Abstract
The social stress often induces fear memory and stress-relevant phobias. Molecular and cellular mechanisms of fear memory and anxiety remain to be addressed for the exploration of therapeutic strategies for these deficits. In social defeat mice induced by the resident/intruder paradigm, we have examined how auditory cortical neurons are recruited to encode stress signals that cause fear memory and anxiety by approaches of behavioral tasks, neural tracing, electrophysiology and molecular biology. The social stress in intruder C57 mice by the attack of resident CD1 mouse causes their fear memory and anxiety-like behaviors. In addition to the interconnections between auditory and somatosensory cortices in the mice of fear memory and anxiety, auditory cortical neurons receive new synapses from the somatosensory cortex and the synapses from the medial geniculate body. These auditory cortical neurons are able to encode the stress signals including the pain stimulus to injury areas and the battle sound in a resident/intruder paradigm. Neuroligin-3 mRNA knockdown in the auditory cortex prevents the recruitment of associative memory neurons that encode fear memory and anxiety-like behaviors. Therefore, neuroligin3-mediated synapse formation is essential for the stress-induced recruitment of associative memory neurons in auditory cortices that encode stress signals, fear memory and anxiety.
Similar content being viewed by others
Introduction
Physical and psychological stressors often evoke fear memory and anxiety, such as posttraumatic stress disorder and phobia to the stress-related events or objects [1,2,3,4,5,6,7,8,9,10,11]. These affective disorders in turn suppress the immune system and the cardiovascular system to induce their secondary diseases [12]. It is important to reveal cellular and molecular mechanisms underlying stress-induced fear memory and affective disorders, which is a primary goal of the therapy of these pathological moods [13]. Endeavor to achieve this goal appears unsatisfied [13,14,15,16,17]. To the less success, the different types of stressors, such as acute severe stress versus chronic mild stress, psychological stress versus physical stress and natural hazard stress versus social stress, may lead to their featured cellular and molecular alterations in the brain specifically correlated to anxiety, depression and their mixtures [4, 5, 13, 18,19,20,21]. The comprehensive maps about molecular cascades and neural circuits between different stressors and their associated affective disorders remain to be revealed to explore their specific therapeutic strategies.
The acute severe stress evokes the fear memory and anxiety [1, 3,4,5, 7, 22,23,24,25,26]. Many brain areas have been presumably involved in the fear memory and anxiety, including the amygdala, the nucleus accumbens and the prefrontal cortex [27,28,29,30,31,32,33,34,35,36,37]. Neuronal circuits in the amygdala and the nucleus accumbens have been thought of as relevance to the balance between fear memory and reward memory [38,39,40,41,42,43]. The attenuation of the nucleus accumbens facilitates the amygdala to memorize negative events, leading to affective disorders [44,45,46,47,48,49,50,51,52,53,54,55,56]. In addition, the interconnection between the amygdala and the auditory cortex appears formed and strengthened in the fear memory induced by the associations of the bell ring and the electrical foot shock [23, 57,58,59,60,61]. It is possible that stressful fear signals are programmed by the interactions between the amygdala and the auditory cortex. How auditory cortical neurons are recruited to encode the stress signals for the fear memory and anxiety is hypothetically taken into our study.
The auditory cortex has been indicated to encode the joint storage of auditory signals and other signals in associative learning and memory [7, 62,63,64,65,66,67,68,69,70,71,72]. The primary auditory cortex discriminates those stimulations from threat and nonthreat signals and then to regulate the specificity of threat memory, implying its effectiveness on subsequent fear memory [73]. The cellular mechanisms for the information storage in the auditory cortex are largely unknown [74,75,76,77,78]. The associative memory neurons have been identified in sensory cortices including the somatosensory cortices, the piriform cortex, and the gustatory cortex, which are featured by synapse interconnections among these cross-modal sensory cortices as well as the encoding of multiple associated signals [79,80,81,82,83,84]. Whether the associative memory neurons are recruited in the auditory cortex to encode those fear signals in the social stress and to cause fear memory and anxiety will be examined in our study.
Taken these questions above, we intend to examine the roles of the formation of new synapses and the recruitment of associative memory neurons in the auditory cortex in encoding stress signals, fear memory and anxiety by multiple approaches of behavioral tasks, electrophysiology and neural tracing and molecular biology. In terms of the strategies, C57 mice as intruders were subjected to the social stress by the resident/intruder paradigm, in which they were attacked by a resident CD1 mouse [4, 85,86,87,88,89,90]. The C57 mice in the groups of social stress and control were placed in a social interaction cage to measure the formation of fear memory and on an elevated-plus maze to assess anxious state. Both intruder mice with the stress-induced fear memory/anxiety and control mice were investigated by the neural tracing to track the new synapse formation and axon interconnections and by electrophysiological recordings to analyze the encoding of auditory cortical neurons to the stressful signals, such as the battle sound and the pain signal from somatic injury regions. The interconnections of the associative memory neurons among cross-modal cortices were examined by microinjecting anterograde and retrograde adeno-associated viruses that carried genes of encoding fluorescent proteins in one of brain areas and by detecting their expression in its interconnected cortical areas, or the other way around. The recruitment of associative memory neurons was ensured when new synapse contacts along with those presented synapse contacts were detected on the dendritic spines of auditory cortical neurons in the convergent manner [79, 81, 91] and when the auditory cortical neurons showed a raised spike-encodings in response to stress signals [79, 84, 92]. The essential role of associative memory neurons recruited by neuroligin-3, a synapse linkage protein, in stress-induced fear memory and anxiety was examined by using short-hairpin RNAs (shRNA) specific to silence neuroligin-3 mRNA, which were carried by adeno-associated virus (AAV).
Materials and methods
Studies approved in mice and animals feeding
Experiments were conducted in accordance with guidelines and regulations by the Administration Office of Laboratory Animals in Beijing China. All of the experiment protocols were approved by the Institutional Animal Care and Use Committee in the Administration Office of Laboratory Animals in Beijing China (B10831).
In our study, two species of mice were applied. C57BL/6J Thy1-YFP mice (Jackson Lab. USA) were used and divided into intruder and control groups as well as neuroligin-3 knockdown and its scramble control. The glutamatergic neurons in the cerebral brain of these mice were genetically labeled by the yellow fluorescent protein (YFP), hereafter referred as C57 mice in our paper. CD-1 (ICR) mice were used as resident mice. These mice were accommodated in specific pathogen-free facilities (SPF) with the circadian about twelve hours for night and day plus the self-help feeding. Well-developed C57 mice in postnatal 21 days were selected to experience the resident/intruder paradigm (also named as intruder) or control. Male CD-1 mice with the stronger aggressive above three months old were chosen as residents.
Behavioral study
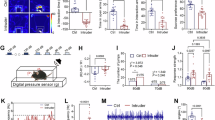
The C57 mice were taken into the laboratory to accommodate experiment operators and the training apparatus for two days and were randomly assigned into control group and intruder group. In the resident/intruder paradigm [85,86,87,88,89], an intruder C57 mouse was placed in a cage of male CD-1 mouse, where this male CD-1 mouse has been housed with a female CD-1 mouse above three days. When placing an intruder C57 mouse into this cage and taking female CD-1 mouse out, this male resident CD-1 mouse attacked and bit intruder C57 mouse at least 10 times or 5 min per day for 12 days (Fig. 1A, lower left). Subsequently, C57 mice were examined by the social interaction (SI) test to identify their fear memory to CD-1 mouse and by elevated-plus maze (EPM) test to evaluate the anxious state. The social interaction test was performed in an open field box (50*50 cm) that included a transparent and perforated box (10*10 cm). One CD1 mouse or a tiny audio recorder that broadcasted the battle sound were placed in this small box about five minutes to examine the avoidance behavior of C57 mice as the indicator of fear memory to CD-1 mouse or the battle sound. The area within 5 cm around the small box was defined as an interaction zone (Fig. 1A, lower median). The stay duration of C57 mice in the interaction zone was named as the interaction time in this test. The EPM consisted of two open arms (30 × 5 cm) opposite to two closed arms (30 × 5 × 15.25 cm). The arms extended from a central platform (5 × 5 cm). The EPM was located 40 cm above the floor (Fig. 1A, lower right). The data of behavior test about the time on open arms and interaction time of C57 mice were analyzed by Matlab. The detailed method was described in our previous studies [4, 11, 93, 94].
