Abstract
Gamma oscillations are essential for brain communication. The 40 Hz neural oscillation deficits in schizophrenia impair left frontotemporal connectivity and information communication, causing auditory hallucinations. Transcranial alternating current stimulation is thought to enhance connectivity between different brain regions by modulating brain oscillations. In this work, we applied a frontal-temporal-parietal 40 Hz-tACS stimulation strategy for treating auditory hallucinations and further explored the effect of tACS on functional connectivity of brain networks. 32 schizophrenia patients with refractory auditory hallucinations received 20daily 20-min, 40 Hz, 1 mA sessions of active or sham tACS on weekdays for 4 consecutive weeks, followed by a 2-week follow-up period without stimulation. Auditory hallucination symptom scores and 64-channel electroencephalograms were measured at baseline, week2, week4 and follow-up. For clinical symptom score, we observed a significant interaction between group and time for auditory hallucinations symptoms (F(3,90) = 26.964, p < 0.001), and subsequent analysis showed that the 40Hz-tACS group had a higher symptom reduction rate than the sham group at week4 (p = 0.036) and follow-up (p = 0.047). Multiple comparisons of corrected EEG results showed that the 40Hz-tACS group had higher functional connectivity in the right frontal to parietal (F (1,30) = 7.24, p = 0.012) and right frontal to occipital (F (1,30) = 7.98, p = 0.008) than the sham group at week4. Further, functional brain network controllability outcomes showed that the 40Hz-tACS group had increased average controllability (F (1,30) = 6.26, p = 0.018) and decreased modality controllability (F (1,30) = 6.50, p = 0.016) in the right frontal lobe compared to the sham group. Our polit study indicates that 40Hz-tACS combined with medicine may be an effective treatment for targeting symptoms specific to auditory hallucinations and altering functional connectivity and controllability at the network level.
Similar content being viewed by others
Introduction
Schizophrenia (SZ), one of the most severe disorders primarily characterized by positive, negative, and cognitive symptoms [1,2,3], has a lifetime prevalence of about 1% [4] and accounts for a massive health care burden [5]. Although disease pathophysiology is not fully understood, the core symptoms of schizophrenia are thought to be caused by the faulty transmission of nerve impulses among brain networks [6,7,8]. Particularly, auditory hallucinations were regarded as a hallmark positive symptom of schizophrenia [9, 10]. It may be associated with the reduced frontotemporal functional connectivity in schizophrenia [11,12,13], i.e., the failure of information generated in the frontal cortex about internal speech to integrate with sensory processing in the temporal-parietal junction (TPJ) [14]. Several studies have shown that 60 to 80% of patients with schizophrenia exhibit auditory hallucinations [15], and up to 30% of patients who use antipsychotic drugs still experience auditory hallucinations [16]. Over time, they would develop schizophrenia with refractory auditory hallucinations (RAH-SZ). Thus, clinical challenges compel clinicians to consider incorporating additional therapeutic techniques.
In recent years, non-invasive brain stimulation techniques have shown potential in the field of mental illness and could be considered complementary therapy to improve the effectiveness of treatment. Specifically, tACS is a non-invasive technology of brain stimulation that applies a weak current with a sine-wave pattern to the scalp to modulate endogenous neural oscillations [17,18,19]. With sufficient dosage (usually 2–4 mA), tACS can modulate neural spike timing [20], alter neuroplasticity [21], and adjust long-range connectivity in the network [22]. The use of stimulation customized to abnormal neural oscillations had more experimental applications from a clinical standpoint. After 40Hz-tACS at 2 mA, patients with Alzheimer’s disease showed a significant increase in blood perfusion in the bilateral temporal lobes [23]. A case showed that 40Hz-tACS applied at 2 mA to the bilateral dorsolateral prefrontal lobes was effective in improving negative symptoms and cognitive deficits in patients with schizophrenia [24]. Multiple studies demonstrated that RAH-SZ patients exhibit abnormal gamma oscillation [25,26,27], particularly specific decreases in their ability to facilitate synchronized gamma-band oscillations when exposed to auditory stimuli at a frequency of 40 Hz (abnormal 40 Hz auditory steady-state response) [28,29,30,31,32,33].
Given that gamma-band neural oscillations in the 30–50 Hz range (centered at 40 Hz) play a key role in cortical transmission and the integration of sensory and cognitive information [34,35,36,37]. We hypothesize that applying 40Hz-tACS to schizophrenia may improve their abnormal frontotemporal connectivity in response to 40 Hz auditory stimulation. As for how 40Hz-tACS affected brain activity, two main aspects can be considered: (1) In addition to modulating endogenous brain oscillations and inducing changes in synaptic plasticity [19], tACS in two or more regions can strengthen or weaken long-range connectivity by synchronizing affected regions to the same or different AC electrical phases [38]. (2) Based on the theory that the state of a complex system can be regulated by energy input [39], 40Hz-tACS could also drive the transition of brain states. Consideration of both connectivity and dynamics of brain activity may better characterize brain dysfunction in psychiatric disorders [40]. However, whether 40Hz-tACS is effective for refractory auditory hallucinations and its mechanism of action remain unclear, requiring further investigation.
Thus, we aimed to evaluate the effectiveness of 40Hz-tACS in treating refractory auditory hallucinations and explore the underlying physiological mechanism of action. In the present study, patients diagnosed with schizophrenia were randomly assigned to the 40Hz-tACS and pseudo-stimulation groups, and participants received a total of four weeks (five weekly sessions of 20 min each) of 40Hz-tACS (or sham) treatment with repeated assessments of symptoms and electrophysiological changes. The change in auditory hallucination symptom scores from baseline to follow-up were our primary outcome, which reflected the effect of the 40Hz-tACS intervention. Changes in functional brain connectivity and controllability as measured by EEG were our secondary outcome used to illustrate the mechanism of action of 40Hz-tACS.
