Abstract
Obsessive-compulsive disorder (OCD) is a highly heterogeneous disorder, with notable variations among cases in structural brain abnormalities. To address this heterogeneity, our study aimed to delineate OCD subtypes based on individualized gray matter morphological differences. We recruited 100 untreated, first-episode OCD patients and 106 healthy controls for structural imaging scans. Utilizing normative models of gray matter volume, we identified subtypes based on individual morphological abnormalities. Sensitivity analyses were conducted to validate the reproducibility of clustering outcomes. To gain deeper insights into the connectomic and molecular underpinnings of structural brain abnormalities in the identified subtypes, we investigated their associations with normal brain network architecture and the distribution of neurotransmitter receptors/transporters. Our findings revealed two distinct OCD subtypes exhibiting divergent patterns of structural brain abnormalities. Sensitivity analysis results confirmed the robustness of the identified subtypes. Subtype 1 displayed significantly increased gray matter volume in regions including the frontal gyrus, precuneus, insula, hippocampus, parahippocampal gyrus, amygdala, and temporal gyrus, while subtype 2 exhibited decreased gray matter volume in the frontal gyrus, precuneus, insula, superior parietal gyrus, temporal gyrus, and fusiform gyrus. When considering all patients collectively, structural brain abnormalities nullified. The identified subtypes were characterized by divergent disease epicenters. Specifically, subtype 1 showed disease epicenters in the middle frontal gyrus, while subtype 2 displayed disease epicenters in the striatum, thalamus and hippocampus. Furthermore, structural brain abnormalities in these subtypes displayed distinct associations with neurotransmitter receptors/transporters. The identified subtypes offer novel insights into nosology and the heterogeneous nature of OCD.
Similar content being viewed by others
Introduction
Obsessive-compulsive disorder (OCD) stands as a prevalent and serious mental health condition, affecting approximately 1–3% of adults over their lifetime [1]. This disorder exhibits remarkable heterogeneity, with patients presenting diverse clinical manifestations and variable responses to pharmacotherapy [2, 3] to extent that cases with OCD can exhibit non-overlapping symptom profiles [4]. To address the heterogeneity, clinical psychiatrist usually stratifies OCD patients into subtypes based on clinical symptoms, such as washing and checking [5]. However, while this approach has been successful, it also possesses limitations. Patients across different subtypes may still share similar aberrant patterns [6, 7], and a universally accepted classification system remains elusive [2]. Furthermore, clinical symptoms interact intricately with biological mechanisms and may evolve throughout the course of the illness [8, 9]. The identification of neurophysiological subtypes within OCD holds promise for gaining a more objective comprehension of the disorder’s underlying biological mechanisms and enhancing personalized diagnostic and therapeutic strategies. Nonetheless, reliably pinpointing such subtypes remains a challenge. In recent years, researchers have increasingly turned to objective neuroimaging data to tackle this issue within mental health disorders [10].
Previous structural neuroimaging investigations have explored group-level gray matter morphological disparities in OCD, highlighting abnormalities in brain regions including the medial frontal gyrus, hippocampus, precuneus, and insula [11,12,13,14]. However, the considerable intersubject heterogeneity poses challenges in identifying reproducible neuroimaging biomarkers for clinical decision-making [15, 16]. Despite this heterogeneity, many neuroimaging studies in OCD continue to utilize traditional case-control methodologies, primarily aimed at capturing group-level effects [17]. Yet, it’s been demonstrated that such approaches overlook neuroimaging characteristics unique to individuals [18,19,20,21]. To address this heterogeneity, the normative model has emerged as a potential solution [20]. This model determines individualized neuroimaging anomalies by evaluating deviations from the normal distribution [20]. Leveraging the normative model, neuroimaging inquiries have successfully pinpointed subject-level structural brain alterations in various mental disorders such as autism, schizophrenia, and depression [8, 18, 22,23,24]. However, whether individualized structural deviations could reveal OCD subtypes remains to be elucidated.
Recent neuroimaging studies have shown that neuropsychiatric disorders affect intrinsic brain networks. Structural brain abnormalities, originating in specific local brain regions known as ‘disease epicenters’, spread to other brain regions along with the pathways defined by normal brain network architecture, supporting the network-based spreading hypothesis [25,26,27,28]. Different psychiatric disorders and their subtypes have distinct disease epicenters, reflecting their unique biological underpinnings and progression patterns [29, 30]. In OCD, a similar association between structural brain abnormalities and normal brain network has also been observed [31, 32]. Furthermore, the availability of a whole-brain atlas of neurotransmitter receptors and transporters has enabled researchers to explore the molecular substrates underlying these structural and functional brain abnormalities [33]. Both local molecular factors and the global brain network architecture shape cortical abnormalities in psychiatric disorders [29]. Understanding the molecular bases of these structural abnormalities helps connect neuroimaging findings to the biological mechanisms underlying OCD.
In this study, we aimed to identify distinct OCD subtypes based on individualized gray matter morphological abnormalities compared to normative expectations. We recruited 100 first-episode, untreated patients diagnosed with OCD and 106 healthy controls, who underwent structural imaging scans. First, we created individualized deviation maps to capture gray matter morphological abnormalities using normative modeling and used these maps to determine OCD subtypes. We then performed a series of sensitivity analyses to assess the reproducibility and generalizability of the identified subtypes. Subsequently, we examined the disease epicenters of these subtypes, hypothesizing that they would exhibit divergent disease epicenters. Additionally, to gain deeper insights into the molecular underpinnings of these structural abnormalities, we explored the associations between the structural brain abnormalities in these subtypes and the distribution of neurotransmitter receptors and transporters.
Methods
Participants
This study adhered to the principles outlined in the Helsinki Declaration and received approval from the research ethics committee of the First Affiliated Hospital of Zhengzhou University. Before participating, each individual provided informed consent.
