by the different genotypes in Trial 2 and 3, Shank3 KO mice exhibited increased social interaction, particularly nose-to-nose contacts (Table S3), and huddling behaviors when encountering only Shank3 KO mice (Fig. 4A, B) compared to interaction with a mix of genotypes and WT animals. However, social motivation did not differ for both genotypes and trials (Fig. 4C). In Trial 3, social exploration and motivation in Shank3 KO mice decreased proportionally with the increasing number of mice per cage, compared to WT animals (Fig. 4D–F, Table S3). However, these effects may reflect underlying sex differences, as Shank3 KO females exhibited increased social exploration compared to Shank3 KO males and WT females (Fig. S5J–L). These results suggest that, over the long term, Shank3 KO females may prefer the company of only a few conspecifics. In conclusion, these findings provide evidence that social environments differentially influence social skills of Shank3 KO mice.
Chronic social isolation enhances vomeronasal neuron signaling in WT and Shank3 KO mice
Considering that limited access to social odors from cage mates is a key aspect of chronic social isolation, we investigated whether this condition would impact olfactory function and the expression of oxytocin receptors (Oxtr) in olfactory tissues [38, 39]. Indeed, social isolation induced an increase in Oxtr mRNA levels in the vomeronasal organ (VNO), but not in the main olfactory epithelium (MOE) or in the olfactory bulb (OB; Fig. 5A–C).
A The relative quantitative expression of Oxtr transcripts is increased in the VNO of WT isol (burgundy; n = 7, 3 males and 4 females) compared to WT group (gray; n = 6, 3 males and 3 females), with no significant changes in Gapdh transcripts or in MOE or OB tissues. Both transcripts are normalized to Actb housekeeping transcript levels. B Subcellular quantification of fluorescent in situ hybridization puncta in WT group (n = 4, 2 males and 2 females) and WT isol (burgundy, n = 4, 1 male and 3 females), along with representative pictures (C) confirmed an enrichment of Oxtr transcripts (green; DAPI counterstaining, blue) in the VNE (arrowheads) of WT isol (right) compared to WT group males (left), the difference was not statistically significant. Calcium imaging showed that a higher percentage of VSN cells from WT isol (burgundy; n = 4 males) responded to urine (D), but not to oxytocin (1 µM; E), compared to WT group (gray; n = 5 males). In contrast, KO group (turquoise; n = 5 males) displayed a decreased percentage of cells responding to both stimuli, which was restored by chronic social isolation (KO isol; purple; n = 5 males). F Representative calcium imaging traces showed VSN responses to urine and oxytocin stimulations. Data are presented as mean ± sd. Statistical significance was determined using Kruskal-Wallis tests (A), a two-tailed Student’s t-test (B) or Fisher’s exact test (D–E) between groups. *: p value < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001. MOE, main olfactory epithelium; NSE, non-sensory tissue; OB, olfactory bulb; KO group, Shank3 KO raised in groups; KO isol, Shank3 KO exposed to 4-week chronic social isolation; Oxtr, oxytocin receptor; VNE, vomeronasal sensory epithelium; VNO, vomeronasal organ; VSN, vomeronasal sensory neurons; WT group, WT raised in groups; WT isol, WT exposed to 4-week chronic social isolation.
Therefore, we wondered whether vomeronasal sensory neuron (VSN) responses to social cues were affected in WT isolated animals or Shank3 KO mice, and whether social isolation of Shank3 KO mice would also normalize any deficits. To address this, we analyzed VSN responses to urine from male conspecifics and oxytocin, using live-cell calcium imaging. Both WT and Shank3 KO VSNs were activated by urine as well as oxytocin. However, VSNs of Shank3 KO mice responded less to urine (from 3 to 1.6%; 1.9-fold) and oxytocin (from 3 to 1.9%; 1.6-fold; Fig. 5D–F) compared to WT mice, indicating a sensory dysfunction. Chronic isolation increased the number of VSNs responding to urine in both WT mice and Shank3 KO mice, with a more pronounced increase observed in Shank3 KO mice (1.7-fold and 5-fold increase in WT and KO mice, respectively). However, responses to oxytocin did not differ between housing conditions (from 3 to 3.7% and 1.9 to 2.4%, respectively).