A Schematic diagram of experiment timeline. A resident/intruder paradigm (lower left under the timeline) as the social stress protocol was applied to C57 mice aged 24 days. The social interaction test (lower median) was used to evaluate fear memory retrievals by CD-1 mice and battle sound, respectively. The elevated plus maze test (lower right) was used to evaluate the anxiety-like behaviors of C57 mice. B The hot-spot maps of C57 mice in response to CD-1 resident mouse in an interaction cage. C The interaction time of C57 mice in intruder group is 34.80 ± 7.69 s, and the interaction time of C57 mice in control group is 119.7 ± 6.14 s. C57 mice in intruder group show less interaction time with this CD-1 mouse in open field (control, n = 11; intruder, n = 13; ****, P < 0.0001; one-way ANOVA). D The hot-spot maps of C57 mice in response to the battle sound in an interaction cage. E The interaction time of C57 mice in intruder group is 47.22 ± 6.51 s, and the interaction time of C57 mice in control group is 91.23 ± 3.95 s. C57 mice in intruder group show less interaction time with the battle sound in an interaction cage (control, n = 9; intruder, n = 12; ****, P < 0.0001; one-way ANOVA). F The hot-spot maps of C57 mice on an elevated plus maze. G The time on open arms of C57 mice in intruder group is 7.01 ± 1.34%, and the time on open arm of C57 mice in control group is 19.7 ± 1.55%. C57 mice in intruder group show less duration of staying open arms (control, n = 19; intruder, n = 13; ****, P < 0.0001; one-way ANOVA). Error bars indicate SEM.
Retrograde and anterograde neural tracing
To trace the formation and change of neuronal circuits in intruder C57 mice induced by the social stress with the resident/intruder paradigm, we used adeno-associated viruses (AAV purchased from OBiO Inc, Shanghai, China) for neural tracing. The AAV2/retro-CMV-mCherry was applied for a retrograde tracing. The AAV2/8-CMV-tdTomato and AAV2/8-CMV-EBFP were used as an anterograde tracing. The locations for the microinjection of AAVs and the confocal view of neural images were based on the brain map for mouse [95].
In tracing the interconnections between the auditory cortex and the primary somatosensory cortex for a trunk area (S1-Tr cortex), 0.2 µL AAV2/retro-CMV-mCherry was microinjected into the auditory cortex (2.30 mm posterior to the bregma, 4.00 mm lateral to the middle line and 0.65 mm depth away from the cortical surface) or the S1-Tr cortex (1.50 mm posterior to the bregma, 1.50 mm lateral to the middle line and 0.50 mm depth away from the cortical surface) in the mice from groups of intruders before a resident/intruder paradigm and control three weeks before the neural tracing. The injection of AAVs into cerebral cortices was conducted by using a glass pipette. The microinjection quantity and duration were controlled by a microsyringe system held with the three-dimensional stereotaxic apparatus (RWD Life science, Shenzhen, China). The glass pipettes were reserved no less than 15 min before withdrawing from the mouse brain. In principle, AAV2/retro-CMV-mCherry injected into the auditory cortex or the S1-Tr cortex was uptaken by axon terminals and boutons of these cortical neurons, and was subsequently transported toward their somata to express red fluorescent protein, so that the somata of neurons as the source area and their projected axons as the target areas (or AAV injection area) were traced in a retrograde manner. It is noteworthy that the transfection efficiency of AAVs in the S1-Tr cortex is presented in Figure S4. The averaged percentage of the AAV transfected neurons is similar to the averaged percentage of cFos-labelled neurons (Fig. 2K), indicating AAV transfection to active neurons.
A Retro-AAVs-mCherry are microinjected in the S1-Tr cortex. B The retrogradely traced neurons in the auditory cortex (post bregma 2.30 mm, scale bar, 50 µm) in samples of control mouse (left panel) and intruder mouse (right panel). C Statistical analyses show mCherry-labeled cells per mm3 in the auditory cortices from control mice (blue symbols) and intruder mice (red symbols; ***, P < 0.001, one-way ANOVA). D Retro-AAVs-mCherry are microinjected in the auditory cortex. E The retrogradely traced neurons in the S1-Tr cortex (post bregma 1.70 mm, scale bar, 50 µm) in samples of control mouse (left panel) and intruder mouse (right panel). F Statistical analyses show mCherry-labeled cells per mm3 in the S1-Tr cortices from control mice (blue symbols) and intruder mice (red symbols; *, P < 0.05, one-way ANOVA). G An image shows the injection site of retro-AAVs-mCherry in the auditory cortex. H An immunofluorescent staining image with low power, in which the white frame shows the S1-Tr cortex. I Images with high power in the S1-Tr cortices from control mice (top panels) and intruder mice (bottom panels). The images show DAPI-labeled neurons, mCherry-labeled neurons, cFos-labeled neurons and their merges, respectively, in left-to-right panels. J mCherry-labeled neurons in the S1-Tr cortex are significantly higher in intruder mice (red symbols) than in control mice (blue symbols; *, P < 0.05, one-way ANOVA). K cFos-labeled neurons in the S1-Tr cortex in control mice (blue symbols) and intrude mice (red symbols). L The neurons labeled by both mCherry and cFos in the S1-Tr cortex are higher in intruder mice (red symbols) than control mice (blue symbols; ***, P < 0.001, one-way ANOVA). Error bars indicate SEM.
In the study of the convergent synapse innervations on auditory cortical neurons from the S1-Tr cortex and the medial geniculate body (MG), 0.2 µL AAV2/8-CMV-tdTomato was injected into the S1-Tr cortex (1.5 mm posterior to the bregma, 1.5 mm lateral to the middle line and 0.5 mm depth below the cortical surface), and 0.2 µL AAV2/8-CMV-EBFP was injected into the MG (3.2 mm posterior to the bregma, 1.9 mm lateral to the middle line and 2.7 mm depth away from the cortical surface). CMV-coded AAVs transfected nerve cells in the injected areas and expressed their carried-genes and fluorescent proteins. Subsequently, these fluorescent proteins produced in neuronal somata were transported over entire axons in an anterograde manner, such that their axonal boutons and terminals labelled by these fluorescent proteins were detected in the target areas. The contacts by these axon boutons and terminals on dendritic spines of auditory cortical neurons was deemed as synapse contacts. The raised contacts in the experiments included newly formed synapse contacts. The auditory cortical neurons with convergent synapses newly from the S1-Tr cortex and innately from the medial geniculate body were presumably associative memory neurons [79, 81, 84, 91].
The resident/intruder paradigm was conducted three days after the surgical operation and microinjections in order to allow C57 mice recovery from the operation. In the next two weeks, fluorescent proteins were transported to entire axon boutons and terminals along with the stress -induced axon prolongation. At last, the mice were anesthetized by the intraperitoneal injections of urethane (1.5 g/kg) and perfused through the left ventricle with 25 ml 0.01 M phosphate buffer solution (PBS) followed by 25 ml of 4% paraformaldehyde until their bodies were rigid. The brains were quickly isolated and soaked in 4% paraformaldehyde for the fixation no less in 24 h. The cerebral brains were sliced by a vibratome in a series of coronal sections with a thickness of 100 µm in PBS. These slices were air-dried and cover-slipped with 50% glycerin in PBS. The images of neurons, dendrites, spines and axonal boutons were taken and collected at a 60X lens for high magnification in a confocal microscope (Nikon A1R plus). The anatomic images of the cerebral brain were taken by a 4X lens for a low magnification under this confocal microscope.
In C57BL/6JThy1-YFP mice, postsynaptic neuron dendrites and spines were labelled by the YFP. The presynaptic axon boutons whose somata were infected AAV were labelled by either RFP (mCherry or tdTomato) or BFP. The wavelength of an excitation laser-beam 561 nm was used to activate RFP. The wavelength of an excitation laser-beam 405 nm was used to activate the BFP. The wavelengths of the emission spectra of the BFP, YFP and RFP are 412–482 nm, 522–552 nm and 572–652 nm, respectively. The contacts between yellow dendritic spines and red or blue axon boutons with less than 0.1 µm space cleft were presumed as chemical synapses. The images of dendritic spines, axon boutons and synapse contacts were analyzed quantitatively by ImageJ and Imaris [79, 81, 84, 91]. Associative memory neurons were accepted by detecting at least two sources of boutons onto the dendritic spines of YFP-labelled auditory cortical neurons.
Immunofluorescence
Mice were anesthetized by the intraperitoneal injections of urethane (1.5 g/kg) and perfused through the left ventricle with 25 ml 0.1 M phosphate buffer solution (PBS) followed by 25 ml of 4% paraformaldehyde until their bodies were rigid. Brains were postfixed with 4% paraformaldehyde for 20 min and then kept in PBS. The cerebral brains were sliced by a vibratome in a series of coronal slices with the thickness of 30 µm in PBS. These slices were washed in PBS, blocked by a buffer solution containing 5% goat serum for 1 h, incubated with the buffer solution that contained the primary antibody in 1% bull serum albumin (free for cFos staining) and 0.3% Triton X-100 at 4 °C for overnight. After washed three times in PBS, these slices were incubated with the secondary antibody for two hours and then, if necessary, incubated with 4′,6-diamidino-2- phenylindole for 20 min at room temperature. The secondary antibody for the staining was Alexa Fluor 647 goat anti-rabbit immunoglobulin G (4414S, CST). The slices were lastly washed three times, mounted on microscope slides, air-dried and cover-slipped with 50% glycerin in PBS. Images were captured under a confocal laser-scanning microscope (Nikon A1R plus) and colocalization analysis and merged images were processed according to our previous work [79, 81, 84, 91].