A total of 100 first-episode, untreated patients diagnosed with OCD were recruited from outpatient services within the Department of Psychiatry at the First Affiliated Hospital of Zhengzhou University. Diagnosis was established independently by two experienced psychiatrists according to the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) for OCD. The severity of symptoms was evaluated using the Yale–Brown Obsessive Compulsive Scale (Y-BOCS) [34]. Exclusion criteria ensured that none of the patients had a history of neurological disorders, brain trauma, concurrent mental or psychotic disorders, or first-degree relatives with similar conditions. Healthy controls (HCs, n = 106) were enlisted from the community via poster advertisements. All participants were of Han Chinese ethnicity, right-handed, and underwent screening to ensure they met specified exclusion criteria. These criteria included abstaining from medications such as anesthetics and analgesics within the preceding month, no history of substance abuse, brain tumors, traumatic brain injury, surgeries, or organic body diseases, as well as the absence of cardiovascular diseases, diabetes, or hypertension. Additionally, individuals with contraindications for MRI scanning or other structural brain abnormalities were excluded from the study.
Scan acquisition
High-resolution T1-weighted anatomical images of participants were acquired using on 3-Tsela GE Discovery MR750 scanner (General Electric, Fairfield Connecticut, USA) using the following parameters: repetition time = 8164 ms, echo time = 3.18 ms, inversion time = 900 ms, flip angle = 7 degrees, resolution matrix = 256 × 256, slices = 188, thickness = 1.0 mm and voxel size = 1 × 1 × 1 mm3.
Imaging data processing
Voxel-wise gray matter volume (GMV) for each participant was derived using voxel-based morphometry in the CAT 12 toolbox (http://dbm.neuro.uni-jena.de/cat12/). The standard CAT 12 pipeline was followed, as detailed in reference [35]. This pipeline encompassed bias-field correction, brain segmentation into gray and white matter and cerebrospinal fluid, correction for partial volume effects, normalization to Montreal Neurological Institute space, resampling to 1.5 mm³, and nonlinear modulation [35, 36]. Subsequently, the gray matter maps underwent smoothing with a 6-mm full width at half maximum Gaussian kernel. Additionally, the total intracranial volume (TIV) of each participant was computed. To ensure data quality, the Image Quality Rating (IQR) was recorded [24].
Subtyping analysis based on individualized gray matter morphological abnormalities
First, we estimated individualized gray matter morphological abnormalities by employing the normative modeling. Following methods used in previous studies [18, 20, 24], we trained a Gaussian process regression model to estimate the normative range of regional GMVs from age and sex in HCs according to the brain connectome atlas [37]. This trained model was then applied to each patient to generate predicted GMVs. Subsequently, Z scores were calculated to quantify deviations of the predicted GMVs from normal distributions. Positive Z scores indicated higher GMVs in patients compared to HCs, and vice versa. This process resulted in a Z-score vector (246 × 1) for each patient, representing their individualized gray matter morphological abnormalities.
Next, we employed the k-means algorithm to identify OCD subtypes, with the Z scores serving as features and the squared Euclidean distance as the distance metric. The optimal number of subtypes, ranging from 2 to 10, was determined using silhouette values [38]. For each number, the k-means algorithm was repeated 100 times to avoid local minima during the initialization of centroid positions [39].
Subsequently, for each subtype, we examined the gray matter morphological abnormalities relative to HCs using a two-sample t-test, with sex, age, and TIV as covariates.
Reproducibility analysis
A series of sensitivity analyses were undertaken to evaluate the reproducibility of the clustering outcomes. Firstly, to mitigate the influence of a small subset of patients, we randomly selected 90% of the patients and conducted k-means clustering on this subset. We then computed the Adjusted Rand Index (ARI) between the subtype assignments based on this subset and those derived from the entire patient cohort. This process was repeated 1000 times. Secondly, we validated clustering outcomes by employing another brain atlas with different resolutions, specifically the Automated Anatomical Labeling (AAL) atlas. The AAL atlas contains 90 cortical and subcortical regions (excluding the cerebellum) [40]. This validation step ensured that our results were not contingent on the specific brain parcellation scheme used in the analysis, thereby enhancing the reproducibility of our findings. Thirdly, we validated our results using another distance metric in the k-means algorithm, namely correlation distance (1 min Pearson’s correlation coefficient).
Disease epicenter mapping for each subtype
Subsequently, we explored whether structural brain abnormalities of the identified subtypes exhibited distinct disease epicenters. We evaluated the normal brain network using the structural covariance network. This network characterizes the coordination of regional volumes among brain regions, potentially reflecting shared developmental pathways [41,42,43] and has been implicated in the pathology of OCD [43, 44]. Notably, our previous research has indicated that the structural covariance network demonstrated a stronger association with gray matter morphological abnormalities compared to the functional network in mental disorders including OCD [32, 45].
The normal structural covariance network was constructed using another extensive dataset from a single site, comprising 492 healthy individuals aged 19–80 years. Further details about this dataset are available elsewhere [46]. The structural covariance network was constructed by computing pairwise correlation coefficients of regional gray matter volumes across subjects, resulting in a 246 × 246 structural covariance (SC) network where negative correlations were set to zero [30]. We examined the relationship between the SC network and the gray matter morphological abnormalities of the identified subtypes, employing methodologies established in prior studies [28, 30]. For each subtype, the normalized collective abnormalities/differences of structural neighbors of region i (\({D}_{i}^{{sc}}\)) were obtained as follows:
Where \({D}_{i}^{{sc}}\) represents the normalized collective abnormalities of the structural neighbors of region i, Di is regional gray matter morphological abnormalities (unthresholded t statistic) of region i, Ni is the number of neighbors of region i with a structural covariance connection, SCi,j is the strength of structural covariance between region i and region j. For each subtype, the \({D}_{i}^{{sc}}\) was predicted using regional abnormalities of the neighboring regions. The Pearson’s correlation coefficient between true abnormalities (Di) and predicted ones (\({D}_{i}^{{sc}}\)) across all brain regions was calculated.