Given that the VNO is specialized in detecting chemosignals, including peptides, that trigger various social behaviors, which are also modulated by oxytocin receptor (for review see [40, 41]), we hypothesized that mice could detect oxytocin peptide and that chronic isolation or Shank3 deletion could affect those responses (Fig. 6). Using an olfactory two-choice preference test, we observed a preference for oxytocin (OT) over saline, both diluted in a mix of urine and saline solution, in WT isolated and grouped animals (Fig. 6A), indicating the detection of oxytocin in this complex odor environment. To our knowledge, this is the first report showing that mice are able to detect oxytocin. OT preference was not significant due to the interindividual variability and lower number of animals in both genotypes. Shank3 KO mice displayed a lack of preference for urine of opposite sex (Usex), but no difference in the latency to sniff the Usex paper compared to WT mice (Fig. 6B, C). Interestingly, WT mice raised in groups showed a strong preference for urine from isolated sex-matched animals (Uisol) over urine from animals raised in groups (Ugroup; Fig. 6D), as an index of interest for an unknown odor. The OT preference was particularly pronounced in WT females and is unlikely to be attributed to oxytocin concentrations in the urine. Indeed, oxytocin concentrations were similar in the urine of isolated animals, while higher in the plasma (2.8-fold increase) compared to WT animals raised in groups (Fig. 5E, F), with no correlation between the two concentrations. In conclusion, chronic social isolation in Shank3 KO mice restored olfactory processing in the VNO, with a potential contribution of oxytocin receptors to this effect.
A In the olfactory two-choice preference test, WT group (gray, n = 46, 28 males and 18 females) and WT isol (burgundy, n = 22, 13 males and 9 females) showed a preference for oxytocin (OT, 60 µg) over saline (both diluted in 1:1 saline and sex-matched urine) and for opposite sex urine (Usex) over saline. B KO group (turquoise; n = 16, 8 males and 8 females) and WT group (gray; n = 15, 7 males and 8 females) displayed no significant difference for OT preference (lower statistical power in WT group) while only WT group showed a preference for Usex. C KO group showed no difference in latency to sniff the paper with the Usex compared to WT group. D Interestingly, WT group (n = 20, 10 males and 10 females) showed a preference for urine from chronically isolated animals (Uisol) over urine from animals raised in groups (Ugroup). E The concentration of OT in both urine samples did not differ between conditions (Uisol, n = 5, 2 males and 3 females; Ugroup, n = 6, 3 males and 3 females). F In contrast, plasma levels of OT were increased in WT isol (n = 8, 5 males and 3 females) compared to WT group (n = 14, 6 males and 8 females). Data are presented as mean ± sd (Table S4). Kruskal-Wallis tests followed by Dunn post hoc tests were conducted, with asterisks indicating significant housing effects. *: p < 0.05, **: p < 0.01, ****: p < 0.0001. KO group, Shank3 KO raised in groups; OT, oxytocin; Ugroup, urine of mice raised in groups; Uisol, urine of mice exposed to 4-week chronic social isolation; Usex, urine of opposite sex mice; WT group, WT raised in groups; WT isol, WT exposed to 4-week chronic social isolation.
Discussion
In this study, we demonstrated that WT mice exposed to 4-week chronic social isolation exhibited enhanced social interactions, contrary to the expectation (reviewed in [26]). This suggests that social isolation for 4 weeks likely triggers a craving for social contacts, as evidenced by increased social interactions, which lasts over 20 min upon reunion. Our results align with earlier reports in mice [42], but also diverge from impairment on social novelty preference observed in rats [27]. These differences suggest that social isolation in WT mice may be influenced by housing in different animal facilities and test conditions, rather than the duration of isolation (1 week intermediate vs. 4 weeks established effect), sex or mouse background, as demonstrated in this study. Chabout and colleagues showed that isolated animals emit comparable amounts of ultrasound vocalizations as those raised in groups, and even more upon reunion [43]. The environment may explain the enhanced social motivation and seeking for conspecifics. Indeed, a semi-naturalistic enriched environment improved core ASD-like features in BTBR inbred mice [44]. Furthermore, the well-being of animals has improved over the last decades, with successive generations raised in enriched social environments, potentially mitigating the adverse effects of social isolation. Interestingly, male and female mice express sex-specific responses when exposed to distinct social environments. While WT females tended to exhibit increasing sociability with an increasing number of mice per cage, WT males displayed a U-shaped pattern, with no differences in aggression. This suggests that dominant/subordinate dynamics in males (absent in female lab mice [45]) may underlie this effect. Therefore, exploring the impact of social isolation across generations with different levels of enrichment and investigating the effect of sex or dominance might provide clarity to these questions.