Materials and methods
Study participants
This study was a double-blind, randomized, placebo-controlled pilot clinical trial conducted at the Tianjin Anding Hospital from October 2020 to May 2022 and was approved by the Ethics Committee of the Tianjin Anding Hospital. Fifty schizophrenic participants with refractory auditory hallucinations were recruited from the inpatient department of Tianjin Anding Hospital. Of these, 32 participants who completed all study sessions were included in the analysis (see Fig. S1). The study protocol was approved by the Ethics Committee of Tianjin Anding Hospital [Approval Number: 2020(2020–30)]. All methods were performed in accordance with the relevant guidelines and regulations. All participants gave written informed consent after being informed about the study.
Inclusion and exclusion criteria
All participants were diagnosed with schizophrenia or schizoaffective disorder (confirmed by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders 5th edition [41] (DSM-V)) with symptoms during this treatment present for greater than 12 weeks and who also met the following criteria: 18–70 years old (male and female are not limited); junior high school or above; treated with ≥ 2 different antipsychotic medications (chlorpromazine equivalent dose (The drug equivalent dosage instructions see Table S1) > 600 mg), last medication measure lasting ≥ 6 weeks with poor outcome (CGI ≥ 4 points or PANSS reduction rate < 20% since this treatment) [PANSS reduction rate = pre/post difference/(baseline score - 30)]; Consistent PANSS hallucinatory behavior (P3) item score of ≥ 4 or PSYRATS score change of ≤ 20% in the last two weeks prior to patient enrollment as assessed by the supervising physician, exhibiting stable and significant hallucinatory symptoms; no transcranial magnetic stimulation, transcranial direct current stimulation, or electroconvulsive therapy in the last four weeks.
Participants were excluded from the study for the following criteria: have a mental illness other than the study illness (according to DSM-V); severe physical or neurological illness; any brain device or implant, including cochlear implants and aneurysm clips; previous autoimmune disease or family history of immune disease; antibiotics and immunosuppressive drugs in the last four weeks.
Demographics and baseline scale statistical analysis
For demographic analysis, chi-square tests were conducted for categorical variables, including gender, family history, marital status, clozapine use, and the use of antipsychotic drugs (APD). Data normality was confirmed with the Shapiro-Wilk test (P > 0.05). Independent samples t-tests were employed to assess continuous variables such as years of education, duration of illness, and equivalent drug dosage. Regarding baseline scores on the PANSS and PSYRATS scales for both groups, we also performed independent samples t-tests.
Study design and blinding procedures
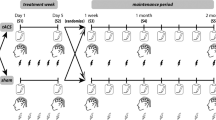
Participants who completed the study attended a total of four weeks of the treatment period and two weeks of the non-treatment period (Fig. 1A). In the treatment period, participants in the active tACS group received 20 min of 40Hz-tACS per day for five days (Monday to Friday) per week for four consecutive weeks in hospital settings, whereas participants in the sham tACS group received 20 sessions of sham stimulation for the same duration (see “Electrode Montage and Stimulation Protocol” below for details). Participants in the active tACS group received 40 Hz stimulation (3 s ramp up, 3 s ramp down) for 20 min. Participants in the sham tACS group only received 3 s ramp-up and a 3 s ramp-down to simulate the sensation of active tACS on the skin for the purpose of blinding. Clinical assessments were conducted at baseline, week2, week 4 and the follow-up (week6) of the treatment period. Resting-state EEG and task-related EEG recordings were conducted at baseline (before the stimulation), week2, week4 (after the stimulation) and follow-up (week6). (labeled in Fig. 1A). Participants were randomly assigned following simple randomization procedures (computerized random numbers) to one of treatment groups.
A Experimental design consisted of two stages: the treatment period (left) and the non-treatment period (right). During the treatment period, participants were randomly assigned to the tACS group (Bottom right, red frame) or the sham group (Bottom right, blue frame). Ramp-in and ramp-out is 3 s for all conditions, with 0 s of active stimulation for sham stimulation, 20 min of active stimulation for 40Hz-tACS. Participants received 40Hz-tACS or sham stimulation five times a week (Monday through Friday) for four consecutive weeks. Clinical assessments and EEG recordings were conducted on baseline (before the stimulation), week2 and week4 (after the stimulation). After the end of treatment, Clinical assessments and EEG recordings were conducted on week6. Our analyses focused on the clinical assessment and task-related EEG of the four key times. B Gamma-band (40 Hz) auditory steady-state response paradigm. C Transcranial alternating current stimulation (tACS) configuration for all participants. The first figure (from left to right) is shown that two stimulators were used; one connected to the electrode over AF3 (red pad), one connected to the electrode over CP5 (red pad), and both connected to the electrode over Cz (blue pad). tACS at AF3 and CP5 has an amplitude of 1 mA (zero-to-peak), while the return current at Cz has an amplitude of 2 mA (zero-to-peak). The second and third figures show the stimulated voltage distribution over the brain from two different views (left and top) when currents at AF3 and CP5 reach their positive peak.
The randomization of all participants, group assignment, and preparation of the stimulation equipment (40Hz-tACS or sham) were conducted by a single researcher. Once the stimulation equipment was set up, the experimenter performed electrical stimulation on the patient. The stimulation interface was oriented only towards the experimenter, ensuring that the patient was unaware of the specific type of stimulation being performed. In addition, the rater who assessed the patient’s symptoms was unaware of the group assignment.
Electrode montage and stimulation protocol
Transcranial alternating stimulation was delivered via Starstim 32 (Neuroelectrics, Spain). Three carbon-silicone electrodes were applied to the scalp with a saline-soaked round sponge pad for all participants. One electrode was placed at AF3 (in the International 10–20 system) in the area of the dorsolateral prefrontal cortex (dlPFC); another electrode of the same dimensions was placed at CP5 in the area of the left TPJ. These two electrodes are shown in red in Fig. 1C. A third return electrode, the same as two additional electrodes, was placed over Cz (the blue pad in Fig. 1C). For participants who received tACS, 40 Hz alternating currents were applied to the dlPFC and TPJ electrodes in phase with each other (red waves in Fig. 1C), with a zero-to-peak amplitude of 1 mA; 40 Hz alternating currents at the return electrode were antiphase to those of the dlPFC and TPJ electrodes (blue waves in Fig. 1C). The placement of electrodes was based on previous work using tACS for the treatment of schizophrenia [33, 42]. The simulated voltage distribution and the electric field strength shown in Fig. 1C were simulated using ROAST3.0 [43] on the MNI 152 Head. During the stimulation, participants sat comfortably in a quiet room and were asked to keep their eyes open, with the aim of maintaining a constant brain state. At the same time, the operator in the room avoided interaction with the patient as much as possible. After each stimulation, the participant was asked about the sensation of the stimulation. All participants enrolled in the experiment reported no discomfort throughout the stimulation process.
