Abstract
The relationship between sleep quality, neurofilament light chain (NFL), and cognitive impairment, including the potential effect of plasma NFL in this association, remains unclear. Using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort, we excluded individuals with dementia or a history of sleep-related medication use at baseline, including 640 participants with complete sleep assessments and covariates. Sleep quality was assessed using the Neuropsychiatric Inventory sleep subscale, which includes ratings of frequency, severity, and their product, with higher scores indicating poorer sleep quality. Baseline and follow-up demographics, sleep indices, plasma NFL levels, and cognition scores (including Mini-Mental State Examination [MMSE], Montreal Cognitive Assessment [MoCA], Alzheimer’s Disease Assessment Scale-Cognitive Subscale [ADAS13], Clinical Dementia Rating Scale-Sum of Boxes [CDRSB], Executive Function [EF], Language [LAN], and Memory [MEM]) were also collected. Multivariable linear regression examined the associations between baseline sleep quality, plasma NFL, and cognition, as well as the relationship between sleep quality and longitudinal cognitive decline, calculated using linear mixed-effects models. Mediation analysis evaluated the role of plasma NFL in the sleep-cognition association. Multiple testing significance was corrected using false discovery rate, with results presented as Q-values. Poor sleep quality scores were associated with elevated plasma NFL levels (β: 0.055 to 2.645, P < 0.05), poorer cognition (ADAS13, CDRSB, EF, LAN, MEM; β: −0.188 to 1.279, Q < 0.05), and accelerated longitudinal cognitive decline (MoCA; β: −0.005, Q < 0.05) in both models, with sensitivity analyses supporting these findings. Furthermore, plasma NFL levels partially mediated the relationship between sleep quality and both baseline cognition (ADAS13, CDRSB, LAN, MEM; P < 0.05) and longitudinal cognitive decline (MoCA; P < 0.05), with mediation proportions ranging from 9.2% to 26.7%. Poorer sleep quality was associated with cognitive impairment and accelerated cognitive decline, suggesting its potential role in Alzheimer’s disease. These associations may be partially mediated by neuroaxonal injury.
Similar content being viewed by others
Introduction
Cognitive impairment is a key component of neurodegeneration disease, including Alzheimer’s disease (AD) [1, 2]. With an aging population, delaying or preventing cognitive decline has become a major focus in neuroscience and geriatric medicine [3]. Traditionally, lifestyle factors such as exercise, diet, smoking, and alcohol consumption have been considered primary modifiable risk factors for cognitive health [4]. Recently, sleep, a dynamic and adjustable biological behavior, has gained increasing attention as a critical risk factor for cognitive impairment.
Sleep disturbances are common in older adults, with aging associated with changes in sleep patterns, including reduced total sleep time and efficiency, increased fragmentation and difficulty falling asleep, decreased rapid eye movement (REM) sleep and slow-wave sleep, excessive daytime sleepiness, sleep-disordered breathing, and circadian rhythm disruptions [5]. These alterations are closely linked to cognitive decline. While ample evidence suggests that good sleep quality enhances memory consolidation in younger individuals, its benefits for older adults remain unclear [5,6,7]. Thus, exploring the relationship between sleep quality and cognition in older adults is crucial. However, most studies focus on individual sleep patterns, with limited research on overall sleep disturbances in populations. Notably, the relationship between the frequency and severity of sleep/nighttime behavioral disturbances and cognitive impairment remains underexplored. A systematic evaluation of this association is needed.