One of our main discoveries lies in the amelioration of both social interaction and some stereotyped behaviors in Shank3 KO mice when exposed to social isolation. Consistent with the first study [6], we observed a substantial impairment in social novelty preference in Shank3 KO mice, accompanied with motor stereotypies. Additionally, Maloney and colleagues reported diminished social motivation in Shank3 KO mice [46]. Therefore, chronic isolation likely reshapes brain circuitry and restores social motivation in Shank3 KO mice, notably intensifying the seeking of a conspecific and the need for social comfort, as evidenced by the time spent huddling. Furthermore, we found that chronic isolation normalized self-grooming but not head shake episodes in Shank3 KO mice. While the former likely reflects motor impairment in the striatum of this model [6], our results suggest that self-grooming behavior is acquired through life in social groups. Specifically, Shank3 KO mice raised in groups exhibited exacerbated synchronized grooming, potentially induced by specific ultrasound vocalizations from conspecifics. Hence, chronic isolation may prevent this acquired behavior. Social touch has been shown to elicit aversive and atypical responses in the Fmr1 KO mice, a model of Fragile X syndrome, and in a mouse model of maternal immune activation, potentially via the novel concept of brain endogenous noise [47,48,49]. If abnormal and aversive social touch processing also occurs in Shank3 KO mice, it could contribute to the social and grooming phenotype, induced by living with several conspecifics. Indeed, we showed that Shank3 KO mice exposed to a high number of animals display reduced social interaction and motivation. This finding has implications for refining housing conditions (the 3Rs) to enhance the welfare and use of this mouse model in research. Further study should elucidate the precise mechanism underlying the impact of chronic social isolation in Shank3 KO mice. It might involve similar mechanisms to those elucidated in WT animals, such as the paraventricular nucleus (PVN) oxytocin-ventral tegmental area (VTA) dopamine-medial prefrontal cortex circuitry [42]. Indeed, dysfunctions in the prefrontal cortex, cortico-striatal circuit and oxytocin neuron activation in the paraventricular nucleus have been previously reported in Shank3 KO mouse and rat [6, 50, 51].
Additionally, in mice, olfactory cues play a pivotal role in triggering social interaction, recognition, and motivation for conspecifics (for review, see ref. [40]) To our knowledge, this is the first report of the detection of oxytocin by VSNs. However, it is not yet clear how oxytocin receptor expression could impact olfaction. The olfactory function was affected in Shank3 KO mice, likely due to VNO dysfunction. Fewer VNO neurons responded to urine and oxytocin, suggesting potential dysfunction in VNO-dependent social behaviors in Shank3 KO mice, such as maternal or male-pup aggression (for review, see ref. [40]), and potentially impacting social interaction. Our results are consistent with the olfactory impairment in a rich odor environment observed in Shank3 heterozygous mice [52]. Interestingly, we showed that chronic isolation strongly modifies VSN responses in Shank3 KO mice. Exploring the comprehensive sensory dysfunction in these mice, including aspects of social touch and olfaction, will enable us to understand their contribution to the social phenotype.
Two studies have reported positive effects of chronic social isolation in two other mouse models of ASD. Chronic social isolation in Nlgn3R451C KI males increased social interaction with females and normalized male aggression phenotype towards females, along with female urine exploration to WT levels [53]. Additionally, the G protein-coupled receptor CX3CR1, implicated in ASD [11], exhibits increased protein levels due to social isolation in the prefrontal cortex, nucleus accumbens and hippocampus [54]. Chronic isolation reduced prepulse inhibition only in WT and also increased locomotion in both WT and Cx3cr1 KO male mice [54]. Although not tested on social interaction with the same sex, these studies align with our results in Shank3 KO mice. Indeed, we demonstrated that Shank3 KO mice are sensitive to subtle behavioral differences, prompting huddling behaviors when exposed to other KO individuals. Our study focuses on short-term interactions, contrasting with previous research that investigates the long-term effects of various genotypes on mouse models. Positive effects of co-housing with WT were observed in Homer1a KO mice [55], while detrimental outcomes were observed in 16p11.2 deletion mouse models [56]. However, we found that WT interacted more when exposed to WT conspecifics, which aligns with the effect observed in WT mice co-housed with Nlgn3 KO mice [57]. Nonetheless, further exploration of the impact of social isolation in other mouse models of ASD is necessary to generalize the effects on ASD-like features.