EEG tasks and acquisition
Stimulus procedure
Auditory steady-state stimuli were conducted binaurally through noise-cancelling headphones (WH-1000XM3, SONY) at a sound pressure level (SPL) of 90 dB. After fitting the headphones, the operator asked participants for readiness. Upon confirmation, the operator initiated the program, and the sound playback commenced. The response to auditory steady-state stimuli (click-trains) was recorded (Fig. 1B). Before the formal stimulation, a 0.5 s beep signaled the start of the experiment. Considering environmental factors and the specificity of the participants, during the click stimulation, participants were required to look at a picture of the headphones on a computer monitor while listening to a 3 s long click train at a rate of 40 Hz, with two click trains separated by a 1.5 s interval, for a total of 56 click trains. Participants underwent EEG acquisition while receiving the auditory stimuli in order to record the response to the auditory click-trains.
EEG acquisition
EEGs were continuously digitized at a rate of 1000 Hz using a 64-channel SynAmps2 system (Neuroscan, USA), and the interelectrode impedance was kept below 10 kΩ. The electrode montage was based on standard positions in the International 10–20 electrode system [44]. The system acquisition bandpass was 0.1–200 Hz. Data recording was referenced to a linked left mastoid electrode (“M1”) with the ground electrode located at “AFz”.
Clinical outcome measures and statistics
The primary outcome measure was the change in the auditory hallucination symptom scores measured by Psychotic Symptom Rating Scales (PSYRATS) from baseline to week2, week4, and follow-up. In addition, the outcome measures also measured by the positive and negative syndrome scale (PANSS) were assessed at baseline, week 2, week4, and follow-up. For statistical analysis, custom-built scripts in R (R Foundation for Statistical Computing, Vienna, Austria) were used and were available by request. Data normality was confirmed with the Shapiro-Wilk test (P > 0.05) (see Table S3). To assess equality of variance, we used Levene’s test (P > 0.05). We adopted a two-way repeated-measure ANOVA (rm-ANOVA), with the intergroup factor being “condition” (40Hz-tACS and sham), and the intragroup factor being “time” (baseline, week2, week4, follow-up). This study mainly focused only on scale scores, for which there was a significant interaction. Further analysis was performed using the Students’ test to compare the severity of symptoms between the two groups; a one-way ANOVA was used to compare the change in symptoms between the two groups over the treatment and non-treatment periods, and a paired Student’s test (Bonferroni corrected) was chosen for the post-hoc test. For scores where there was a significant interaction, we further calculated the symptom score reduction rates for the two groups with the formula:
Two-samples t-tests were used to compare the rate of score reduction between the two groups at different time points (baseline, week2, week4, follow-up). To control for baseline differences in AH symptom scores, baseline scores were included as a covariate in the analysis of reduction rates at week2, week4, and follow-up. An analysis of covariance (ANCOVA) was performed to compare the rate of symptom reduction between the two groups across these time points.
EEG data analysis and statistic
Preprocessing
EEG data analyses were performed in Matlab using the EEGLAB toolbox and a combination of custom MATLAB scripts (MathWorks, Natick, MA). First, electrodes that were not used, such as CB1, CB2, HEO, VEO, EKG, and EMG, were removed from the experiment. All data were down-sampled to 250 Hz. Offline, EEG data recorded immediately after stimulation were imported into EEGLAB 2022.1 running under MATLAB 2020a and re-referenced to the average of the left and right mastoids. The function pop_eegfiltnew.m was applied for a 0.5–120 Hz bandpass filter (finite impulse response filter, cutoff frequency (−6 dB): [0.25 Hz 120.25 Hz], zero-phase, non-causal). Notch filtered at 50 and 100 Hz was utilized to remove industrial frequency interference. Artifact subspace reconstruction in the plugin clean_rawdata() of EEGLAB was used to automatically reject high-amplitude artifacts [45]. The parameters applied were: flat line removal, 10 s; electrode correlation, 0.7; ASR, 100 (this value was chosen to consider the actual data quality); window rejection, 0.5. The rejected channels are spatially interpolated by spherical. Further, independent component analysis (ICA) was used to remove eye blinks, eye movement, muscular artifacts, and heartbeats. To select brain ICs among all types of ICs, the EEGLAB plugin ICLabel() was used [46].
Continuous EEG data were segmented into epochs that started at –1 s and ended at 4 s relative to sound stimulus onset. Bad epochs were rejected by thresholding the magnitude (±100 μV) of each epoch. We found no significant difference in the epochs and components between all conditions (two-way rm-ANOVA, α = 0.05, see Table S2). Moreover, we set eight regions of interest (i.e., ROIs) according to the anatomical location (Fig. 3A) [47], namely the left frontal region (green area: FP1, AF3, F7, F5, F3, F1, FC5, FC3, FC1, C1), right frontal region (green area: FP2, AF4, F8, F6, F4, F2, FC6, FC4, FC2, C2), left temporal region (yellow area: FT7, T7, TP7), right temporal region (yellow area: FT8, T8, TP8), left parietal region (blue area: C5, C3, CP5, CP3, CP1, P7, P5, P3, P1), right parietal region (blue area: C6, C4, CP6, CP4, CP2, P8, P6, P4, P2), left occipital region (purple area: PO7, PO5, PO3, O1), and right occipital region (purple area:PO8, PO6, PO4, O2).