A brain region was considered as the disease epicenter if, along with its connected neighbors, it exhibited pronounced atrophy than other regions [28, 30]. Brain regions were ranked based on their atrophy, and SC-informed abnormalities (\({D}_{i}^{{sc}}\)) in ascending order. Then, the average ranking values were considered as the disease epicenter likelihood rankings, and significance was determined through permutation testing (10,000 times).
Association between neurotransmitter receptors/transporters with gray matter morphological abnormalities of the identified subtypes
We then investigated the link between neurotransmitter receptors/transporters profiles and gray matter morphological abnormalities of the identified subtypes. These profiles were obtained from PET-derived neurotransmitter receptors/transporters atlas shared by Hansen et al. [33]. The neurotransmitter receptors/transporters include serotonin (5HT1A [47], 5HT1B [47,48,49,50,51,52,53], 5HT2A [54], 5HT4 [54], 5HT6 [55, 56], 5HTT [54]), norepinephrine (α4β2 [53, 57], M1 [58], VAChT [59, 60]), cannabinoid (CB1 [61,62,63,64]), dopamine (D1 [65], D2 [66,67,68,69], DAT [70]), GABA (GABAa [71]), histamine (H3 [72]), glutamate (mGluR5 [73, 74], NMDA [75, 76]), opioid (MOR [77]), and norepinephrine (NET [78,79,80,81]). PET images were averaged across participants within each study, normalized to the MNI-ICBM 152 non-linear 2009 template, and then parcellated into 246 brain regions. The average regional neurotransmitter receptor/transporter densities were then Z-scored [29, 33].
For each subtype, we developed a multilinear model linking neurotransmitter receptors/transporters profiles with gray matter differential pattern. The significance of these models was assessed through permutation testing (10,000 times), false discovery rate (FDR) correction.
Subsequently, to better understand the relative importance of each predictor (in this case, neurotransmitter receptor/transporter profile) in contributing to the model’s overall predictive power, we conducted a dominance analysis for each multilinear model. Dominance analysis aids in determining how each predictor influences the model’s fit by considering all possible predictor combinations within the model [82]. This involves applying the multilinear model to every potential subset of predictors, resulting in 2n–1 subset models for a model with n predictors. In our study, we utilized the total dominance to assess each predictor’s relative significance [29, 82]. Total dominance is the mean increase in explained variance (R2) contributed by each predictor across all subset models in which it is included [82, 83].
Results
Clinical demographics
Patients with OCD did not demonstrate significant differences from HCs regarding age, sex, TIV and image quality (IQR). However, in comparison to HCs, individuals with OCD showed significantly fewer years of education (p < 0.001). These results are outlined in Table 1.
Two subtypes manifesting opposite gray matter morphological abnormalities are identified
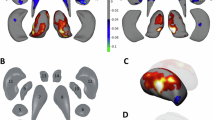
Using individualized gray matter morphological abnormalities as features, we identified two OCD subtypes, exhibiting contrasting patterns of gray matter morphological abnormalities relative to HCs, as shown in Fig. 1. Subtype 1 had significantly increased gray matter volume in the frontal gyrus, precuneus, insula, hippocampus, parahippocampal gyrus, amygdala, and temporal gyrus (FDR corrected p < 0.05). Conversely, subtype 2 showed significantly decreased gray matter volume in the frontal gyrus, precuneus, insula, superior parietal gyrus, temporal gyrus, and fusiform (FDR corrected p < 0.05). When considering all patients together, there were no significant gray matter volume differences compared to HCs.
Regarding demographic and clinical variables, subtype 1 had a higher proportion of male patients than subtype 2 (χ2 = 16.161, p < 0.001). Other variables, such as age of onset, illness duration, and symptom severity, did not differ significantly between the subtypes (p > 0.05). Additionally, there were no significant differences between the subtypes in TIV and IQR, indicating that these factors did not influence our results. These findings are summarized in Table S1.
Reproducibility analysis results
To assess the reproducibility of the clustering results, we validated them in randomly selected subsamples and using another brain atlas with different resolutions. These results confirmed the robustness and reproducibility of our results. Specifically, the average ARI values (Fig. 2A) subtyping results from the entire patient cohort and those from randomly selected subsets were 0.98 (±0.04). This high ARI alleviates concerns about biases potentially introduced by individual patients. Furthermore, the optimal number of subtypes remained two when using an alternative brain atlas (AAL), as evidenced by silhouette values in Fig. 2B. The two subtypes identified with the AAL brain atlas also exhibited contrasting gray matter morphological abnormalities (FDR corrected p < 0.05) compared to HCs, as shown in Fig. 2C. The ARI between subtype assignments using different brain atlases reached 0.74, demonstrating a good level of consistency across different parcellation schemes. Thirdly, when using correlation metric in the k-means clustering, the optimal number of subtypes was still two for both brain atlases. The corresponding silhouette values are presented in Fig. S1. The ARI between subtype assignments using different distant metrics was 0.62 for brain connectome atlas and 0.62 for AAL. Overall, these results confirm the reproducibility and robustness of our subtyping outcomes.
A Adjusted Rand Index (ARI) values between the subtyping results based on randomly selected subsamples and those based on all patients. B Silhouette values for each number of subtypes using the AAL brain atlas. C Significant gray matter morphological abnormalities (FDR corrected p < 0.05) relative to healthy controls for the identified subtypes using the AAL brain atlas.
Subtype exhibit divergent structural covariance network-informed disease epicenters
In both subtypes, we found significant correlations between regional gray matter morphological abnormalities and SC-informed abnormalities (subtype 1: r = 0.44, permutation pFDR < 0.001, as shown in Fig. 3A; subtype 2: r = 0.50, permutation pFDR < 0.001, see Fig. 3A). These results indicate that the observed gray matter morphological abnormalities in both subtypes are influenced by the normal SC network.