While our findings cannot be directly applied to autistic individuals, this study suggests that careful monitoring of the social environment during the implementation of inclusive classrooms could enhance the successful integration of children with ASD. For instance, children with ASD may face challenges when exposed to an entire classroom of 20–30 children, whereas exposure to fewer children (of their choice) might facilitate the implementation and sustainability of inclusive classrooms. In this study, we demonstrated that various social contexts influence social skills in both WT and Shank3 KO mice, highlighting the suitability of Shank3 KO mice as a model for back-translating behavioral interventions or mimicking inclusive classroom programs for children with ASD. Subsequent studies could explore the impact of short- or long-term exposure to a mix of Shank3 KO and WT mice, as well as different durations of isolation and reunion on social interaction and stereotyped behaviors, providing valuable insights. Finally, our observations could be extended to other mouse models of ASD, such as Fmr1 KO mice, which also exhibit abnormal sensory processing [47,48,49].
Data availability
All the raw data are available in supplementary tables and the movies and code are available upon request.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Association, 2013. https://doi.org/10.1176/appi.books.9780890425596.
Jiang M, Lu T, Yang K, Li X, Zhao L, Zhang D, et al. Autism spectrum disorder research: knowledge mapping of progress and focus between 2011 and 2022. Front Psychiatry. 2023;14:1096769.
Shenouda J, Barrett E, Davidow AL, Sidwell K, Lescott C, Halperin W, et al. Prevalence and disparities in the detection of autism without intellectual disability. Pediatrics. 2023;151:e2022056594.
Talantseva OI, Romanova RS, Shurdova EM, Dolgorukova TA, Sologub PS, Titova OS, et al. The global prevalence of autism spectrum disorder: a three-level meta-analysis. Front Psychiatry. 2023;14:1071181.
Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–57.
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42.
Yoo H. Genetics of autism spectrum disorder: current status and possible clinical applications. Exp Neurobiol. 2015;24:257–72.
McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–21.
Varni JW, Handen BL, Corey-Lisle PK, Guo Z, Manos G, Ammerman DK, et al. Effect of aripiprazole 2 to 15 mg/d on health-related quality of life in the treatment of irritability associated with autistic disorder in children: a post hoc analysis of two controlled trials. Clin Ther. 2012;34:980–92.
Kumar B, Prakash A, Sewal RK, Medhi B, Modi M. Drug therapy in autism: a present and future perspective. Pharmacol Rep. 2012;64:1291–304.
Annamneedi A, Gora C, Dudas A, Leray X, Bozon V, Crépieux P et al. Towards the convergent therapeutic potential of G protein‐coupled receptors in autism spectrum disorders. Br J Pharmacol. 2023; bph.16216.
Tobe R, Zhu Y, Gleissl T, Rossomanno S, Veenstra-VanderWeele J, Smith J, et al. Predictors of placebo response in three large clinical trials of the V1a receptor antagonist balovaptan in autism spectrum disorder. Neuropsychopharmacology. 2023;48:1201–16.
Lord C, Charman T, Havdahl A, Carbone P, Anagnostou E, Boyd B, et al. The Lancet Commission on the future of care and clinical research in autism. Lancet. 2022;399:271–334.
Curie A, Oberlander TF, Jensen KB. Placebo effects in children with autism spectrum disorder. Dev Med Child Neurol. 2023;65:1316–20.
Waters CF, Amerine Dickens M, Thurston SW, Lu X, Smith T. Sustainability of early intensive behavioral intervention for children with autism spectrum disorder in a community setting. Behav Modif. 2020;44:3–26.
Guthrie W, Wetherby AM, Woods J, Schatschneider C, Holland RD, Morgan L et al. The earlier the better: an RCT of treatment timing effects for toddlers on the autism spectrum. Autism. 2023; 136236132311591.
Asta L, Persico AM. Differential predictors of response to early start denver model vs. early intensive behavioral intervention in young children with autism spectrum disorder: a systematic review and meta-analysis. Brain Sci. 2022;12:1499.