Functional connectivity
Functional connectivity (phase synchrony) between two-channel locations was measured by the phase locking value (PLV), the absolute value of the mean phase difference between the two signals expressed as a complex unit-length vector [48, 49]. When analyzing bio-signals, especially electrical brain activities, PLV represented a crucial factor to consider in terms of synchronization. It gauged the frequency-specific synchronization, which was not directional and denotes long-range integrations. It evaluated how the phase difference between two signals varies over time [48]. We computed PLV as:
where θ (t, n) was the phase difference \({\emptyset}_{1}({\rm{t}},{\rm{n}})-{\emptyset}_{2}({\rm{t}},{\rm{n}})\). PLV measured the intertrial variability of this phase difference at t: If the phase difference varies little across the trials, PLV was close to 1; otherwise, it was close to 0 [50]. We selected 3 s post-stimulation EEG recordings (30–50 Hz bandpass filtering) from each epoch of 40 Hz click stimulation for further analysis. The mean connectivity between every electrode in a specific region and all the electrodes in the other region was computed for every ROI. The connectivity between both regions was determined by averaging all the electrode pairs between them.
Functional brain network controllability
The functional brain network controllability analysis was based on brain connectivity networks. Brain network controllability reflected the possibility of driving a current network state to other desired target states with external energy input. Average controllability and modal controllability were the most frequently employed metrics to measure network controllability [39]. Average controllability was a measure of the ability of a node to drive the brain to all possible, easily reachable states considering the average input energy cost [51]. Average controllability could be measured by the trace of the Gramian matrix, which was inversely proportional to the control energy required to drive shifts in brain states [52]. Brain regions with high average controllability could switch the brain to many easily achievable states with less input energy. Modal controllability quantified the ease with which a single control node could drive the brain into difficult-to-reach states [53]. Areas with high modal controllability were not hubs of the network but instead had a low degree of control, which imposed high energy costs for completing complex goal-specific operations [54]. For a more detailed derivation of the formula and the code, please refer to the study by Gu et al. [39]. For times with significant differences, the Pearson correlation between average controllability and modal controllability was explored for all participants.
Statistic
The statistical analysis of EEG data was performed using custom R scripts. The neural data met the assumptions of the statistical tests used. Data normality was confirmed with the Shapiro-Wilk test (P > 0.05). To assess equality of variance, we used Levene’s test (P > 0.05). Similar to clinical scale score statistics, two-way rm-ANOVA (α = 0.05) was selected to statistically analyze the PLV, average controllability and modal controllability within the eight ROIs obtained, and the results focused only on the regions with significant interactions. Further analysis was performed using the Students’ test to compare the values of PLV, average controllability and modal controllability between the two groups. A one-way ANOVA was used to compare the change in EEG features between the two groups over time of treatment and non-treatment period. Post-hoc comparisons within two-way rm-ANOVAs were Bonferroni-corrected.
Results
Demographic and baseline symptom scores
The demographics, baseline symptom scores, and antipsychotic medication of participants were presented in Table 1. There was no statistically significant difference in age, education, gender, family history, matrimony, and the information of medicine among participants across the two groups (Table 1, Demographics). Similarly, there was no statistically significant difference in baseline symptom measures across the two groups (Table 1, Baseline Measures).
Clinical outcomes
Group-averaged symptom scores in four sessions, including baseline, week2, week4 and follow-up, were shown in Table S4. Two-way rm-ANOVA was used to determine the effect of different interventions (40Hz-tACS, sham) over time on clinical symptom scores. Statistical results showed that the interaction of group*time had a statistically significant effect on auditory hallucinations (Table 2), demonstrating the overall trajectories of symptom changes were significantly different between the 40Hz-tACS and sham groups. Subsequently, a simple effects analysis was performed for group and time. There was no significant difference between the 40Hz-tACS and sham group in auditory hallucination symptom scores at baseline (F (1,30) = 1.600, p = 0.216), week2 (F (1,30) = 0.127, p = 0.724), week4 (F (1,30) = 0.677, p = 0.417), follow-up (F (1,30) = 0.518, p = 0.477). Notably, as can be seen from the positive and negative statistical values, mean auditory hallucination scores were slightly higher in the tACS group than in the sham group at baseline and week2, but slightly lower in the tACS group than in the sham group at week4 and at follow-up.
Further analysis revealed that for the 40Hz-tACS group, the effect of the time factor on auditory hallucination symptom scores was statistically significant (F (3,45) = 20.000, pBonf < 0.001). Post-hoc comparisons revealed that only baseline auditory hallucination scores were significantly different from week2 (p = 0.008), week4 (p < 0.001) and follow-up (p < 0.001). For the sham group, the effect of the time factor on auditory hallucination symptom scores was statistically significant (F (3,45) = 7.670, pBonf < 0.001). Post-hoc tests showed that only baseline auditory hallucination scores were significantly different from week2 (p = 0.042), week4 (p = 0.022) and follow-up (p = 0.003) (Fig. 2A). As treatment time proceeds, medication alone (sham group) and medication overlaid with 40Hz-tACS could significantly reduce auditory hallucination symptoms.
Subsequently, we examined the changes (reduction rates) in the auditory hallucinations, PSYRATS, from the baseline to week2, week4 and follow-up (Fig. 2B). The baseline AH symptoms score did not have a significant impact, suggesting that adjusting for baseline differences did not substantially alter the results (see Table S5). An independent-samples t-test was used to determine the difference in the reduction rate between the 40Hz-tACS and the sham group at different time points. The results revealed that at week4, the 40Hz-tACS group had a significantly higher reduction rate than the sham group (t = 2.203, p = 0.036); at follow-up, the 40Hz-tACS group also had a significantly higher reduction rate than the sham group (t = 2.082, p = 0.047). It was easy to see that the reduction rate of auditory hallucinations in the 40Hz-tACS group was significantly elevated compared with that in the sham group at week4, and this improvement had continued to the follow-up, which also revealed that drug superimposed 40Hz-tACS treatment was more effective than drug treatment alone (sham group).