Additionally, we observed distinct disease epicenters in the two subtypes (see Fig. 3B). Specifically, subtype 1 exhibited disease epicenters mainly in the middle frontal gyrus (permutation p < 0.01). In contrast, subtype 2 displayed disease epicenters in the striatum, thalamus and hippocampus (permutation p < 0.01).
Gray matter morphological abnormalities of subtypes exhibit distinct associations with neurotransmitter receptors/transporters
We further investigated the connections between neurotransmitter receptors/transporters and gray matter morphological abnormalities in the identified subtypes. This investigation involved constructing two multilinear models that accounted for the spatial distributions of receptors/transporters and the differential patterns of gray matter morphological abnormalities in each subtype. The goodness-of-fit of these models (adjusted R2) was 0.43 (F-statistic (246,226) = 10.80) for subtype 1 and 0.34 (F-statistic (246,226) = 7.76) for subtype 2, as illustrated in Fig. 4A. Importantly, all FDR-corrected permutation p-values were less than 0.001 (Fig. 4B). Moreover, our analysis utilizing dominance analysis revealed key neurotransmitter receptors associated with each subtype. Specifically, 5HT1A emerged as significant for subtype 1, whereas 5HT2A was identified as crucial for subtype 2 (see Fig. 4C).
A Multilinear models are constructed to analyze the association between neurotransmitter receptors/transporters and gray matter morphological abnormalities of the identified subtypes. The bar plot displays the corresponding model goodness-of-fit (adjusted R2), with all FDR-corrected permutation p-values being less than 0.001. B Permutation testing results. C Dominance analysis is employed to determine the relative importance of predictors for each multilinear model. Total dominance values, indicative of the predictors’ relative importance, are depicted.
Discussion
To address the heterogeneity of structural brain abnormalities in OCD, we identified two robust subtypes using subject-level gray matter abnormalities, assessed using the normative model of gray matter. These subtypes demonstrated opposite patterns of gray matter abnormalities relative to HCs, but shared indistinguishable clinical profiles. Interestingly, when combined, all patients showed no significantly differential gray matter abnormalities. Furthermore, subtype 1 exhibited disease epicenters in the frontal gyrus, while subtype 2 showed them in the striatum, thalamus and hippocampus. Additionally, we found distinct associations between gray matter morphological abnormalities in these subtypes and neurotransmitter receptors/transporters. Specifically, the abnormal pattern in subtype 1 was primarily linked to 5HT1A, whereas that in subtype 2 was mainly associated with 5HT2A. These findings provide insights into the underlying molecular mechanisms of these subtypes. In summary, our results reveal two reproducible subtypes characterized by distinct gray matter morphological differential patterns and underlying molecular bases. These insights contribute significantly to the nosology of OCD, enhancing our understanding of its heterogeneous nature.
Psychiatric disorders, including OCD, are well-known for their heterogeneity among cases. Patients with OCD exhibit a wide range of symptom profiles, disease trajectories and treatment responses [2, 3]. This intersubject variability leads to conflicting findings in neuroimaging studies [84, 85]. For instance, while the hippocampus is a key region in OCD pathology, studies report both increased and decreased gray matter volumes in this area [86,87,88]. Inconsistent findings about structural abnormalities hint the existence of OCD subtypes. In the current study, we unveiled two distinct OCD subtypes with contrasting anormal patterns. Subtype 1 only demonstrated increased gray matter volume, while subtype 2 only decreased gray matter volume in widespread brain regions. Most of these brain regions showing structural abnormalities are overlapped (e.g. hippocampus). However, when combining all patients, no significant structural abnormalities emerged. These results, indicating that previous inconsistent findings may stem from differing subtype proportions [86,87,88]. These subtypes align with previous studies using various clustering algorithms and neuroimaging metrics [87, 89]. In spite of tremendous differences in structural brain abnormalities, these two subtypes demonstrated indistinguishable clinical profiles. Despite structural differences, the subtypes had similar clinical profiles, suggesting that symptom-based subtyping alone may miss underlying neuroanatomical distinctions. Moreover, sensitivity analysis results excluded the potential factors (e.g. imaging quality) on our results and verified the reproducibility of the identified subtypes.
Another significant finding is the observation of divergent disease epicenters within the identified subtypes, as informed by the structural covariance network. Previous neuroimaging researches suggest that structural brain abnormalities in psychiatric disorders tend to adhere to the normal connectome architecture rather than being randomly distributed [28, 32]. Typically, these abnormalities originate from specific disease epicenters and then propagate to other brain regions, likely reflecting shared developmental or maturation trajectories [25, 27, 28, 42, 43]. Our previous studies have established a link between normal brain networks and gray matter morphological abnormalities, supporting the network-based spreading model in OCD [31, 32]. In the current study, we found that the identified subtypes exhibited divergent disease epicenters in regions such as the hippocampus, striatum, and insula, which are consistently implicated across various psychiatric disorders [90]. These regions display structural abnormalities early in the course of illness and may exert causal effects on the structural abnormalities observed in other brain regions [91]. Divergent disease epicenters in these subtypes hint distinct pathological progression trajectories in the identified subtypes. This hypothesis could be confirmed in future studies using longitudinal data.