Pujol CN, Pellissier LP, Clément C, Becker JAJ, Le Merrer J. Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities. Transl Psychiatry. 2018;8:197.
Yingling ME, Bell BA. Underutilization of early intensive behavioral intervention among 3-Year-Old children with autism spectrum disorder. J Autism Dev Disord. 2019;49:2956–64.
Eckes T, Buhlmann U, Holling H-D, Möllmann A. Comprehensive ABA-based interventions in the treatment of children with autism spectrum disorder – a meta-analysis. BMC Psychiatry. 2023;23:133.
Siller M, Morgan L, Fuhrmeister S, Wedderburn Q, Schirmer B, Chatson E et al. Feasibility and acceptability of a low-resource-intensive, transdiagnostic intervention for children with social-communication challenges in early childhood education settings. Autism 2023;136236132311792.
Vivanti G, Bent C, Capes K, Upson S, Hudry K, Dissanayake C, et al. Characteristics of children on the autism spectrum who benefit the most from receiving intervention in inclusive versus specialised early childhood education settings. Autism Res. 2022;15:2200–9.
Boujut E, Dean A, Grouselle A, Cappe E. Comparative study of teachers in regular schools and teachers in specialized schools in france, working with students with an autism spectrum disorder: stress, social support, coping strategies and burnout. J Autism Dev Disord. 2016;46:2874–89.
Zahid N, Jamil A, Nawaz I. Behavioral problems and academics of children in inclusive education – A cross-sectional survey. Heliyon. 2023;9:e13496.
Koenig KP, Feldman JM, Siegel D, Cohen S, Bleiweiss J. Issues in implementing a comprehensive intervention for public school children with autism spectrum disorders. J Prevent Intervent Community. 2014;42:248–63.
Padilla-Coreano N, Tye KM, Zelikowsky M. Dynamic influences on the neural encoding of social valence. Nat Rev Neurosci. 2022;23:535–50.
Lavenda-Grosberg D, Lalzar M, Leser N, Yaseen A, Malik A, Maroun M, et al. Acute social isolation and regrouping cause short- and long-term molecular changes in the rat medial amygdala. Mol Psychiatry. 2022;27:886–95.
de Chaumont F, Ey E, Torquet N, Lagache T, Dallongeville S, Imbert A, et al. Real-time analysis of the behaviour of groups of mice via a depth-sensing camera and machine learning. Nat Biomed Eng. 2019;3:930–42.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878.
Trouillet A-C, Keller M, Weiss J, Leinders-Zufall T, Birnbaumer L, Zufall F, et al. Central role of G protein Gαi2 and Gαi2+ vomeronasal neurons in balancing territorial and infant-directed aggression of male mice. Proc Natl Acad Sci USA. 2019;116:5135–43.
Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, et al. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902.
Russell V Lenth. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2023. https://CRAN.R-project.org/package=emmeans.
Artig F, Lohse L DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2022.http://florianhartig.github.io/DHARMa/.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
Kassambara A rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. https://CRAN.R-project.org/package=rstatix.
Gilbert C, McCafferty D, Le Maho Y, Martrette J-M, Giroud S, Blanc S, et al. One for all and all for one: the energetic benefits of huddling in endotherms. Biol Rev Camb Philos Soc. 2010;85:545–69.
Ibarra-Soria X, Levitin MO, Saraiva LR, Logan DW. The olfactory transcriptomes of mice. PLoS Genet. 2014;10:e1004593.
Nakahara TS, Camargo AP, Magalhães PHM, Souza MAA, Ribeiro PG, Martins-Netto PH, et al. Peripheral oxytocin injection modulates vomeronasal sensory activity and reduces pup-directed aggression in male mice. Sci Rep. 2020;10:19943.
Chamero P, Leinders-Zufall T, Zufall F. From genes to social communication: molecular sensing by the vomeronasal organ. Trends Neurosci. 2012;35:597–606.
Dudas A, Nakahara TS, Pellissier LP, Chamero P. Parenting behaviors in mice: olfactory mechanisms and features in models of autism spectrum disorders. Neurosci Biobehav Rev. 2024;161:105686.
Musardo S, Contestabile A, Knoop M, Baud O, Bellone C. Oxytocin neurons mediate the effect of social isolation via the VTA circuits. Elife. 2022;11:e73421.