40Hz-tACS altered functional connectivity
To investigate the potential 40Hz-tACS effects over several brain areas on network connectivity, we computed PLV for each pair of scalp electrodes at 30–50 Hz. The functional connectivity in the ROIs was defined by the average PLV over all scalp electrode pairs in this region (Fig. 3A), and then we performed a statistical analysis of PLV change at the region level. The results showed that the interaction of group*time had a statistically significant effect on left frontal to right frontal (LFRF), right intra-frontal (RFRF), right frontal-to-parietal (RFRP), and right frontal-to-occipital (RFRO), indicating the 40Hz-tACS intervention produced inconsistent changes in the functional connectivity of brain networks in the two groups of schizophrenia patients. Subsequently, a simple effects analysis was performed for group and time. The results revealed that at week4, there was a significant difference in PLV between the two groups in RFRF (F (1,30) = 7.24, p < 0.05) and RFRP (F (1,30) = 7.98, p < 0.05), while LFRF (F (1,30) = 4.15, p = 0.051) and RFRO (F (1,30) = 4.13, p = 0.051) showed a trend toward significance.
A The 60 electrodes of the whole scalp of brain were divided into eight different regions (i.e. ROI) according to the location of the anatomical structures (midline regions were not included in the analysis): left frontal, right frontal (green region); left temporal, right temporal (yellow regions); left parietal, right parietal (blue regions); left occipital, right occipital (purple regions). B Significant and marginally significant differences between the 40Hz-tACS group and sham group at Week4. The red color represents a higher value, while the blue color represents a lower value. C Statistical results of the two groups in LFRF, RFRF, RFRP, RFRO (*p < 0.05, **p < 0.01).
Next, we performed further statistical analysis. For LFRF, no effect of time was observed between the 40Hz-tACS and sham group (40Hz-tACS: F (3,45) = 2.35, pBonf = 0.172; sham: F (3,45) = 1.85, pBonf = 0.304). For RFRF, only the effect of time in the 40Hz-tACS group was statistically significant (F (3,45) = 3.63, pBonf = 0.04). Post-hoc tests showed that only PLV of week2 was significantly lower than that at week4 (t (15) = −3.55, pBonf = 0.017). For RFRP, only the effect of time factor in the sham group was statistically significant (F (3,45) = 4.44, pBonf = 0.042). Post-hoc comparisons revealed that only baseline PLV was significantly higher than that at week4 (t (15) = 3.09, pBonf = 0.045). For RFRO, only the effect of time in the sham group was statistically significant (F (3,45) = 3.58, pBonf = 0.044). Post-hoc tests revealed that there was no statistically significant difference between any two groups. The concept proposed that higher coherence in oscillatory networks will facilitate both intra- and inter-regional communication [55]. These findings imply that 40Hz-tACS successfully modulate brain functional connectivity based on network effects in a frequency-specific way.
40Hz-tACS changed functional brain network controllability
Average controllability and modal controllability were calculated based on the mean functional connectivity adjacent matrix (8*8) to probe the transition of brain states, and a two-way rm-ANOVA was chosen for statistical tests (see Fig. 4). The interaction of group*time had a statistically significant effect on the right frontal region, indicating the overall trajectories of functional brain network controllability changes were significantly different between the 40Hz-tACS and sham groups. For average controllability, there was merely a significant difference between the 40Hz-tACS and sham groups at week4 (F (1,30) = 6.26, p = 0.018). For modal controllability, there was a significant difference between the 40Hz-tACS and sham groups at week4 (F (1,30) = 6.50, p = 0.016). The negative correlation between average and modal controllability was observed in all the participants at week4 (r = −0.82, p < 0.01), manifesting that the two metrics characterize different and relatively independent aspects of brain function. Under the existing stimulation strategy, the average controllability of the right frontal region was elevated, and modal controllability was decreased in schizophrenia in the 40Hz-tACS group compared to the sham group. These findings suggest that the stimuli altered the right frontal region in connection with other brain regions. Inputting the right frontal lobe with lower energy was more likely to drive transitions between states that were easily accessible to the patient, but it was more difficult to direct the system to states that were difficult for the patient to reach.
The altered controllability of right frontal cortex was found in patients with schizophrenia during treatment period (the first figure from left to right). Average controllability and modal controllability comparisons between 40Hz-tACS group and sham group at Week4 (the second and third figure). Correlation between average controllability and modal controllability (the last figure, p < 0.001).
Discussion
This work adopted the frontal-temporal-parietal 40Hz-tACS intervention strategy for treating auditory hallucinations of RAH-SZ, further exploring the neurophysiological mechanism of action of 40Hz-tACS. At the symptom level, compared to the sham group, 40Hz-tACS treatment reduced auditory hallucinations to a greater extent in patients with refractory auditory hallucinations. At the neurophysiological level, during auditory steady-state stimulation, 40Hz-tACS treatment increased functional connectivity in the gamma-band (30–50 Hz), while average controllability in the right frontal lobe increased and modal controllability decreased. Taken together, our work demonstrated reproducible target engagement by 40Hz-tACS in schizophrenic patients with refractory auditory hallucinations and provided initial evidence for the use of tACS as a complementary treatment for clinical treatment.
In the present study, four consecutive weeks (20 sessions in total) of 40Hz-tACS treatment resulted in changes in functional connectivity and network controllability of the brain in the gamma-band over the same time scale (week4). Many studies demonstrated that synchronous gamma oscillation deficits in schizophrenia were exhibited by 40 Hz auditory steady-state stimuli [56,57,58]. The findings indicated that long-range synchrony of gamma oscillations was distributed in schizophrenia and that this deficit was related to auditory hallucinations [28, 59]. Alterations in patients with schizophrenia included dysfunction in the ability to adaptively switch in and out of brain states [60, 61]. Previous 10Hz-tACS at dlPFC and TPJ failed to reduce auditory hallucination symptoms in schizophrenia [42, 62]. In this study, the auditory hallucination symptoms as measured by clinician-administered assessments (PSYRATS) gradually decreased over time as treatment progressed. Thus, 40Hz-tACS treatment may be specific to the auditory hallucination symptom of schizophrenia.