To explore the biological mechanisms underlying these structural abnormalities, we examined their associations with neurotransmitter receptor/transporter profiles. Neurotransmitter dysfunction is a key aspect of psychiatric disorders, with current antidepressants and antipsychotics targeting neurotransmitter functions [29]. The serotonergic system, in particular, plays a crucial role in OCD pathogenesis, with serotonin reuptake inhibitors being the first-line pharmacotherapy [92, 93]. While neurotransmitter receptor profiles are linked to structural and functional dysfunctions in psychiatric disorders [29, 33], up to half of all OCD patients do not benefit from this combination treatment in a clinically meaningful way [93]. This prompts efforts aimed at identifying diagnostic subgroups with specific pathogenic mechanisms that may mediate treatment resistance, as well as improving treatment options. Our study found that subtype 1 abnormalities were predominantly linked to 5HT1A, while subtype 2 was mainly associated with 5HT2A, providing insights into the distinct molecular mechanisms of these subtypes. These receptors are implicated in the neuropathophysiology of OCD. For instance, increased 5HT2A availability in the caudate nucleus has been reported in OCD patients [94]. Second-generation antipsychotics may induce or worsen obsessive-compulsive symptoms through 5-HT2A receptor blockade in regions like the anterior cingulate cortex, the dorsal posterior prefrontal cortex and the orbitofrontal cortex [95, 96]. Modulation of 5HT1A and 5HT2A is also critical to the antidepressant efficacy of 5-HT reuptake inhibitors [97, 98]. These findings suggest that the identified subtypes may exhibit distinct treatment responses, which could be explored in future research.
This study has several limitations. First, it was conducted using a cross-sectional sample, raising questions about the stability and generalizability of the identified subtypes. Future studies should validate our results with independent and longitudinal datasets to confirm their robustness. Second, the limited clinical information available restricted our ability to explore potential differences in other clinical aspects between the subtypes. Comprehensive clinical data in future studies could provide deeper insights into subtype characteristics. Especially, the multivariate correlation analysis between neuroimaging feature and clinical characteristics, and the potential of individualized abnormalities to predict the prognosis can be further investigated [99,100,101,102]. Third, individual variations in neurotransmitter systems may affect the observed associations between structural abnormalities and neurotransmitter systems, which were based on publicly available datasets [103]. Finally, patients with OCD in our study had lower educational levels compared to healthy controls. However, we found no significant differences in educational level between the subtypes, suggesting minimal impact on the clustering results. Further investigation into the effects of educational level would still be valuable.
In conclusion, we identified two robust OCD subtypes based on individualized gray matter morphological abnormalities. These subtypes exhibit contrasting patterns of gray matter morphological abnormalities relative to normal population and have divergent structural covariance network-informed disease epicenters. Additionally, these abnormalities are distinctly associated with neurotransmitter receptors/transporters, indicating distinct molecular underpinnings. These findings offer novel insights into nosology and heterogeneous nature of OCD.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code availability
Gray matter volume is assessed with the Computational Anatomy Toolbox (CAT12, http://dbm.neuro.uni-jena.de/cat12/). The normative model is freely available (GAUSSIAN PROCESS REGRESSION AND CLASSIFICATION Toolbox version 4.2, http://www.GaussianProcess.org/gpml/code). Group-level differences in GMV are obtained using SPM12 (http://www.fil.ion.ucl.ac.uk/spm). Dominance analysis is performed with python toolbox (https://github.com/kaladharprajapati/dominance_analysis).
References
Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol Psychiatry. 2010;15:53–63.
McKay D, Abramowitz JS, Calamari JE, Kyrios M, Radomsky A, Sookman D, et al. A critical evaluation of obsessive-compulsive disorder subtypes: symptoms versus mechanisms. Clin Psychol Rev. 2004;24:283–313.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81.
Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:228–38.
Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76.
Ravindran A, Richter M, Jain T, Ravindran L, Rector N, Farb N. Functional connectivity in obsessive-compulsive disorder and its subtypes. Psychol Med. 2020;50:1173–81.
Yoo SY, Roh MS, Choi JS, Kang DH, Ha TH, Lee JM, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci. 2008;23:24–30.
Sun X, Sun J, Lu X, Dong Q, Zhang L, Wang W, et al. Mapping neurophysiological subtypes of major depressive disorder using normative models of the functional connectome. Biol Psychiatry. 2023;94:936–47.
Haas SS, Ge R, Agartz I, Amminger GP, Andreassen OA, Bachman P, et al. Normative modeling of brain morphometry in clinical high risk for psychosis. JAMA Psychiatry. 2024;81:77–88.
Beijers L, Wardenaar KJ, van Loo HM, Schoevers RA. Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol Psychiatry. 2019;24:888–900.
Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877–87.
Menzies L, Williams GB, Chamberlain SR, Ooi C, Fineberg N, Suckling J, et al. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry. 2008;165:1308–15.
Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, et al. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res. 2003;122:37–47.
Luo LK, You WF, DelBello MP, Gong QY, Li F. Recent advances in psychoradiology. Phys Med Biol. 2022;67:23TR01.
Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin N Am. 2000;23:563–86.
Boedhoe PSW, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA obsessive-compulsive disorder working group. Am J Psychiatry. 2018;175:453–62.
Luo LK, Li Q, Wang YX, He N, Wang YX, You WF, et al. Shared and disorder-specific alterations of brain temporal dynamics in obsessive-compulsive disorder and schizophrenia. Schizophr Bull. 2023;49:1387–98.
Wolfers T, Doan NT, Kaufmann T, Alnæs D, Moberget T, Agartz I, et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75:1146–55.
Lv JL, Di Biase M, Cash RFH, Cocchi L, Cropley VL, Klauser P, et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. Mol Psychiatry. 2021;26:3512–23.
Marquand AF, Rezek I, Buitelaar J, Beckmann CF. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 2016;80:552–61.
Cai M, Ma J, Wang Z, Zhao Y, Zhang Y, Wang H, et al. Individual-level brain morphological similarity networks: current methodologies and applications. CNS Neurosci Ther. 2023;29:3713–24.
Wolfers T, Beckmann CF. Individual differences v. the average patient: mapping the heterogeneity in ADHD using normative models. Psychol Med. 2020;50:314–23.