Electrophysiology
Before the electrophysiological recording of auditory cortical neurons in vivo, the mice in control or intruder were anesthetized by intraperitoneal injections of urethane (1.5 g/kg) for surgical operations after training paradigms had been done. The body temperature was kept at 37 °C by a computer-controlled heating blanket. The craniotomy (1 mm in diameter) was done on the mouse skull above the left side of the auditory cortex (−2.40 mm posterior to the bregma and 4.00 mm lateral to the midline). The location for electrophysiological recordings was based on the brain mapping for mouse [95]. Electrophysiological recordings to auditory cortical neurons in vivo were conducted in the mice under a light anesthetic condition with a withdrawal reflex by pinching, the eyelid blinking reflex by the air-puffing and the muscle relax. The electrical discharges of unitary cortical neurons were recorded in layers IV-V of the auditory cortex by using glass pipettes filled with the standard solution (150 mM NaCl, 3.5 mM KCl and 5 mM HEPES). The resistance of those recording pipettes was 40–50 MΩ. The electrical signals of auditory cortical neurons in their spontaneous spikes and evoked-spikes by the battle sounds or the stimulus to injury areas were recorded and acquired by AxoClamp-2B amplifier and Digidata 1322 A, and were analyzed by pClamp 10 system (Axon Instrument Inc. CA, USA). Spike signals were digitized at 20 kHz and filtered by low-pass at 5 kHz. A 100–3000 Hz band-pass filter and a second-order Savitzky-Golay filter were used to isolate the spike signal. Spiking frequencies were quantitatively analyzed [79, 81, 84, 91].
Normalized spike frequency in response to either one of stimuli was the number of the spike frequency in response to the stimulus in 20 s divided by spontaneous discharge frequency in 20 s before the stimulation. When the ratio reached 1.5 or above, the auditory cortical neurons was deemed to be response to this stimulus. Associative memory neurons (AMN) were accepted by detecting a situation that auditory cortical neurons responded to both stimulations. The identification of associative memory neurons versus those neurons in response to a stimulus is presented in Figure S5. The spectra of spike frequencies in these neurons are presented as the Z-score in Figure S5.
Neuroligin-3 knockdown by shRNA carried by AAV
In the study of the role of neuroligin-3 in the formation of new synapse innervations, one of the proteins for the synapse linkage [84, 96,97,98,99,100,101], the approach of its mRNA knockdown was used by the short-hairpin RNA (shRNA) specific for neuroligin-3 mRNA which was carried by AAV (AAV2/8- CMV-EGFP-shNlg3), in which the AAV was injected into the auditory cortex. The piece of scramble sequence carried by AAV (AAV2/8-CMV-EGFP-scramble) as a control was injected in the auditory cortex too. The microinjections were operated three days before the resident/intruder paradigm. Theoretically, this approach suppresses the expression level of neuroligin-3 in auditory cortical neurons, the formation of new synapse innervations from S1-Tr neurons and the recruitment of associative memory neurons in the auditory cortex.
Similarly, the behavior tests, AAV-mediated neural tracing and electrophysiological recording were done to evaluate the effectiveness of neuroligin-3 knockdown on the fear memory, synapse formation and associative memory neuron recruitment in the auditory cortex. Specifically, the encoding capability of neurons in response to the battle sound and the somatic pain signals were analyzed and compared in two subgroups. The effectiveness of shRNA specific for a neuroligin-3 inhibition on new synapse formation and associative memory cell recruitment was confirmed if the number of new synapse contacts and associative memory neurons in the mice of neuroligin-3 knockdown was lowered significantly in comparison with the subgroup of scramble control mice.
Statistics
All data are presented as arithmetic mean ± SEM. The statistical analyses of all our data were conducted by using GraphPad Prism 9. One-way ANOVA was used for the statistical comparisons of the changes in behavioral and morphology study between the groups of control and intruder as well as between the neuroligin-3 knockdown subgroup and scramble subgroup in the intruder group. A Chi-test was used for the statistic comparison of changes in the percentage of recruited associative memory neurons in the electrophysiological study among these groups. P values equally and above 0.05 in the comparisons among the groups were set to be no statistical differences, or vice versa. The one asterisk, two asterisks, three asterisks and four asterisks were presented to be P < 0.05, 0.01, 0.001 and 0.0001 respectively.
Results
In this section, we present our studies about the essential role of associative memory cells at the auditory cortex in stress-induced fear memory and anxiety. In the resident/intruder paradigm for the social stress, the fear memory and anxiety-like behaviors in intruder C57 mice was induced by attacks from a resident CD-1 mouse. In this resident/intruder paradigm, the stressful signals included the pain signal due to their trunk injury bitten by resident CD-1 mouse and the battle sound. The neural tracing was applied to examine the mutual synapse innervations between the auditory cortex and the S1-Tr cortex induced by this social stress. The electrophysiological recording and neural tracing were jointly utilized to identify the recruitment of associative memory neurons in the auditory cortex. Associative memory neurons were surely recruited when the auditory cortical neurons became to encode these stressful signals during the electrophysiological recordings in vivo and when the convergent synapse innervations were made onto auditory cortical neurons by the axon boutons of S1-Tr cortical neurons and medial geniculate neurons. The shRNA specific for the neuroligin-3 knockdown was used to test the essential roles of neuroligin-3 in the formation of new synapses, the recruitment of associative memory neurons and the emergence of fear memory and anxiety-liker behavior induced by this social stress.
In the resident/intruder paradigm, one of intruder C57 mice was placed into the home cage of a resident CD1 mouse. The resident mouse recognized this intruder mouse as a stranger and attacked it. Intruder mice experienced this stress situation once a day and twelve days in total. Control C57 mice in this period were treated without the exposure to the resident CD1 mouse. Subsequently, the social interaction (SI) test and elevated-plus maze (EPM) test were conducted in such two groups of mice to evaluate the emergence of fear memory specific to this resident mouse and of anxiety-like behaviors (Fig. 1A).
In the social interaction test, intruder mice appear to stay away from this resident mouse placed in a small box of the interaction cage (right panel in Fig. 1B), compared to control mice (left panel). The stay durations in the interaction zone (interaction time) in response to this resident CD1 mouse are 34.8 ± 7.69 s in intruder mice (red symbols in Fig. 1C, n = 13) and 119.7 ± 6.14 s in control mice (blue symbols; n = 11, p < 0.0001, ANOVA). These intruder mice also appear to stay away from the battle sound broadcasted by an audio recorder in the small box of the interaction cage (right panel in Fig. 1D), compared with control mice (left panel). The interaction time in response to the battle sound is 47.22 ± 6.51 s in intruder mice (red symbols in Fig. 1E, n = 12) and 91.23 ± 3.95 s in control mice (blue symbols; n = 9, p < 0.0001, ANOVA). This result indicates that the social stress to intruder mice induces their fear memory specific to the resident mouse and the battle sound. Furthermore, in the elevated-plus maze test, intruder mice appear to avoid the open fields since they prefer to stay in the closed arms (right panel in Fig. 1F), compared to control mice (left panel). The percentages of the stay time in open arms are 7.01 ± 1.34% in intruder mice (red symbols in Fig. 1G, n = 13) and 19.7 ± 1.55% in control mice (blue symbols, n = 19, p < 0.0001, ANOVA). That is, intruder mice become more anxious after experienced the resident/intruder paradigm. The data above indicate that the social stress leads to fear memory and anxiety-like behaviors.
In terms of the cellular mechanism underlying the fear memory to associative stressful signals in a resident/intruder paradigm, we assumed that the battle sound activated the auditory cortex by the auditory system and the pain signal in their injury trunk areas activated the somatosensory cortex by the somatic system simultaneously. Based on the principle of coactivity together and interconnection together that recruits associative memory neurons [13, 83, 84], we further assumed that such stressful signals included in the resident/intruder paradigm to induce fear memory and anxiety instigated the new synapse interconnections between auditory and S1-Tr cortices as well as recruited the auditory cortical neurons to be the associative memory neurons that encode the fear memory to stressful signals and the anxiety-like behaviors.