While our findings suggest that 40Hz-tACS may have a specific therapeutic effect on auditory hallucinations in schizophrenia, it is important to acknowledge the limitations and potential side effects associated with tACS. A study investigated 40Hz-tACS targeting the left prefrontal cortex (F3) and the right orbitofrontal cortex (Fp2), and found no enhancement in working memory [63]. Every coin has two sides. The broad range of adjustable parameters in tACS allowed for greater customization to meet individual patient needs, yet this flexibility also contributed to reproducibility challenges. To date, there are no formal safety guidelines for the selection of stimulus parameters in tACS [64]. However, numerous studies have reported transient phosphenes or skin sensations in participants undergoing tACS [65]. In our study, participants were questioned about their experiences after each stimulation session, and none reported any discomfort. It is plausible that patients with schizophrenia may exhibit reduced sensitivity to visual illusions and skin sensations due to the long-term effects of the disorder. Therefore, applying tACS to patients should carefully tailor stimulation parameters to the specific characteristics of the illness to ensure patient safety. All in all, with increasing awareness of the disease, tACS will have more potential for clinical application.
In this study, we did not perform a correlation analysis between auditory hallucination symptom scores and electrophysiological results of RAH-SZ. First of all, our primary purpose was to explore whether 40Hz-tACS could effectively improve the auditory hallucination symptoms of patients. Obviously, after 4 weeks of intervention, the auditory hallucination symptoms of patients were effectively relieved and this improvement could continue to the follow-up period. Secondly, electrophysiological results also showed that schizophrenic patients had significantly improved brain network connectivity and controllability at four weeks. It also reflected the effectiveness of 40Hz-tACS from the side. However, it was worth noting that the EEG results did not show a delayed effect of the intervention, that was, the consistency between symptom changes and electrophysiological changes was low, which was why we did not conduct correlation analysis. Finally, considering the incomplete synchronization of symptom assessment and EEG data acquisition process and inevitable objective factors, we finally decided not to conduct a correlation analysis between the two. Of course, it is very necessary to conduct correlation analysis. In future research, we will further standardize the experimental process and strictly control the experimental variables, and strive to improve the correlation analysis process in subsequent research.
In accordance with our previous assumptions, homophasic cross-current electrical stimulation of both regions could enhance interregional long-range connectivity [22, 66]. From a functional connectivity perspective, compared to the sham group, 40Hz-tACS treatment significantly altered functional connectivity between the right and left frontal lobes, the right frontal to parietal lobe, the right frontal to occipital lobe, and within the right frontal region at the end of treatment. It was easy to see that the area of functional connectivity changes was not in the left dlPFC and TPJ, where the stimulating electrodes were placed, but mainly in the right hemisphere, with the right frontal lobe as the core. This could be due to the fact that increased coherence within the oscillatory network would promote intra- and inter-regional interactions [55]. Recent studies have shown that patients with schizophrenia exhibited reduced right and left frontal lobes as well as reduced right frontal-to-occipital connectivity [67]. Multiple studies have shown that hallucinatory symptoms in schizophrenia were associated with disconnected connections between the left frontal and temporal lobes [68,69,70,71]. Notably, tACS altered the ongoing activity of networks instead of imposing coherence on unrelated networks [38]. Furthermore, the future stimulation protocol may have preferable adjustments in the selection of stimulation targets and may yield better performance.
To our knowledge, this is the first time we have used functional brain network controllability to analyze the signal measured by EEG. Dynamic is crucial in getting us closer to function [72]. The brain network could produce a wide range of dynamic states, as evidenced by changing patterns of activation and synergistic activity that reflected the interplay of structural connectivity, neuromodulation, and sensory input [73]. It was essential for brain state transitions to promote flexible cognitive, emotional, and behavioral abilities by selectively arranging appropriate brain states in relevant brain networks [74]. From a functional brain network controllability perspective, compared to the sham group, patients in the 40Hz-tACS group had higher average controllability in the right frontal lobe, while at the same time modal controllability decreased at the end of treatment. The high average controllability and low modal controllability exhibited by the right frontal lobe indicate that the right frontal lobe is a “hub” with close connections to other brain regions. In addition, the results of the functional connectivity study also illustrated this. Thus, lower energy input could drive a shift in brain state [75].
There are some limitations to this work. First, the study conducted was a small-scale pilot investigation intended for exploration purposes with limited statistical power for identifying effect sizes except those that were significant. Specifically, we acknowledge that the non-significant baseline differences of auditory hallucinations and the relatively small sample size may have impacted the overall findings. However, in contrast to previous studies, our study fully considered the mechanism of action of tACS, detecting symptoms and neurophysiological changes in patients with schizophrenia at multiple key time points. Second, due to irresistible factors, both EEG signal acquisitions during the treatment period were made after the end of tACS with a short time interval of approximately one hour. Therefore, the immediate effect caused by tACS cannot be completely eliminated [76]. Third, as this was a small pilot study, our results could have been influenced by factors such as medication, therapy, duration of illness, etc. Fourth, in order not to influence our subsequent follow-up results, we did not perform the assessment of blinding. Therefore, our experiments cannot entirely eliminate the influence of individual differences in the subjective perception of various stimuli. Finally, the results for functional connectivity and controllability showed significant differences only at post-treatment (week4), and there were no significant differences in the results at week2 or follow-up. This demonstrated that four weeks of stimulation had yet to alter synaptic plasticity in patients with refractory hallucinatory schizophrenia. Future intervention programs should take full account of the actual situation of patients by appropriately extending the length of stimulation.
To conclude, 40Hz-tACS significantly altered functional connectivity and functional brain network controllability of the gamma-band in schizophrenia with treatment-resistant auditory hallucinations during auditory steady-state stimuli. At the same time, 40Hz-tACS combined with medicine treatment significantly accelerated the improvement of auditory hallucination symptoms. Our findings suggested that 40Hz-tACS combined with medicine would represent a feasible and efficacious treatment for symptoms that were specific to auditory hallucinations. Further, the study provided preliminary evidence that 40Hz-tACS could be used as a clinical complementary treatment for schizophrenia.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Code availability
All code used to analyze the presented results was available upon request.
References
Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–49.
Gross G. The ‘basic’symptoms of schizophrenia. Br J Psychiatry. 1989;155:21–25.
Andreasen N. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346:477–81.
Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141 https://doi.org/10.1371/journal.pmed.0020141
McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020;77:201–10. https://doi.org/10.1001/jamapsychiatry.2019.3360
Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 2013;23:283–90. https://doi.org/10.1016/j.conb.2012.11.004
Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–80. https://doi.org/10.1016/j.neuron.2012.09.004
Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–24. https://doi.org/10.1016/j.neubiorev.2010.11.004
Waters F, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–93. https://doi.org/10.1093/schbul/sbs045
Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26:177–89. https://doi.org/10.1017/S003329170003381X
Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–11.