Zabihi M, Oldehinkel M, Wolfers T, Frouin V, Goyard D, Loth E, et al. Dissecting the heterogeneous cortical anatomy of autism spectrum disorder using normative models. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:567–78.
Han SQ, Cui Q, Zheng RP, Li SY, Zhou BQ, Fang KK, et al. Parsing altered gray matter morphology of depression using a framework integrating the normative model and non-negative matrix factorization. Nat Commun. 2023;14:4053.
Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–27.
Yau Y, Zeighami Y, Baker TE, Larcher K, Vainik U. Network connectivity determines cortical thinning in early Parkinson’s disease progression. Nat Commun. 2018;9:12.
Wannan CMJ, Cropley VL, Chakravarty MM, Bousman C, Ganella EP, Bruggemann JM, et al. Evidence for network-based cortical thickness reductions in schizophrenia. Am J Psychiatry. 2019;176:552–63.
Shafiei G, Markello RD, Makowski C, Talpalaru A, Kirschner M, Devenyi GA, et al. Spatial patterning of tissue volume loss in schizophrenia reflects brain network architecture. Biol Psychiatry. 2020;87:727–35.
Hansen JY, Shafiei G, Vogel JW, Smart K, Bearden CE, Hoogman M, et al. Local molecular and global connectomic contributions to cross-disorder cortical abnormalities. Nat Commun. 2022;13:4682.
Li J, Long ZL, Sheng W, Du L, Qiu J, Chen HF, et al. Transcriptomic similarity informs neuromorphic deviations in depression biotypes. Biol Psychiatry. 2024;95:414–25.
Liu L, Jia DY, Zhang CW, Wu NK, Kong LQ, Han SQ. Predictive spread of obsessive-compulsive disorder pathology using the network diffusion model. J Affect Disord. 2024;351:120–7.
Wen BH, Xu YH, Fang KK, Guo HR, Liu H, Liu L, et al. Gray matter morphological abnormities are constrained by normal structural covariance network in OCD. Prog Neuropsychopharmacol Biol Psychiatry. 2024;129:110884.
Hansen JY, Shafiei G. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat Neurosci. 2022;25:1569–81.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The yale-brown obsessive compulsive scale. I. development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11.
Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. 2009;27:1163–74.
Xue K, Guo L, Zhu W, Liang S, Xu Q, Ma L, et al. Transcriptional signatures of the cortical morphometric similarity network gradient in first-episode, treatment-naive major depressive disorder. Neuropsychopharmacology. 2023;48:518–28.
Fan LZ, Li H, Zhuo JJ, Zhang Y, Wang JJ, Chen LF, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26.
Han S, Zheng R, Li S, Zhou B, Jiang Y, Fang K, et al. Resolving heterogeneity in depression using individualized structural covariance network analysis. Psychol Med. 2023;53:5312–21.
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–76.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Yun JY, Jang JH, Kim SN, Jung WH, Kwon JS. Neural correlates of response to pharmacotherapy in obsessive-compulsive disorder: individualized cortical morphology-based structural covariance. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:126–33.
Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–36.
Han S, Xu Y, Guo HR, Fang K, Wei Y, Liu L, et al. Resolving heterogeneity in obsessive-compulsive disorder through individualized differential structural covariance network analysis. Cereb Cortex. 2023;33:1659–68.
Han SQ, Xue KK, Chen Y, Xu YH, Li SY, Song XQ, et al. Identification of shared and distinct patterns of brain network abnormality across mental disorders through individualized structural covariance network analysis. Psychol Med. 2023;53:6780–91.
Han S, Fang K, Zheng R, Li S, Zhou B, Sheng W, et al. Gray matter atrophy is constrained by normal structural brain network architecture in depression. Psychol Med. 2024;54:1318–28.
Wei D, Zhuang K, Ai L, Chen Q, Yang W, Liu W, et al. Structural and functional brain scans from the cross-sectional Southwest University adult lifespan dataset. Sci Data. 2018;5:180134.
Savli M, Bauer A, Mitterhauser M, Ding YS, Hahn A, Kroll T, et al. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage. 2012;63:447–59.
Gallezot JD, Nabulsi N, Neumeister A, Planeta-Wilson B, Williams WA, Singhal T, et al. Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [(11)C]P943 in humans. J Cereb Blood Flow Metab. 2010;30:196–210.
Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, et al. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry. 2011;68:892–900.
Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, et al. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology. 2011;213:547–53.
Matuskey D, Bhagwagar Z, Planeta B, Pittman B, Gallezot JD, Chen J, et al. Reductions in brain 5-HT1B receptor availability in primarily cocaine-dependent humans. Biol Psychiatry. 2014;76:816–22.
Saricicek A, Chen J, Planeta B, Ruf B, Subramanyam K, Maloney K, et al. Test-retest reliability of the novel 5-HT1B receptor PET radioligand [11C]P943. Eur J Nucl Med Mol Imaging. 2015;42:468–77.
Baldassarri SR, Hillmer AT, Anderson JM, Jatlow P, Nabulsi N, Labaree D, et al. Use of electronic cigarettes leads to significant Beta2-nicotinic acetylcholine receptor occupancy: evidence from a PET imaging study. Nicotine Tob Res. 2018;20:425–33.
Beliveau V, Ganz M. A high-resolution in vivo atlas of the human Brain’s serotonin system. J Neurosci. 2017;37:120–8.
Radhakrishnan R, Nabulsi N, Gaiser E, Gallezot JD, Henry S, Planeta B, et al. Age-related change in 5-HT(6) receptor availability in healthy male volunteers measured with (11)C-GSK215083 PET. J Nucl Med. 2018;59:1445–50.
Radhakrishnan R, Matuskey D, Nabulsi N, Gaiser E, Gallezot JD, Henry S, et al. In vivo 5-HT(6) and 5-HT(2A) receptor availability in antipsychotic treated schizophrenia patients vs. unmedicated healthy humans measured with [(11)C]GSK215083 PET. Psychiatry Res Neuroimaging. 2020;295:111007.