Mutual innervations between S1-Tr and auditory cortical neurons are associated with fear memory
The formation of interconnections between auditory and S1-Tr cortices was examined by neural tracing, in which adeno-associated viruses that carried the genes encoding fluorescent proteins were used. In the retrograde neural tracing, 0.2 µl AAV/retro-CMV-mCherry was microinjected in the trunk area of somatosensory cortex (S1-Tr cortex) and detected in the auditory cortex (Fig. 2A), or the other way around (Fig. 2D). Three days after injections, C57 mice experienced a resident/intruder paradigm or control for twelve days. In microinjections to the S1-Tr cortex, auditory cortices from two groups were scanned under a confocal microscope. mCherry-labelled neurons in the auditory cortex appear higher in intruder mice with the fear memory (right panel in Fig. 2B) than control mice (left panel). mCherry-labelled neurons per mm3 in the auditory cortex are 2577 ± 309.7 in an intruder group (red symbols in Fig. 2C, n = 9 cubes from 9 mice) and 916.2 ± 166.9 in the control group (blue symbols, n = 9 cubes from 9 mice, p < 0.001, ANOVA). Moreover, in microinjections into the auditory cortex, S1-Tr cortices from two groups were scanned under confocal microscope. mCherry-labelled neurons in the S1-Tr cortex appear higher in intruder mice with fear memories (right panel in Fig. 2E) than control mice (left panel). mCherry-labelled neurons per mm3 in the S1-Tr cortex are 34,524 ± 3965 in intruder group (red symbols in Fig. 2F, n = 8 cubes from 8 mice) and 22,402 ± 2431 in the control group (blue symbols, n = 8 cubes from 8 mice, p < 0.001, ANOVA). These results indicate that the interconnections are formed and increased between auditory and S1-Tr cortices in intruder mice with stress-induced fear memory and anxiety. These results also imply that auditory cortical neurons receive new synapse innervations from the S1-Tr cortex alongside synapse inputs from the medial geniculate body as well as encode the battle sound and trunk pain signals, or their recruitment to be associative memory cells, which are presented below.
In order to examine these interconnected neurons being functionally active, we have conducted the experiment of immunolabelling them with proteins coded by activity-dependent genes, e.g., cFos. Three days after microinjections of AAV-mCherry into the auditory cortex (Fig. 2G), these C57 mice experienced a resident/intruder paradigm or control for twelve days. The mCherry- and cFos-labelled S1-Tr cortical neurons appear higher in intruder mice with fear memories (bottom panels in Fig. 2I) than control mice (top panels). mCherry-labelled neurons per mm2 are 11.64 ± 1.04 in intruder group (red symbols in Fig. 2J, n = 25 fields from 3 mice) and 8.64 ± 0.74 in the control group (blue symbols, n = 25 fields from 3 mice, p < 0.05, ANOVA). mCherry/cFos-labelled neurons per mm2 are 8.64 ± 0.67 in intruder group (red symbols in Fig. 2L, n = 25 fields from 3 mice) and 5.0 ± 0.61 in the control group (blue symbols, n = 25 fields from 3 mice, p < 0.001, ANOVA). These results imply that the interconnected neurons between S1-Tr and auditory cortices are functionally active, i.e., a functional interconnection. It is noteworthy that those mCherry-labelled neurons and c-Fos labelled neurons are not fully overlap (Figure S1), or they are not identical in nature.
Associative memory neurons are recruited in the auditory cortex to encode stressful signals
The recruitment of associative memory neurons in the auditory cortex has been morphologically examined by the anterograde neural tracing in that AAV-carried fluorescent genes were microinjected in the medial geniculate body and the S1-Tr cortex in intruder and control mice. As showed in Fig. 3A, 0.2 μl AAV2/8-CMV-tdTomato was injected in the S1-Tr cortex and 0.2 μl AAV2/8-CMV-EBFP was injected in the medial geniculate body. After the resident/intruder paradigm and control periods went through, auditory cortical neurons were examined in their convergent synapse innervations from the S1-Tr cortex and the medial geniculate body.
A Low power images show the injection of AAVs-tdTomato into the S1-Tr cortex (left panel), the injection of AAVs-eGFP in medial geniculate body (middle panel) and the neural tracing at the auditory cortex (white frame). B Images show synaptic connections made on dendritic spines of auditory cortical glutamatergic neurons by axonal boutons from the medial geniculate body (white arrow to blue boutons) and from the S1-Tr cortex (white triangle to red boutons) in samples of control mouse (top panel) and intruder mouse (bottom panel). Boxes show enlarged synapse contacts pointed by arrows. C Synapse contacts made by red axon boutons from the S1-Tr cortex are higher in intruder mice (red symbols) than in control mice (blue symbols, ***, P < 0.001, one-way ANOVA). D Synapse contacts made by blue axon boutons from the medial geniculate body are higher in intruder mice (red symbols) than in control mice (blue symbols, **, P < 0.01, one-way ANOVA). E Schematic diagram shows in vivo electrophysiological recordings. The auditory stimulus (AS) by the battle sound and the tactile stimulation (TS) to trunk injury-areas were given to mice. The auditory cortices (ACtx) were recorded by the electrode. F Illustrates the examples about the responses of single neurons to the battle sound and the pain stimulus recorded from a control mouse (top panel) and intruder mouse (bottom panel), respectively. The auditory cortical neuron in an intruder mouse responds to the battle sound and the tactile stimulus, i.e., associative memory neuron. G Pie charts show the distributions of neurons with different response patterns recorded in the auditory cortices of control mice (left panel, n = 116) and intruder mice (right panel, n = 109), respectively. H The portions of associative memory neurons in response to both stimuli in the intruder group and control group are 17.43% (n = 18/109) and 6.9% (n = 8/116), respectively. A chi-square test yields χ2 = 5.91 (P = 0.0151).
Convergent synapse innervations onto the dendritic spines of auditory cortical neurons appear higher in intruder mice (bottom panel in Fig. 3B) than control mice (top panel), where white arrows point synapse contacts made by presynaptic boutons and postsynaptic spines. Synapse contacts per 100 µm dendrites on auditory cortical neurons made by axonal boutons of S1-Tr cortical neurons are 3.02 ± 0.42 in intruder group (red symbols in Fig. 3C, n = 25 slices from 3 mice) and 1.28 ± 0.22 in control group (blue symbols in Fig. 3C, n = 25 slices from 3 mice, p < 0.001, ANOVA). Synapse contacts per 100 µm dendrites on auditory cortical neurons made by axonal boutons of neurons in the medial geniculate body are 4.94 ± 0.56 in intruder group (Fig. 3D red symbols, n = 25 slices from 3 mice) and 2.76 ± 0.36 in control group (blue symbols in Fig. 3D, n = 25 slices from 3 mice, p < 0.01, ANOVA). The results indicate that auditory cortical neurons in intruder mice with fear memories specific to resident mice receive more convergent synapse innervations from the S1-Tr cortex and the medial geniculate body. It is noteworthy that the rise of axonal buttons in the auditory cortex projected from the S1-Tr cortex in intruder mice with the fear memory and anxiety (Figure S2) supports this result. In addition, these axons are colocalized with type-I of glutamate transporter (Figure S3), indicating that the axons are glutamatergic. Therefore, associative memory neurons are substantially recruited in the auditory cortex during the social stress.
Whether these auditory cortical neurons are able to encode stressful signals including the battle sound and the painful signal from injury trunk regions was examined by electrophysiology in vivo. The experiment was conducted by recording the responses of auditory cortical neurons to the pain signal that was presumably inputted through the newly established pathway from the S1-Tr cortex to the auditory cortex. The spike frequency was used as an index of the strength of neuronal activity. When the auditory cortical neurons responded to both somatosensory signal and auditory signal, they were presumably associative memory neurons, similar to previous studies [13, 79, 83, 84, 92]. The unitary discharges of auditory cortical neurons were electrophysiologically recorded in vivo (Fig. 3E). The battle sound and the somatic pain stimulus (tweezers to injury trunk areas that mimicked the bite of resident mouse) were sequentially given to intruder mice with fear memories and/or control mice. The evoked spikes on the background of spontaneous spikes were recorded and analyzed. The normalized spike frequencies in response to one of stimuli were calculated by the ratio of the frequency of stimulus-induced spikes to the frequency of spontaneous spikes in twenty seconds before the stimulation. If the ratio reached 1.5 or above, the auditory cortical neurons was deemed as the responses to this stimulation.
Auditory cortical neurons appear to respond to both painful and battle sound signals in intruder mice with fear memory (bottom trace in Fig. 3F), but not control mice (top panel). The percentages of associative memory neurons in total recorded neurons were 17.43% in intruder group (right panel in Fig. 3G, n = 18/109 from 9 mice) and 6.90% in control group (left panel, n = 7/116 from 11 mice). The statistical analysis with Chi-test shows χ2 = 5.91 (p < 0.05, Fig. 3H). The result indicates that some auditory cortical neurons are recruited to encode the battle sound and somatic pain signals for their integrative storage in stress-induced fear memory. Both morphological and electrophysiological data verify the stress-induced recruitment of associative memory neurons in the auditory cortex.