Chabout J, Serreau P, Ey E, Bellier L, Aubin T, Bourgeron T, et al. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS One. 2012;7:e29401.
Binder MS, Bordey A. Semi-natural housing rescues social behavior and reduces repetitive exploratory behavior of BTBR autistic-like mice. Sci Rep. 2023;13:16260.
Zilkha N, Chuartzman SG, Sofer Y, Pen Y, Cum M, Mayo A, et al. Sex-dependent control of pheromones on social organization within groups of wild house mice. Curr Biol. 2023;33:1407–1420.e4.
Maloney SE, Sarafinovska S, Weichselbaum C, McCullough KB, Swift RG, Liu Y, et al. A comprehensive assay of social motivation reveals sex-specific roles of autism-associated genes and oxytocin. Cell Rep Methods. 2023;3:100504.
Bhaskaran A, Gauvrit T, Vyas Y, Bony G, Ginger M, Frick A. Endogenous noise of neocortical neurons drives atypical sensoryresponse variability in autism. Review. 2023. https://doi.org/10.21203/rs.3.rs-2572651/v1.
Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1 − /y mice. Nat Neurosci. 2014;17:1701–9.
Chari T, Hernandez A, Portera-Cailliau C. A novel head-fixed assay for social touch in mice uncovers aversive responses in two autism models. J Neurosci. 2023;43:7158–74.
Jacot-Descombes S, Keshav NU, Dickstein DL, Wicinski B, Janssen WGM, Hiester LL, et al. Altered synaptic ultrastructure in the prefrontal cortex of Shank3-deficient rats. Mol Autism. 2020;11:89.
Resendez SL, Namboodiri VMK, Otis JM, Eckman LEH, Rodriguez-Romaguera J, Ung RL, et al. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J Neurosci. 2020;40:2282–95.
Ryndych D, Sebold A, Strassburg A, Li Y, Ramos RL, Otazu GH. Haploinsufficiency of Shank3 in mice selectively impairs target odor recognition in novel background Odors. J Neurosci. 2023;43:7799–811.
Burrows EL, Eastwood AF, May C, Kolbe SC, Hill T, McLachlan NM, et al. Social isolation alters social and mating behavior in the R451C neuroligin mouse model of autism. Neural Plast. 2017;2017:8361290.
Zhou H, Wang J, Zhang Y, Shao F, Wang W. The role of microglial CX3CR1 in Schizophrenia-related behaviors induced by social isolation. Front Integr Neurosci. 2020;14:551676.
Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF, et al. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–54.
Yang M, Lewis F, Foley G, Crawley JN. In tribute to Bob blanchard: divergent behavioral phenotypes of 16p11.2 deletion mice reared in same-genotype versus mixed-genotype cages. Physiol Behav. 2015;146:16–27.
Kalbassi S, Bachmann SO, Cross E, Roberton VH, Baudouin SJ. Male and female mice lacking Neuroligin-3 modify the behavior of their wild-type littermates. eNeuro. 2017;4:ENEURO.0145-17.2017.
Acknowledgements
Mouse breeding and care was performed by the trained technicians of PAO, the rodent INRAE Animal Physiology Facility (https://doi.org/10.15454/1.5573896321728955E12). We thank Dr Guillaume Sallé for the Python script to automatically analyze ANY-maze outputs for the Y-maze. This work has benefited from the facilities and expertise of the “Plateforme d’Imagerie Cellulaire” (PIC) and the “Laboratoire Phenotypage Endocrinologie” (LPE) of the UMR PRC, INRAE. We acknowledge the use of ChatGPT, developed by OpenAI, for assistance with English editing during the preparation of this manuscript. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 851231). This work was supported by the INRAE “SOCIALOME” project (PAF_29). LPP, AD, and CG acknowledge the LabEx MabImprove (grant ANR-10-LABX-53-01) for the financial support of CG and AD PhD’s co-fund. The funders played no role in the design, analysis, or interpretation of the study’s data.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design, the acquisition, analysis, or interpretation of data of this work and all authors have drafted the work or substantively revised it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gora, C., Dudas, A., Court, L. et al. Effect of the social environment on olfaction and social skills in wild-type and a mouse model of autism. Transl Psychiatry 14, 464 (2024). https://doi.org/10.1038/s41398-024-03174-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03174-6