Hillmer AT, Esterlis I, Gallezot JD, Bois F, Zheng MQ, Nabulsi N, et al. Imaging of cerebral α4β2* nicotinic acetylcholine receptors with (-)-[(18)F]Flubatine PET: Implementation of bolus plus constant infusion and sensitivity to acetylcholine in human brain. Neuroimage. 2016;141:71–80.
Naganawa M, Nabulsi N, Henry S, Matuskey D, Lin SF, Slieker L, et al. First-in-human assessment of (11)C-LSN3172176, an M1 muscarinic acetylcholine receptor PET radiotracer. J Nucl Med. 2021;62:553–60.
Aghourian M, Legault-Denis C, Soucy JP, Rosa-Neto P. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [(18)F]-FEOBV. Mol Psychiatry. 2017;22:1531–8.
Bedard MA, Aghourian M, Legault-Denis C, Postuma RB, Soucy JP, Gagnon JF, et al. Brain cholinergic alterations in idiopathic REM sleep behaviour disorder: a PET imaging study with (18)F-FEOBV. Sleep Med. 2019;58:35–41.
Normandin MD, Zheng MQ, Lin KS, Mason NS, Lin SF, Ropchan J, et al. Imaging the cannabinoid CB1 receptor in humans with [11C]OMAR: assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. J Cereb Blood Flow Metab. 2015;35:1313–22.
D’Souza DC, Cortes-Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T, et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:60–67.
Ranganathan M, Cortes-Briones J, Radhakrishnan R, Thurnauer H, Planeta B, Skosnik P, et al. Reduced brain cannabinoid receptor availability in schizophrenia. Biol Psychiatry. 2016;79:997–1005.
Neumeister A, Normandin MD, Murrough JW, Henry S, Bailey CR, Luckenbaugh DA, et al. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–9.
Kaller S, Rullmann M, Patt M, Becker GA, Luthardt J, Girbardt J, et al. Test-retest measurements of dopamine D(1)-type receptors using simultaneous PET/MRI imaging. Eur J Nucl Med Mol Imaging. 2017;44:1025–32.
Smith CT, Crawford JL, Dang LC, Seaman KL, San Juan MD, Vijay A, et al. Partial-volume correction increases estimated dopamine D2-like receptor binding potential and reduces adult age differences. J Cereb Blood Flow Metab. 2019;39:822–33.
Sandiego CM, Gallezot JD, Lim K, Ropchan J, Lin SF, Gao H, et al. Reference region modeling approaches for amphetamine challenge studies with [11C]FLB 457 and PET. J Cereb Blood Flow Metab. 2015;35:623–9.
Zakiniaeiz Y, Hillmer AT, Matuskey D, Nabulsi N, Ropchan J, Mazure CM, et al. Sex differences in amphetamine-induced dopamine release in the dorsolateral prefrontal cortex of tobacco smokers. Neuropsychopharmacology. 2019;44:2205–11.
Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–24.
Dukart J, Holiga Š, Chatham C, Hawkins P, Forsyth A, McMillan R, et al. Cerebral blood flow predicts differential neurotransmitter activity. Sci Rep. 2018;8:4074.
Nørgaard M, Beliveau V, Ganz M, Svarer C, Pinborg LH, Keller SH, et al. A high-resolution in vivo atlas of the human brain’s benzodiazepine binding site of GABA(A) receptors. Neuroimage. 2021;232:117878.
Gallezot JD, Planeta B, Nabulsi N, Palumbo D, Li X, Liu J, et al. Determination of receptor occupancy in the presence of mass dose: [(11)C]GSK189254 PET imaging of histamine H(3) receptor occupancy by PF-03654746. J Cereb Blood Flow Metab. 2017;37:1095–107.
Smart K, Cox SML, Scala SG, Tippler M, Jaworska N, Boivin M, et al. Sex differences in [(11)C]ABP688 binding: a positron emission tomography study of mGlu5 receptors. Eur J Nucl Med Mol Imaging. 2019;46:1179–83.
DuBois JM, Rousset OG, Rowley J, Porras-Betancourt M, Reader AJ, Labbe A, et al. Characterization of age/sex and the regional distribution of mGluR5 availability in the healthy human brain measured by high-resolution [(11)C]ABP688 PET. Eur J Nucl Med Mol Imaging. 2016;43:152–62.
Galovic M, Erlandsson K, Fryer TD, Hong YT, Manavaki R, Sari H, et al. Validation of a combined image derived input function and venous sampling approach for the quantification of [(18)F]GE-179 PET binding in the brain. Neuroimage. 2021;237:118194.
McGinnity CJ, Hammers A, Riaño Barros DA, Luthra SK, Jones PA, Trigg W, et al. Initial evaluation of 18F-GE-179, a putative PET tracer for activated N-methyl D-aspartate receptors. J Nucl Med. 2014;55:423–30.
Kantonen T, Karjalainen T, Isojärvi J, Nuutila P, Tuisku J, Rinne J, et al. Interindividual variability and lateralization of μ-opioid receptors in the human brain. Neuroimage. 2020;217:116922.
Ding YS, Singhal T, Planeta-Wilson B, Gallezot JD, Nabulsi N, Labaree D, et al. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse. 2010;64:30–8.
Li CS, Potenza MN, Lee DE, Planeta B, Gallezot JD, Labaree D, et al. Decreased norepinephrine transporter availability in obesity: positron emission tomography imaging with (S,S)-[(11)C]O-methylreboxetine. Neuroimage. 2014;86:306–10.
Sanchez-Rangel E, Gallezot JD, Yeckel CW, Lam W, Belfort-DeAguiar R, Chen MK, et al. Norepinephrine transporter availability in brown fat is reduced in obesity: a human PET study with [(11)C] MRB. Int J Obes. 2020;44:964–7.