Neuroligin-3 is required for fear memory, anxiety and associative memory cell recruitment
To the emergence of fear memory and the recruitment of associative memory neurons in social stress, new synapses arise in the auditory cortex. The formation of new synapses requires the linkage proteins between presynaptic and postsynaptic membranes, such as neuroligin-3 and neurexin [96, 98,99,100,101]. We hypothesized that this neuroligin-3 played the essential role in the new synapse formation for the stress-induced emergence of fear memory/anxiety and recruitment of associative memory neurons in the auditory cortex. This hypothesis was examined by a neuroligin-3 knockdown in the auditory cortex to observe whether the neuroliginb-3 downregulation precludes the emergence of fear memory and anxiety, the formation of new synapses and the recruitment of associative memory cells in response to the social stress. AAV-carried shRNA specific to neuroligin-3 mRNA [102,103,104,105,106] was injected into the auditory cortex of intruder mice. The shRNA-scramble control was injected in another group of intruder mice. In terms of time line for experiments, pAAV[shRNA]-GFP-U6-mNlgn3 was injected into the auditory cortex (the right panel in Fig. 4A) three days before resident/intruder paradigms. This shRNA ensured to lower neuroligin-3 expression expectedly prevented the stress-induced formation of synapse connection from the S1-Tr cortex to the auditory cortex and the recruitment of associative memory neurons in the auditory cortex. The effectiveness of this pAAV[shRNA]-GFP-U6-mNlgn3 on neuroligin-3 knockdown has been validated and presented in the supporting datum of our previous publication [107]. The experiments in neuroligin-3 knockdown were conducted with the behavior tasks, AAV-mediated neural tracing and electrophysiology in vivo applied in Figs. 1–3. The essential roles of neuroligin-3 in the emergence of fear memory/anxiety, the formation of new synapse innervations and the recruitment of associative memory neurons would be ensured if these processes in the group of intruder plus neuroligin-3 knockdown mice were downregulated.
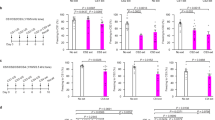
A Lower power images show the injections of AAV-CMV-tdTomato into the S1-Tr cortex (left panel), AAV-CMV- EGBP into the medial geniculate body (middle panel), and AAV-DJ/8-CMV-EGFP-U6-shNlgn3 or AAV-DJ/8-CMV-EGFP-U6-scramble into the auditory cortex (right panel) in intruder mice, respectively. The neural tracing images are taken at the auditory cortex (blue frame). B Images show synaptic connections on dendritic spines of auditory cortical neurons made by axonal boutons from the medial geniculate body (white arrow to blue boutons) and from the S1-Tr cortex (white triangle to red boutons) in samples of scramble group mouse (top panel) and Nlgn3-KD group mouse (bottom panel). Boxes show enlarged synapse contacts pointed by arrows. C The number of red synaptic connections decreases in Nlgn3-KD group, in comparison with scramble group (****, P < 0.0001, one-way ANOVA). D. The number of blue synaptic connections has no difference between two groups (P > 0.5, one-way ANOVA). Error bars denote SEM. E The schematic diagram of electrophysiological recordings in vivo. AAV-DJ/8-CMV-EGFP-U6-shNlgn3 or AAV-DJ/8-CMV-EGFP-U6- scramble was injected into the auditory cortex (ACtx) of intruder C57 mice. After the resident-intruder paradigm, auditory cortical neurons in the intruder mice are electrophysiologically in vivo recorded in response to the battle sound stimulus (auditory signal, AS) and the tactile stimulus (TS). F Illustrates the examples about the responses of single neurons to the battle sound and the pain stimuli recorded from a mouse in scramble group (top panel) and a mouse in Nlgn3-KD group (bottom panel). Auditory cortical neuron in a mouse in the intruder plus scramble group responds to the battle sound and the tactile stimulus, i.e., associative memory neuron. G Pie charts on the left and right panels show the proportions of response types of auditory cortical neurons recorded in mice of scramble and Nlgn3-KD groups, respectively. H The proportions of associative memory neurons are 15.38% (n = 15/91) in the scramble mice and5.56% (n = 5/90) in Nlgn3-KD mice. A chi-square test yielded χ2 = 4.65 (P < 0.05), indicating a significant decrease in the proportion of associative memory neurons in the Nlgn3-KD group compared to the scramble group.
In the meantime of pAAV[shRNA]-GFP-U6-mNlgn3 injections in the auditory cortex, AAV-EBFP and AAV-tdTomato were injected into the medial geniculate body and the S1-Tr cortex, respectively (Fig. 4A). The synapse contacts between the dendritic spines on auditory cortical neurons (yellow) and the axonal boutons of S1-Tr cortical neurons (red) appear lower in intruder plus neuroligin-3 knockdown mice (bottom panel in Fig. 4B) than in intruder plus scramble control mice (top panel). Synapse contacts per 100 µm dendrites are 0.60 ± 0.15 in intruder plus neuroligin-3 knockdown group (green symbols in Fig. 4C, n = 30 dendrites from 3 mice) and 3.02 ± 0.30 in intruder plus scramble control group (red symbols, n = 34 dendrites from 3 mice, p < 0.0001, ANOVA). Moreover, the synapse contacts formed by the dendrite spines of auditory cortical neurons (yellow) and the axon boutons of neurons in the medial geniculate body (blue) appear no difference in these two subgroups. Synapse contacts per 100 µm dendrites are 3.81 ± 0.48 in intruder plus neuroligin-3 knockdown group (green symbols in Fig. 4D, n = 30 dendrites from 3 mice) and 3.66 ± 0.42 in intruder plus scramble control group (red symbols, n = 34 dendrites from 3 mice). This result indicates that neuroligin-3 knockdown in the auditory cortex downregulates the stress-induced formation of new synapse innervation from the S1-Tr cortex, but not synaptic inputs from the media geniculate body. That is, neuroligin-3 is required for the formation of new synapse innervations and the recruitment of associative memory neurons in the auditory cortex induced by the social stress.
The electrophysiological recording in vivo was conducted in the auditory cortices of the intruder plus neuroligin-3 knockdown and intruder plus scrambler control mice (Fig. 4E). The examples in Fig. 4F illustrate the recording of auditory cortical neurons in response to the battle sound and the somatic stimuli in the injury trunk area from an intruder plus scramble control mouse (top trace) and an intruder plus neuroligin-3 knockdown mouse (bottom trace). The percentages of auditory cortical neurons in response to both battle sound and somatic stimulus, i.e., associative memory cells, were 15.38% in intruder plus scramble control subgroup (n = 15/91 from 8 mice) and 5.56% in intruder plus neuroligin-3 knockdown subgroup (n = 5/90 from 8 mice, Fig. 4G). The statistical analysis by Chi-test shows χ2 = 4.65 (p < 0.05, Fig. 4H). The neuroligin-3 knockdown prevents the responses of auditory cortical neurons to the stressful signals including the battle sound and the somatic stimulus to injury trunk area. That is, neuroligin-3 is required for the stress-induced recruitment of associative memory neurons in the auditory cortex.
In the experiment of behavioral tasks, the interaction time in response to the resident mouse is 43.64 ± 8.29 s in those intruder plus scramble control mice (red symbols in Fig. 5C, n = 17) and 35.36 ± 7.39 s in intruder plus neuroligin-3 knockdown mice (green symbols, n = 18). In addition, the interaction time in response to the battle sound appears lower in intruder plus scramble control mice (left panel in Fig. 5D) than in the intruder plus neuroligin-3 knockdown mice (right panel). The interaction time in response to the battle sound is 110.1 ± 16.99 s in intruder plus neuroligin-3 knockdown mice (green symbols in Fig. 5E, n = 10) and 62.81 ± 6.56 s in intruder plus scramble control mice (blue symbols, n = 10, p < 0.05, ANOVA). This result indicates that neuroligin-3 knockdown in the auditory cortices of intruder mice prevents the retrievals of fear memories to the battle sound, but not the appearance of resident CD1 mouse. In other words, the fear memory to stressful signals in the resident/intruder paradigm is mainly caused by the storage and retrieval of the stressful signals in the auditory cortex, which supports our focus on studying the auditory cortex.
A AAV-DJ/8-CMV-EGFP-U6-scramble or AAV-DJ/8-CMV-EGFP-U6-shNlgn3 are injected into the auditory cortex to suppress the expression of neuroligin-3 in intruder mice. B The hot-spot maps of an intruder C57 mouse in response to a CD-1 resident mouse in an interaction cage. C The interaction time of intruder C57 mice with this CD-1 mouse is not significantly difference between a scramble subgroup (red symbols, n = 17) and a Nlgn3-KD subgroup (green symbols, n = 18, P > 0.05, one- way ANOVA). D The hot-spot maps of an intruder C57 mice in response to the battle sound in an interaction cage. E C57 mice in intruder plus Nlgn3-KD subgroup (n = 10) express more interaction time with the battle sound, in comparison with intruder plus scramble subgroup (n = 10; *, P < 0.05, one-way ANOVA). F The hot-spot maps of an intruder C57 mouse on an elevated plus maze. G C57 mice in intruder plus Nlgn3-KD subgroup (n = 14) show more duration of staying open arms, compared with scramble subgroup (n = 17; **, P < 0.01, one-way ANOVA). Error bars indicate SEM.