Moreover, sleep disturbances can lead to dysregulated inflammatory responses, with mechanisms involving cytokine reactions, neuroendocrine and autonomic pathways connecting sleep and the immune system, and the role of inflammatory peptides in sleep regulation [8]. These mechanisms underscore the importance of inflammation in sleep. However, the relationship between neurofilament light chain (NFL), a key biomarker for axonal injury, and sleep disturbances remains to be explored. The NFL, one of the three subunits of neurofilaments, is an important axonal cytoskeletal protein crucial for axon growth. After inflammation-induced axonal damage, NFL is released into the extracellular environment, subsequently entering cerebrospinal fluid (CSF) and blood, making it a key inflammatory biomarker for assessing axonal injury [9]. Recent studies suggest that NFL may predict the progression of sleep disturbances to neurodegenerative diseases [10, 11]. Elevated plasma and CSF NFL concentrations in neurodegenerative patients, compared to healthy controls, further highlight its potential as a biomarker for diseases like AD [12]. However, the exact relationship between sleep disturbances and NFL levels remains unclear. Furthermore, recent studies have shown that poor subjective sleep quality is linked to increased plasma monocyte cytokine secretion, immune activation, and elevated transcription factor activity, providing a basis for further investigation into the relationship between sleep, neuroinflammation, and neuronal damage [13, 14]. These findings support the exploration of sleep/nighttime behavior disturbances, neuroinflammation, and cognitive impairment or AD. Thus, studying the relationship between sleep disturbances and NFL levels is crucial for understanding the mechanisms linking sleep disturbances to cognitive decline or AD. However, research in this area remains in its early stages, with limited evidence available.
In this context, given the close relationship between sleep, NFL, and cognitive function, we hypothesize that sleep disturbances may lead to cognitive decline through axonal damage, reflected by elevated NFL levels. Furthermore, we propose that NFL may mediate the relationship between sleep disturbances and cognitive changes. Therefore, this study aims to: (1) examine the cross-sectional and longitudinal relationships between baseline sleep disturbances, plasma NFL levels, and cognitive function; (2) analyze the mediating role of plasma NFL in the relationship between sleep disturbances and cognitive function in a non-demented population, and clarify how sleep disturbance frequency, severity, and composite scores affect neuroinflammation and neuronal health, leading to cognitive decline or dementia, providing insights for optimizing sleep intervention strategies.
Methods
ADNI study design
The data utilized in our study were sourced from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu) [15, 16]. Launched in 2003, ADNI is a $60 million, 5-year public-private partnership involving the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies, and non-profit organizations. For the most current information, please refer to www.adni-info.org. Written consent was obtained from all participants at enrollment, and the study received approval from the institutional review board at each participating site.
Participants
We categorized the participants into three groups based on cognitive status: the cognitively normal (CN) group, the mild cognitive impairment (MCI) group, and the AD dementia group. Our study included only non-demented individuals, comprising CN controls and individuals with MCI. All participants had baseline data available on Neuropsychiatric Inventory (NPI) scores, plasma NFL levels, cognitive assessments, and relevant covariates from the ADNI database. Detailed inclusion and exclusion criteria can be found online (http://www.adni-info.org) [17]. In terms of cognitive function assessment, participants were tested using a variety of scales and grouped according to the results. CN participants exhibited a mini-mental state examination score exceeding 24 and a clinical dementia rating score of 0. Patients with MCI had a Mini-Mental State Examination (MMSE) score of ≥24 and a Clinical Dementia Rating of 0.5, with preserved activities of daily living and no dementia diagnosis. Additionally, their performance on the Logical Memory II subtest of the Wechsler Memory Scale was at least one standard deviation below the mean of the CN group [18].
In this study, the first inclusion of participants was defined as the baseline, and any subsequent inclusion was considered follow-up. Follow-up was defined as having at least one additional inclusion beyond the baseline. To mitigate the impact of medications on baseline sleep quality, individuals with a history of using sleep-improving, antidepressant,anxiolytic, or mood-enhancing medications were excluded from the study. A detailed summary of sleep-impacting medication history and specific drugs is provided in Additional Table 1. Ultimately, after excluding 55 individuals with a history of sleep-impacting medication use, a total of 640 participants aged between 55 and 91 years were included in the analysis, of whom 607 had follow-up data available. Typically, follow-up visits are conducted every 6 or 12 months, with some participants being followed for up to 10 years.
Sleep quality assessments
Sleep disturbances were assessed at baseline using the sleep subscale of the NPI scale, which is completed by the participant or their caregiver during the interview. This approach was chosen to improve the reliability of the assessment tool. To balance the comprehensiveness of the behavioral assessment with an acceptable testing time, screening questions were used. Based on information provided by an informant, patients were asked whether they had experienced any of the following sleep/nighttime behavioral disturbances within the past month:
-
1.