De Weijer A, Mandl R, Diederen K, Neggers S, Kahn R, Pol HH, et al. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res. 2011;130:68–77.
Wolf ND, Sambataro F, Vasic N, Frasch K, Schmid M, Schönfeldt-Lecuona C, et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36:366–74.
Wible CG. Hippocampal temporal-parietal junction interaction in the production of psychotic symptoms: a framework for understanding the schizophrenic syndrome. Front Hum Neurosci. 2012;6:180 https://doi.org/10.3389/fnhum.2012.00180
Lim A, Hoek HW, Deen ML, Blom JD, Investigators G. Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr Res. 2016;176:493–9. https://doi.org/10.1016/j.schres.2016.06.010
Nathou C, Etard O, Dollfus S. Auditory verbal hallucinations in schizophrenia: current perspectives in brain stimulation treatments. Neuropsychiatr Dis Treat. 2019;15:2105–17. https://doi.org/10.2147/ndt.S168801
Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. 2013;7:317.
Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24:333–9. https://doi.org/10.1016/j.cub.2013.12.041
Tavakoli AV, Yun K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front Cell Neurosci. 2017;11:214 https://doi.org/10.3389/fncel.2017.00214
Alekseichuk I, Wischnewski M, Opitz A. A minimum effective dose for (transcranial) alternating current stimulation. Brain Stimul. 2022;15:1221–2.
Wischnewski M, Engelhardt M, Salehinejad M, Schutter D, Kuo M-F, Nitsche M. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb cortex. 2019;29:2924–31.
Alekseichuk I, Falchier AY, Linn G, Xu T, Milham MP, Schroeder CE, et al. Electric field dynamics in the brain during multi-electrode transcranial electric stimulation. Nat Commun. 2019;10:2573.
Sprugnoli G, Munsch F, Cappon D, Paciorek R, Macone J, Connor A, et al. Impact of multisession 40Hz tACS on hippocampal perfusion in patients with Alzheimer’s disease. Alzheimers Res Ther. 2021;13:1–14.
Haller N, Hasan A, Padberg F, Brunelin J, Valiengo LdCL, Palm U. Gamma transcranial alternating current stimulation in patients with negative symptoms in schizophrenia: a case series. Neurophysiol Clin. 2020;50:301–4.
Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr Bull. 2011;37:659–63.
Sun Y, Farzan F, Barr MS, Kirihara K, Fitzgerald PB, Light GA, et al. Gamma oscillations in schizophrenia: mechanisms and clinical significance. Brain Res. 2011;1413:98–114.
Jadi MP, Behrens MM, Sejnowski TJ. Abnormal gamma oscillations in N-methyl-D-aspartate receptor hypofunction models of schizophrenia. Biol Psychiatry. 2016;79:716–26.
Onitsuka T, Tsuchimoto R, Oribe N, Spencer KM, Hirano Y. Neuronal imbalance of excitation and inhibition in schizophrenia: a scoping review of gamma-band ASSR findings. Psychiatry Clin Neurosci. 2022;76:610–9.
O’Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, Morzorati SL. The auditory steady-state response (ASSR): a translational biomarker for schizophrenia. Suppl Clin Neurophysiol. 2013;62:101–12.
Grent-‘t-Jong T, Gajwani R, Gross J, Gumley AI, Krishnadas R, Lawrie SM, et al. 40-Hz auditory steady-state responses characterize circuit dysfunctions and predict clinical outcomes in clinical high-risk for psychosis participants: a magnetoencephalography study. Biol Psychiatry. 2021;90:419–29. https://doi.org/10.1016/j.biopsych.2021.03.018
Hirano Y, Oribe N, Onitsuka T, Kanba S, Nestor PG, Hosokawa T, et al. Auditory cortex volume and gamma oscillation abnormalities in schizophrenia. Clin EEG Neurosci. 2020;51:244–51.
Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:1–13.
Ahn S, Mellin JM, Alagapan S, Alexander ML, Gilmore JH, Jarskog LF, et al. Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. Neuroimage. 2019;186:126–36.
Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13.
Lee K-H, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Rev. 2003;41:57–78.
Zhou T-H, Mueller NE, Spencer KM, Mallya SG, Lewandowski KE, Norris LA, et al. Auditory steady state response deficits are associated with symptom severity and poor functioning in patients with psychotic disorder. Schizophr Res. 2018;201:278–86.
Uhlhaas PJ, Haenschel C, Nikolić D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–43.
Wischnewski M, Alekseichuk I, Opitz A. Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cognit Sci. 2023;27:189–205.
Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, Kahn AE, et al. Controllability of structural brain networks. Nat Commun. 2015;6:8414.
Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15:683–95.
First MB. DSM-5-TR® Handbook of Differential Diagnosis. American Psychiatric Pub. 2013.
Zhang M, Force RB, Walker C, Ahn S, Jarskog LF, Frohlich F. Alpha transcranial alternating current stimulation reduces depressive symptoms in people with schizophrenia and auditory hallucinations: a double-blind, randomized pilot clinical trial. Schizophrenia. 2022;8:114.
Huang Y, Datta A, Bikson M, Parra LC. Realistic volumetric-approach to simulate transcranial electric stimulation—ROAST—a fully automated open-source pipeline. J Neural Eng. 2019;16:056006.
Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34:1600–11.
Blum S, Jacobsen NS, Bleichner MG, Debener S. A riemannian modification of artifact subspace reconstruction for EEG artifact handling. Front Hum Neurosci. 2019;13:141.
Pion-Tonachini L, Kreutz-Delgado K, Makeig S. ICLabel: an automated electroencephalographic independent component classifier, dataset, and website. Neuroimage. 2019;198:181–97.
Liu X, Liu S, Li M, Su F, Chen S, Ke Y, et al. Altered gamma oscillations and beta–gamma coupling in drug-naive first-episode major depressive disorder: association with sleep and cognitive disturbance. J Affect Disord. 2022;316:99–108.
Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208.