The influence of neuroligin-3 knockdown on anxiety-like behavior in intruder mice was examined by using the elevated-plus maze (EPM). The stay time on the plate of open arms in the EPM appears longer in those intruder plus neurolgin-3 knockdown mice (right panel in Fig. 5F) than intruder plus scramble control mice (left panel). The ratios of the stay time on the plate of open arms to the total time in the EPM are 9.2 ± 1.31% in intruder plus neuroligin-3 knockdown mice (green symbols in Fig. 5G, n = 14) and 4.15 ± 1.04% in intruder plus scramble control mice (red symbols, n = 17, p < 0.01, ANOVA). Neuroligin-3 knockdown in the auditory cortex of intruder mice prevents their anxiety-like behaviors induced by the social stress based on resident/intruder paradigm. The data indicate that neuroligin-3 in the auditory cortex plays essential role in stress-induced fear memory and anxiety-like behaviors.
Discussion
The social stress by the resident/intruder paradigm induces the fear memory and anxiety in intruder mice, in which the stressful signals include the pain from trunk injury area and the battle sound (Fig. 1). In the mice of expressing stress-induced fear memory and anxiety, the synapse interconnections between auditory and Sr-1Tr cortices are newly formed and functionally active (Fig. 2). The auditory cortical neurons receive new synapse innervations from the S1-Tr cortex alongside those synapse innervations from the medial geniculate body (Fig. 3). Some auditory cortical neurons in these mice become to encode the pain signal from trunk injury areas and the sound signal during their battles (Fig. 3). Therefore, the social stress recruits auditory cortical neurons to be associative memory neurons that encode stress signals, fear memories and anxiety. Moreover, the recruitment of associative memory neurons and the emergence of fear memory and anxiety are precluded by knocking down neuroligin-3-mediated synapse linkage (Figs. 4–5). In summary, the social stress makes auditory cortical neurons recruited to be associative memory neurons that encode fear memory and anxiety by neuroligin-3-mediated new synapse formation.
Our data reveal that the social stress induces the fear memory in those intruder mice specific to the stressful signals including the battle sound generated during the attack of a CD-1 resident mouse and the pain signal from trunk injury areas bitten by this CD-1 resident mouse. As a resident/intruder paradigm was commonly used to study the suffering of depression and anxiety [4, 85,86,87,88,89,90], the anxiety and depression in the intruder mice may result secondarily from the fear memory to stressful signals. It is known that the physical and psychological stressors lead to anxiety and depression [1,2,3, 5,6,7,8,9,10,11, 13, 18, 20]. Such affective disorders may suppress the immune system and the cardiovascular system to induce the secondary diseases [12]. Based on our present study, the downregulation of stress-induced memory to negative outcome may be the primary step to reduce these pathological moods and to prevent their relevant secondary diseases.
The auditory cortex encoded the joint storage of auditory signals with other signals in associative learning and memory [7, 62, 64,65,66,67,68,69,70, 72]. The primary auditory cortex has been thought to work for the discrimination of stimulations from threat versus nonthreat signals and to regulate the specificity of threat memory [73]. However, the cellular mechanism underlying the information storage in the auditory cortex is largely unknown [74,75,76,77,78]. Associative memory cells have been identified in the sensory cortices including the barrel cortex, piriform cortex, somatosensory cortex and gustatory cortex, which are characterized to be the coactivity-dependent interconnections among cross-modal cortices and the encoding of relevant associated signals [79,80,81,82,83,84, 108]. By using the resident/intruder paradigm, we discover that the social stress induces the recruitment of associative memory neurons in the auditory cortex that are featured by the formation of synapse interconnections with S1-Tr cortical neurons and the encoding of the fear signals including the battle sound and the painful signal essential for fear memory and anxiety. These associative memory cells can be specified to the fear memory cells and anxiety cells. Whether these associative memory cells also encode the emotional negative valence led by the negative memory of aversive tones in the auditory cortex remains to be examined once the test and the approach for the emotional reaction is well developed. In other words, the auditory cortical neurons may be recruited to have multiple functions, such as the fear memory and anxiety as well as their correlated negative emotional valence. In addition, all types of the associative memories to the signals from the external environment and endogenous brain activities are presumably based on the recruitment of associative memory cells in the brain. This logical prediction encourages researchers to test whether associative memory neurons are widely present in the memory formation as basic units of memory trace [7, 13].
Acute severe stress often induces fear memory and anxiety [1, 3,4,5, 7, 22, 25, 26, 107]. Many brain regions, such as the amygdala, the nucleus accumbens and the prefrontal cortex, have been presumably correlated to the fear memory and anxiety [27,28,29,30,31,32,33,34,35,36,37]. The neural circuits in the amygdala and the nucleus accumbens have been thought of the relevance to the balance between the fear memory and the reward memory [38,39,40,41,42,43]. The imbalance of these structures causes dominant memories to negative events, leading to affective disorders [44,45,46,47,48,49, 52,53,54,55,56]. In addition to the interaction of amygdala neurons with other brain areas, the connections between the amygdala and the auditory cortex appear to be strengthened during the fear memory induced by the association of bell ring and foot shock [23, 57,58,59,60,61]. These data indicate that the stress-related fear signals may be encoded by the interaction between the amygdala and the auditory cortex. Our data here indicate the recruitment of associative memory neurons in the auditory cortex for the stress-induced fear memory and anxiety. Those secondary associative memory neurons [7, 13] may be recruited in the amygdala for stress-induced fear memory and anxiety, because we have observed the convergent synapse innervations onto the neurons in the lateral area of the amygdala projected from the S1-Tr cortex and the auditory cortex in the mice with stress-induced fear memory and anxiety.
In terms of the molecular mechanism underlying the recruitment of associative memory neurons in the auditory cortex for stress-induced fear memory and anxiety, our studies show that neuroligin-3 knockdown in the auditory cortex by its shRNA prevents the emergence of fear memory and anxiety, the formation of new synapse interconnections and the recruitment of associative memory neurons (Figs. 4–5). The co-disappearance of associative memory cells and fear memory/anxiety by knocking down neuroligin-3 suggests the essential role of synapse linkage protein neuroligin-3 in stress-induced fear memory and anxiety by recruiting associative memory neurons. There are two strategies to test the causal relation of the stress-induced interconnections between the auditory cortex and the S1-Tr cortex to the formations of associative memory cells, fear memory and anxiety. In the present study, the prevention of the formation of this interconnection by downregulating neuroligin-3 is conducted to test whether the recruitment of associative memory neurons as well as the emergences of the fear memory and anxiety are precluded. Another strategy worthy to be applied is to examine whether the inhibition of the function of this stress-induced interconnection by an optogenetic approach can block the activity of associative memory neurons as well as the expression of the fear memory and anxiety. It is noteworthy that the neuroligin-3 knockdown in the auditory cortex is unable to prevent the fear memory by the visual system seeing the resident CD-1 mouse, indicating that the associative memory neurons may also be recruited in the visual cortex, which has been observed in other studies from our group.
To the question whether the interconnection between auditory and S1-Tr cortices is functional, we have experimentally examined the colocalization of those interconnection neurons labeled by AAV -fluorescents and the neurons labelled by the antibody of cFos, one of immediate early genes that are used to show active neurons [109,110,111]. As shown in Fig. 2, both interconnection neurons and cFos-labelled neurons are raised in the auditory cortices from the mice with stress-induced fear memory and anxiety, indicating those interconnected neurons as associative memory neurons more active. It is noteworthy that the partial colocalization of the interconnected neurons and the cFos-labelled neurons in the auditory cortex (Figure S1) indicates that they are not the identical population of auditory cortical neurons, or not mutually represented.
The formation of new synapse interconnections among cross-modal cortices and the recruitment of associative memory neurons to encode stress signals for fear memory and anxiety are functionally and morphologically identified in the mouse model of resident/intruder paradigm. The stress-induced psychological behaviors and cellular changes are based on neuroligin3-mediated new synapse linkage. A diagram in Fig. 6 illustrates that the social stress induces auditory cortical neurons to receive new synapse innervations from the S1-Tr cortex alongside synapses from the geniculate body as well as to interconnect S1-Tr cortex, so that auditory cortical neurons become able to encode all of these stress signals inputted from auditory and somatosensory systems. The associative memory neurons in the auditory cortex may play a central role between the input of fear signals from the sensory system and the processing of fear memory and pathological mood in the amygdala and the prefrontal cortex. The stress-induced recruitment of associative memory neurons is strengthening the concept of associative memory neurons being recruited in all types of associative learning [7]. Our studies also reveal that the cellular working principle for fear memory and anxiety is based on the associative memory neurons in the auditory cortex to encode stress signals and psychological deficits, which has not been indicated in previous studies.