Belfort-DeAguiar R, Gallezot JD, Hwang JJ, Elshafie A, Yeckel CW, Chan O, et al. Noradrenergic activity in the human brain: a mechanism supporting the defense against hypoglycemia. J Clin Endocrinol Metab. 2018;103:2244–52.
Budescu, David V. Dominance analysis: a new approach to the problem of relative importance of predictors in multiple regression. Psychol Bull. 1993;114:542–51.
Azen R, Budescu DV. The dominance analysis approach for comparing predictors in multiple regression. Psychol Methods. 2003;8:129–48.
Okasha A, Rafaat M, Mahallawy N, El Nahas G, El Dawla AS, Sayed M, et al. Cognitive dysfunction in obsessive-compulsive disorder. Acta Psychiatr Scand. 2000;101:281–5.
Lázaro L, Castro-Fornieles J, Cullell C, Andrés S, Falcón C, Calvo R, et al. A voxel-based morphometric MRI study of stabilized obsessive-compulsive adolescent patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1863–9.
Tao QY, Dang JH, Niu XY, Gao XY, Zhang MZ, Yang ZG, et al. White matter microstructural abnormalities and gray matter volume alterations in obsessive-compulsive disorder: a coordinate-based meta-analysis. J Affect Disord. 2023;320:751–61.
Han SQ, Xu YH, Guo HR, Fang KK, Wei YR, Liu L, et al. Two distinct subtypes of obsessive compulsive disorder revealed by heterogeneity through discriminative analysis. Hum Brain Mapp. 2022;43:3037–46.
Fouche JP, Groenewold NA, Sevenoaks T, Heany S, Lochner C, Alonso P, et al. Shape analysis of subcortical structures in obsessive-compulsive disorder and the relationship with comorbid anxiety, depression, and medication use: a meta-analysis by the OCD brain imaging consortium. Brain Behav. 2022;12:e2755.
Han SQ, Xu YH, Guo HR, Fang KK, Wei YR, Liu L, et al. Two distinct subtypes of obsessive compulsive disorder revealed by a framework integrating multimodal neuroimaging information. Hum Brain Mapp. 2022;43:4254–65.
Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. Jama Psychiatry. 2015;72:305–15.
Han SQ, Zheng RP, Li SY, Liu L, Wang CH, Jiang Y, et al. Progressive brain structural abnormality in depression assessed with MR imaging by using causal network analysis. Psychol Med. 2023;53:2146–55.
Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15:410–24.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RC, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5:52.
Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, et al. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401.
Del Casale A, Kotzalidis GD, Rapinesi C, Serata D, Ambrosi E, Simonetti A, et al. Functional neuroimaging in obsessive-compulsive disorder. Neuropsychobiology. 2011;64:61–85.
Del Casale A, Rapinesi C, Kotzalidis GD, De Rossi P, Curto M, Janiri D, et al. Executive functions in obsessive-compulsive disorder: an activation likelihood estimate meta-analysis of fMRI studies. World J Biol Psychiatry. 2016;17:378–93.
El Mansari M, Blier P. Responsiveness of 5-HT1A and 5-HT2 receptors in the rat orbitofrontal cortex after long-term serotonin reuptake inhibition. J Psychiatry Neurosci. 2005;30:268–74.
Lesch KP, Hoh A, Schulte HM, Osterheider M, Muller T. Long-term fluoxetine treatment decreases 5-HT1A receptor responsivity in obsessive-compulsive disorder. Psychopharmacology. 1991;105:415–20.
Li Q, Zhao YJ, Hu YB, Liu Y, Wang YX, Zhang Q, et al. Linked patterns of symptoms and cognitive covariation with functional brain controllability in major depressive disorder. Ebiomedicine. 2024;106:105255.
Vieira S, Bolton TAW, Schöttner M, Baecker L, Marquand A, Mechelli A, et al. Multivariate brain-behaviour associations in psychiatric disorders. Transl Psychiatry. 2024;14:231.
You W, Luo L, Yao L, Zhao Y, Li Q, Wang Y, et al. Impaired dynamic functional brain properties and their relationship to symptoms in never treated first-episode patients with schizophrenia. Schizophrenia. 2022;8:90.
Long F, Chen Y, Zhang Q, Li Q, Wang Y, Wang Y, et al. Predicting treatment outcomes in major depressive disorder using brain magnetic resonance imaging: a meta-analysis. Mol Psychiatry. 2024.
Dukart J, Holiga S, Rullmann M, Lanzenberger R. JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum Brain Mapp. 2021;42:555–66.
Acknowledgements
This research study was supported by the Natural Science Foundation of China (81601467, 81871327, 62106229, 62476252, 82471962), Medical science and technology research project of Henan province (201701011, SBGJ202102103, SBGJ202101013, LHGJ20230217, SBGJ202302068, S20240045) and Key Research Projects of Higher Education Institutions in Henan Province (24A320069, 25A320032).
Author information
Authors and Affiliations
Contributions
Shaoqiang Han, Baohong Wen, Yong Zhang and Jingliang Cheng conceived and designed the study. Hui-Rong Guo, Qiuying Tao, Ya Tian, Wenqing Shi, Jin Sun, Ruiping Zheng, Zijun Liu and Liang Liu collected the data. Shaoqiang Han, Baohong Wen, Keke Fang, Yarui Wei, Lianjie Niu and Xiaopan Zhang analyzed the data. Baohong Wen, Keke Fang and Shaoqiang Han drafted the work. All authors approved the final version to be submitted for publication and agree to be accountable for all aspects of this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wen, B., Fang, K., Tao, Q. et al. Individualized gray matter morphological abnormalities unveil two neuroanatomical obsessive-compulsive disorder subtypes. Transl Psychiatry 15, 23 (2025). https://doi.org/10.1038/s41398-025-03226-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03226-5