Mormann F, Lehnertz K, David P, Elger CE. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys D: Nonlinear Phenom. 2000;144:358–69.
di Biase L, Ricci L, Caminiti ML, Pecoraro PM, Carbone SP, Di Lazzaro V. Quantitative high density EEG brain connectivity evaluation in Parkinson’s disease: the phase locking value (PLV). J Clin Med. 2023;12:1450.
Cornblath EJ, Tang E, Baum GL, Moore TM, Adebimpe A, Roalf DR, et al. Sex differences in network controllability as a predictor of executive function in youth. Neuroimage. 2019;188:122–34.
Jeganathan J, Perry A, Bassett DS, Roberts G, Mitchell PB, Breakspear M. Fronto-limbic dysconnectivity leads to impaired brain network controllability in young people with bipolar disorder and those at high genetic risk. Neuroimage Clin. 2018;19:71–81.
Tang E, Ju H, Baum GL, Roalf DR, Satterthwaite TD, Pasqualetti F, et al. Control of brain network dynamics across diverse scales of space and time. Phys Rev E. 2020;101:062301.
Deng, S & Gu, S Controllability analysis of functional brain networks. arXiv preprint arXiv:2003.08278 (2020).
Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–35. https://doi.org/10.1016/j.neuron.2015.09.034
Koenig T, van Swam C, Dierks T, Hubl D. Is gamma band EEG synchronization reduced during auditory driving in schizophrenia patients with auditory verbal hallucinations? Schizophr Res. 2012;141:266–70.
Sivarao DV. The 40-Hz auditory steady-state response: a selective biomarker for cortical NMDA function. Ann N Y Acad Sci. 2015;1344:27–36.
Coffman BA, Ren X, Longenecker J, Torrence N, Fishel V, Seebold D, et al. Aberrant attentional modulation of the auditory steady state response (ASSR) is related to auditory hallucination severity in the first-episode schizophrenia-spectrum. J Psychiatr Res. 2022;151:188–96.
Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol. 2011;79:55–63.
Narr KL, Leaver AM. Connectome and schizophrenia. Curr Opin psychiatry. 2015;28:229–35.
Andalman AS, Burns VM, Lovett-Barron M, Broxton M, Poole B, Yang SJ, et al. Neuronal dynamics regulating brain and behavioral state transitions. Cell. 2019;177:970–85. e920
Mellin JM, Alagapan S, Lustenberger C, Lugo CE, Alexander ML, Gilmore JH, et al. Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. Eur Psychiatry. 2018;51:25–33.
Hoy KE, Whitty D, Bailey N, Fitzgerald PB. Preliminary investigation of the effects of γ-tACS on working memory in schizophrenia. J Neural Transm. 2016;123:1205–12.
Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol. 2015;126:2181–8.
Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: a review. Clin Neurophysiol Pract. 2017;2:19–25.
Violante IR, Li LM, Carmichael DW, Lorenz R, Leech R, Hampshire A, et al. Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife. 2017;6:e22001.
Koshiyama D, Miyakoshi M, Joshi YB, Molina JL, Tanaka-Koshiyama K, Sprock J, et al. Neural network dynamics underlying gamma synchronization deficits in schizophrenia. Prog Neuro-psychopharmacol Biol Psychiatry. 2021;107:110224.
Stripeikyte G, Potheegadoo J, Progin P, Rognini G, Blondiaux E, Salomon R, et al. Fronto-temporal disconnection within the presence hallucination network in psychotic patients with passivity experiences. Schizophr Bull. 2021;47:1718–28.
Allen P, Modinos G, Hubl D, Shields G, Cachia A, Jardri R, et al. Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012;38:695–703.
Zhang L, Li B, Wang H, Li L, Liao Q, Liu Y, et al. Decreased middle temporal gyrus connectivity in the language network in schizophrenia patients with auditory verbal hallucinations. Neurosci Lett. 2017;653:177–82.
Li B, Cui L-B, Xi Y-B, Friston KJ, Guo F, Wang H-N, et al. Abnormal effective connectivity in the brain is involved in auditory verbal hallucinations in schizophrenia. Neurosci Bull. 2017;33:281–91.
Breakspear M. Dynamic models of large-scale brain activity. Nat Neurosci. 2017;20:340–52.
Sporns O. The complex brain: connectivity, dynamics, information. Trends Cogn Sci. 2022;26:1066–7.
Dolan RJ. Emotion, cognition, and behavior. science. 2002;298:1191–4.
Li Q, Yao L, You W, Liu J, Deng S, Li B, et al. Controllability of functional brain networks and its clinical significance in first-episode schizophrenia. Schizophr Bull. 2023;49:659–68.
Liu A, Vöröslakos M, Kronberg G, Henin S, Krause MR, Huang Y, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. 2018;9:5092.
Acknowledgements
We thank the doctors of Tianjin Anding Hospital for their help with participant recruitment for this study, as well as two assessors assessed the participants on a regular symptom scale. We also thank Xinmeng Guo for her help in calculating the functional brain network controllability in this study. This research reported in this publication was supported by the National Natural Science Foundation of China (Grant Nos. 81925020), the Sci-Tech Innovation 2030-Major Project of Brain science and brain-inspired intelligence technology (Grant Nos. 2021ZD0202000), and the National Natural Science Foundation of China (Grant Nos. 81801786). Finally, we thank our research colleagues for their help in this research.
Author information
Authors and Affiliations
Contributions
Xiaojuan Wang, Jingmeng Liao and Shuang Liu designed the study and wrote the protocol. Shuang Liu and Dong Ming supervised data collection. Yuan Chang managed the literature searches. Xiaojuan Wang, and Shuang Liu analyzed data and undertook the statistical analysis. Xiaojuan Wang wrote the first draft of the manuscript. Xiaochen Zhang and Shuang Liu critically reviewed the results and provided substantial input to the manuscript. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Zhang, X., Chang, Y. et al. Double-blind, randomized, placebo-controlled pilot clinical trial with gamma-band transcranial alternating current stimulation for the treatment of schizophrenia refractory auditory hallucinations. Transl Psychiatry 15, 36 (2025). https://doi.org/10.1038/s41398-025-03256-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03256-z