A Auditory neurons turn into encoding stressful auditory and somatic signals. These stressful signals are transmitted to amygdala, leading to the onset of fear memory and anxiety-like behaviors. B After the social stress, the auditory neurons form synapse interconnection with the somatosensory neurons, alongside receiving synapse innervations from the medial geniculate body.
Data availability
The datasets used and analyzed in the current study are available from the corresponding author based on the request without commercial purpose.
Code availability
The datasets used and analyzed in the current study are available from the corresponding author based on the request without commercial purpose.
References
Baldi E, Bucherelli C. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci Biobehav Rev. 2015;53:160–90.
Coutellier L, Usdin TB. Enhanced long-term fear memory and increased anxiety and depression-like behavior after exposure to an aversive event in mice lacking TIP39 signaling. Behav Brain Res. 2011;222:265–9.
Desmedt A, Marighetto A, Piazza PV. Abnormal fear memory as a model for posttraumatic stress disorder. Biol Psychiatry. 2015;78:290–7.
Du K, Lu W, Sun Y, Feng J, Wang JH. mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress. J Psychiatr Res. 2019;118:44–65.
Izquierdo I, Furini CR, Myskiw JC. Fear memory. Physiol Rev. 2016;96:695–750.
Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35:1625–52.
Wang JH. Associative memory cells: basic units of memory trace. the first edn. Singapore Pre Ltd: Springer Nature: Springer; 2019.
Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–802.
Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16:146–53.
Si Y, Song Z, Sun X, Wang JH. microRNA and mRNA profiles in nucleus accumbens underlying depression versus resilience in response to chronic stress. Am J Med Genet B Neuropsychiatr Genet. 2018;177:563–79.
Sun J, Lu Y, Yang J, Song Z, Lu W, Wang JH. mRNA and microRNA profiles in the amygdala are relevant to susceptibility and resilience to psychological stress induced in mice. J Mol Neurosci. 2020;70:1771–96.
Thrall G, Lane D, Carroll D, Lip GY. A systematic review of the effects of acute psychological stress and physical activity on haemorheology, coagulation, fibrinolysis and platelet reactivity: implications for the pathogenesis of acute coronary syndromes. Thromb Res. 2007;120:819–47.
Wang JH. Searching basic units in memory traces: associative memory cells. F1000Res. 2019;8:457.
de Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci. 2017;18:7–19.
Flores A, Fullana MA, Soriano-Mas C, Andero R. Lost in translation: how to upgrade fear memory research. Mol Psychiatry. 2018;23:2122–32.
Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–45.
Sandkuhler J, Lee J. How to erase memory traces of pain and fear. Trends Neurosci. 2013;36:343–52.
Ma K, Xu A, Cui S, Sun M, Xue Y, Wang J-H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl Psychiatry. 2016;6:1–10.
Sutoo D, Akiyama K. Neurochemical changes in mice following physical or psychological stress exposures. Behav Brain Res. 2002;134:347–54.
Xu A, Cui S, Wang J. Incoordination among subcellular compartments is associated to depression-like behavior induced by chronic mild stress. Int J Neuropsychopharmacol. 2015;19:pyv122.
Zhang F, Liu B, Lei Z, Wang J. mGluR1,5 activation improves network asynchrony and GABAergic synapse attenuation in the amygdala: implication for anxiety-like behavior in DBA/2 mice. Mol Brain. 2012;5:20.
Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17:1644–54.
Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2012;480:331–5.
Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–4.
Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82.
Sun X, Song Z, Si Y, Wang JH. microRNA and mRNA profiles in ventral tegmental area relevant to stress-induced depression and resilience. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:150–65.
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71.
Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–34.
Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52.
Garrett A, Chang K. The role of the amygdala in bipolar disorder development. Dev Psychopathol. 2008;20:1285–96.
Haruno M, Kimura M, Frith CD. Activity in the nucleus accumbens and amygdala underlies individual differences in prosocial and individualistic economic choices. J Cogn Neurosci. 2014;26:1861–70.
Keele NB. The role of serotonin in impulsive and aggressive behaviors associated with epilepsy-like neuronal hyperexcitability in the amygdala. Epilepsy Behav. 2005;7:325–35.
Kostopoulos P, Petrides M. Selective memory retrieval of auditory what and auditory where involves the ventrolateral prefrontal cortex. Proc Natl Acad Sci USA. 2016;113:1919–24.
Lee JH, Lee S, Kim JH. Amygdala circuits for fear memory: a key role for dopamine regulation. Neuroscientist. 2016;23:542–53.
Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–63.
Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58.
Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–42.
Fadok JP, Darvas M, Dickerson TMK, Palmiter RD. Long-term memory for pavlovian fear conditioning requires dopamine in the nucleus accumbens and basolateral amygdala. PLoS One. 2010;5:e12751.
Hikida T, Morita M, Macpherson T. Neural mechanisms of the nucleus accumbens circuit in reward and aversive learning. Neurosci Res. 2016;108:1–5.
Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78.
Kochenborger L, Zanatta D, Berretta LM, Lopes APF, Wunderlich BL, Januario AC, et al. Modulation of fear/anxiety responses, but not food intake, following alpha-adrenoceptor agonist microinjections in the nucleus accumbens shell of free-feeding rats. Neuropharmacology. 2012;62:427–35.
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–76.
Thomas KL, Hall J, Everitt BJ. Cellular imaging with zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci. 2002;16:1789–96.
Anderson EM, Larson EB, Guzman D, Wissman AM, Neve RL, Nestler EJ, et al. Overexpression of the histone dimethyltransferase G9a in nucleus accumbens shell increases cocaine self-administration, stress-induced reinstatement, and anxiety. J Neurosci. 2018;38:803–13.
Barrot M, Wallace DL, Bolanos CA, Graham DL, Perrotti LI, Neve RL, et al. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci USA. 2005;102:8357–62.
Bosch-Bouju M, Larrieu T, Linders L, Manzoni OJ, Laye S. Endocannabinoid-mediated plasticity in nucleus accumbens controls vulnerability to anxiety after social defeat stress. Cell Rep. 2016;16:1237–42.
Feng J, Pena CJ, Purushothaman I, Engmann O, Walker D, Brown AN, et al. Tet1 in nucleus accumbens opposes depression- and anxiety-like behaviors. Neuropsychopharmacology. 2017;42:1657–69.
Fu KQ, Miyamoto Y, Sumi K, Saika E, Muramatsu S, Uno K, et al. Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS One. 2017;12:e0189006.
Kim KS, Lee KW, Baek IS, Lim CM, Krishnan V, Lee JK, et al. Adenylyl cyclase-5 activity in the nucleus accumbens regulates anxiety-related behavior. J Neurochem. 2008;107:105–15.
Heshmati M, Golden SA, Pfau ML, Christoffel DJ, Seeley EL, Cahill ME, et al. Mefloquine in the nucleus accumbens promotes social avoidance and anxiety-like behavior in mice. Neuropharmacology. 2016;101:351–7.
Levita L, Hoskin R, Champi S. Avoidance of harm and anxiety: a role for the nucleus accumbens. Neuroimage. 2012;62:189–98.
Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–9.
Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–8.
Zhu Z, Wang G, Ma K, Cui S, Wang JH. GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression. Oncotarget. 2017;8:35933–45.
Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41.
Wang H, Li F, Zheng X, Meng L, Chen M, Hui Y, et al. Social defeat drives hyperexcitation of the piriform cortex to induce learning and memory impairment but not mood-related disorders in mice. Transl Psychiatry. 2022;12:380.
Grosso A, Cambiaghi M, Concina G, Sacco T, Sacchetti B. Auditory cortex involvement in emotional learning and memory. Neuroscience. 2015;299:45–55.
Cambiaghi M, Renna A, Milano L, Sacchetti B. Reversible inactivation of the higher order auditory cortex during fear memory consolidation prevents memory-related activity in the basolateral amygdala during remote memory retrieval. Front Behav Neurosci. 2017;11:138.
Reinhard SM, Rais M, Afroz S, Hanania Y, Pendi K, Espinoza K, et al. Reduced perineuronal net expression in Fmr1 KO mice auditory cortex and amygdala is linked to impaired fear-associated memory. Neurobiol Learn Mem. 2019;164:107042.
Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–8.
Yang Y, Liu DQ, Huang W, Deng J, Sun Y, Zuo Y, et al. Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat Neurosci. 2016;19:1348–55.
Bigelow J, Rossi B, Poremba A. Neural correlates of short-term memory in primate auditory cortex. Front Neurosci. 2014;8:250.
Choi I, Rajaram S, Varghese LA, Shinn-Cunningham BG. Quantifying attentional modulation of auditory-evoked cortical responses from single-trial electroencephalography. Front Hum Neurosci. 2013;7:115.
Colombo M, D’Amato MR, Rodman HR, Gross CG. Auditory association cortex lesions impair auditory short-term memory in monkeys. Science. 1990;247:336–8.
Gottlieb Y, Vaadia E, Abeles M. Single unit activity in the auditory cortex of a monkey performing a short term memory task. Exp Brain Res. 1989;74:139–48.
Huang Y, Matysiak A, Heil P, Konig R, Brosch M. Persistent neural activity in auditory cortex is related to auditory working memory in humans and nonhuman primates. eLife. 2016;5:e15441.
Nomura H, Hara K, Abe R, Hitora-Imamura N, Nakayama R, Sasaki T, et al. Memory formation and retrieval of neuronal silencing in the auditory cortex. Proc Natl Acad Sci USA. 2015;112:9740–4.
Scheich H, Stark H, Zuschratter W, Ohl FW, Simonis CE. Some functions of primary auditory cortex in learning and memory formation. Adv Neurol. 1997;73:179–93.
Yu L, Hu J, Shi C, Zhou L, Tian M, Zhang J, et al. The causal role of auditory cortex in auditory working memory. eLife. 2021;10:e64457.
Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms. Audiol Neurootol. 1998;3:145–67.
Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem. 1998;70:226–51.
Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–90.
Wigestrand MB, Schiff HC, Fyhn M, LeDoux JE, Sears RM. Primary auditory cortex regulates threat memory specificity. Learn Mem. 2017;24:55–58.
Michalka SW, Rosen ML, Kong L, Shinn-Cunningham BG, Somers DC. Auditory spatial coding flexibly recruits anterior, but not posterior, visuotopic parietal cortex. Cereb Cortex. 2016;26:1302–8.
Moczulska KE, Tinter-Thiede J, Peter M, Ushakova L, Wernle T, Bathellier B, et al. Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proc Natl Acad Sci USA. 2013;110:18315–20.
Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proc Natl Acad Sci USA. 2011;108:15516–21.
Weinberger NM. Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem. 2007;14:1–16.
Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007;229:54–68.
Feng J, Lu W, Wang D, Ma K, Song Z, Chen N, et al. Barrel cortical neuron integrates triple associated signals for their memory through receiving epigenetic-mediated new synapse innervations. Cereb Cortex. 2017;27:5858–71.
Gao Z, Chen L, Fan R, Lu W, Wang D, Cui S, et al. Associations of unilateral whisker and olfactory signals induce synapse formation and memory cell recruitment in bilateral barrel cortices: cellular mechanism for unilateral training toward bilateral memory. Front Cell Neurosci. 2016;10:1–16.
Gao Z, Wu R, Chen C, Wen B, Liu Y, Lu W, et al. Coactivations of barrel and piriform cortices induce their mutual synapse innervations and recruit associative memory cells. Brain Res. 2019;1721:146333.
Liu Y, Gao Z, Chen C, Wen B, Huang L, Ge R, et al. Piriform cortical glutamatergic and GABAergic neurons express coordinated plasticity for whisker-induced odor recall. Oncotarget. 2017;8:95719–40.
Wang D, Zhao J, Gao Z, Chen N, Wen B, Lu W, et al. Neurons in the barrel cortex turn into processing whisker and odor signals: a cellular mechanism for the storage and retrieval of associative signals. Front Cell Neurosci. 2015;9:320.
Xu Y, Cui TL, Li JY, Chen B, Wang JH. Associative memory neurons of encoding multi-modal signals are recruited by neuroligin-3-mediated new synapse formation. eLife. 2023;12:RP87969.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–42.
Hammels C, Pishva E, De Vry J, van den Hove DL, Prickaerts J, van Winkel R, et al. Defeat stress in rodents: from behavior to molecules. Neurosci Biobehav Rev. 2015;59:111–40.
Martinez M, Calvo-Torrent A, Pico-Alfonso MA. Social defeat and subordination as models of social stress in laboratory rodents: a review. Aggress Behav. 1998;24:241–56.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25.
Vasconcelos M, Stein DJ, de Almeida RM. Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade. Trends Psychiatry Psychother. 2015;37:51–66.
Lei Z, Wang D, Chen N, Ma K, Lu W, Song Z, et al. Synapse innervation and associative memory cell are recruited for integrative storage of whisker and odor signals in the barrel cortex through miRNA-mediated processes. Front Cell Neurosci. 2017;11:1–11.
Wu R, Cui S, Wang JH. miRNA-324/-133a essential for recruiting new synapse innervations and associative memory cells in coactivated sensory cortices. Neurobiol Learn Mem. 2020;172:107246.
Sun Y, Lu W, Du K, Wang JH. microRNA and mRNA profiles in the amygdala are relevant to fear memory induced by physical or psychological stress. J Neurophysiol. 2019;122:1002–22.
Yang J, Sun J, Lu Y, An T, Lu W, Wang JH. Correction to: mRNA and microRNA profiles are associated with stress susceptibility and resilience induced by psychological stress in the prefrontal cortex. Psychopharmacology (Berl). 2020;237:3095.
Paxinos G, Watson C The Mouse Brain: in stereotaxic coordinates. Amsterdam: Elsevier Academic Press; 2005.
Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52.
Hamilton SM, Green JR, Veeraragavan S, Yuva L, McCoy A, Wu Y, et al. Fmr1 and Nlgn3 knockout rats: novel tools for investigating autism spectrum disorders. Behav Neurosci. 2014;128:103–9.
Li H, Guo R, Guan Y, Li J, Wang Y. Modulation of trans-synaptic neurexin-neuroligin interaction in pathological pain. Cells. 2022;11:1940.
Lise MF, El-Husseini A. The neuroligin and neurexin families: from structure to function at the synapse. Cell Mol Life Sci. 2006;63:1833–49.
Sudhof TC. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell. 2017;171:745–69.
Uchigashima M, Cheung A, Futai K. Neuroligin-3: a circuit-specific synapse organizer that shapes normal function and autism spectrum disorder-associated dysfunction. Front Mol Neurosci. 2021;14:749164.
Chang K, Elledge SJ, Hannon GJ. Lessons from nature: microRNA-based shRNA libraries. Nat Methods. 2006;3:707–14.
Khatri N, Rathi M, Baradia D, Trehan S, Misra A. In vivo delivery aspects of miRNA, shRNA and siRNA. Crit Rev Ther Drug Carrier Syst. 2012;29:487–527.
Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev. 2007;59:141–52.
Pushparaj PN, Aarthi JJ, Manikandan J, Kumar SD. siRNA, miRNA, and shRNA: in vivo applications. J Dent Res. 2008;87:992–1003.
Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009;61:746–59.
Chen B, Zhang Y, Xiao H, Wang L, Li J, Xu Y, et al. Associative memory cells of encoding fear signals and anxiety are recruited by neuroligin-3-mediated synapse formation. Commun Biol. 2024;7:1464.
Huguenard JR. Neuronal circuitry of thalamocortical epilepsy and mechanisms of antiabsence drug action. Adv Neurol. 1999;79:991–9.
Josselyn SA, Kohler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16:521–34.
Josselyn SA, Tonegawa S. Memory engrams: recalling the past and imagining the future. Science. 2020;367:eaaw4325.
Kim JI, Cho HY, Han JH, Kaang BK. Which neurons will be the engram - activated neurons and/or more excitable neurons? Exp Neurobiol. 2016;25:55–63.
Acknowledgements
This study is funded by the Natural Science Foundation of China (81971027, U2241209 and 81930033) to Jin-Hui Wang (JHW).
Author information
Authors and Affiliations
Contributions
Conceptualization: JHW. Methodology: YZ, BC, LW, JL, YX, JHW. Validation: YZ, BC, LW, JL, YX, JHW. Formal analysis: YZ, BC. Investigation: YZ, BC, LW, JL, YX, JHW. Resources: JHW. Data curation: YZ, BC. Writing-Original draft: JHW. Writing-Review & Editing: JHW. Visualization: YZ, BC, JHW. Supervision: JHW. Project administration: JHW. Funding acquisition: JHW.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Experiments were done in accordance with guidelines and regulations by the Administration Office of Laboratory Animal in Beijing China. All experiment protocols were approved by Institutional Animal Care and Use Committee in Administration Office of Laboratory Animal at Beijing China (B10831).
Consent for publication
In the publication of this manuscript, no individual’ data, such as personal details, images, videos and voice are included. All necessary consent for publication has been secured.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Chen, B., Li, J. et al. Auditory cortical neurons are recruited to encode fear signals and anxiety by neuroligin-3-mediated synapse formation. Transl Psychiatry 15, 140 (2025). https://doi.org/10.1038/s41398-025-03357-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03357-9