Does the patient have difficulty falling asleep?
-
2.
Does the patient get up during the night? (If the patient only wakes up once or twice to use the bathroom and quickly falls back asleep, this is not considered a disturbance.)
-
3.
Does the patient walk around, pace, or engage in other inappropriate activities at night?
-
4.
Does the patient wake you during the night?
-
5.
Does the patient wake up, get dressed, and prepare to go out, thinking it is morning and time to start the day?
-
6.
Does the patient wake up too early in the morning (earlier than their usual time)?
-
7.
Does the patient nap excessively during the day?
-
8.
Are there other disruptive nighttime behaviors not yet discussed?
If no disturbances were reported, the score was 0; if any disturbances were reported, the score was 1. If disturbances were present, the frequency [Frequency ratings (FR)] and severity [Severity ratings (SR)] of the sleep/nighttime behavior were further assessed. FR: 0 = none; 1 = occasional (no more than once a week); 2 = frequent (about once a week); 3 = very frequent (several times a week, but not every night); 4 = extremely frequent (every night or multiple times a night). SR: 0 = none; 1 = mild (behavior occurs but is not particularly disruptive); 2 = moderate (behavior disrupts both the patient’s and caregiver’s sleep; may involve one or more behaviors); 3 = severe (multiple behaviors; the patient is highly distressed at night, and the caregiver’s sleep is significantly affected). For patients with multiple sleep disturbances, the most impactful disturbance was used for evaluation. This ensures a comprehensive assessment of sleep issues while avoiding excessive detail that might interfere with the overall interpretation of the results.
The sleep subscale score (TOTAL) was calculated by multiplying FR by SR. This calculation results in an asymmetrical distribution of scores, where scores of 5, 7, 10, and 11 cannot be obtained. Previous research has shown that the results of parametric and non-parametric analyses with or without consideration of this asymmetry are very similar, and missing scores do not significantly affect the interpretation of the total or dimension scores [19]. We believe that the TOTAL score provides a more comprehensive assessment of sleep/nighttime behavioral disturbances than using FR or SR alone, making it the preferred metric in our study [19].
Plasma NFL measurement
Plasma NFL concentration was measured at baseline using an internal ultrasensitive enzyme-linked immunosorbent assay and a single-molecule array platform (Quanterix Corp), with a lower limit of quantification of 6.7 ng/L and an upper limit of 1620.0 ng/L, as previously described [20]. All samples, except one, were within the quantification range. For the low-concentration quality control sample (11.0 ng/L), the within-run coefficient of variation was 6.2%, and the between-run coefficient of variation was 9.0%. For the high-concentration quality control sample (173.0 ng/L), the within-run coefficient of variation was 4.9%, and the between-run coefficient of variation was 7.2%. Measurements were performed between January 1 and April 1, 2018, by a certified laboratory technician using a single batch of reagents.
Cognition assessments
All participants underwent comprehensive cognitive assessments at baseline and follow-up using multiple scales, including global cognition measures such as the MMSE, Montreal Cognitive Assessment (MoCA), Clinical Dementia Rating Sum of Boxes (CDRSB), and Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13 (ADAS13). Domain-specific cognitive functions were evaluated through neuropsychological assessments, including language (LAN), memory (MEM), and executive function (EF) from the Alzheimer’s Disease Sequencing Project Phenotype Harmonization Consortium. Due to over 50% missing data, visual-spatial performance (VSP) was excluded from this study. Except for CDRSB and ADAS13, where higher scores indicate poorer cognitive function, lower scores on MMSE, MoCA, EF, LAN, and MEM indicate worse cognitive performance.
Covariates
The study established two covariate models. Model 1 adjusted for key factors associated with cognitive impairment and AD, including age, sex, years of education, and apolipoprotein E (APOE) ε4 status. Model 2 included these adjustments and further accounted for smoking status, alcohol status, history of hypertension, and history of stroke. Smoking and alcohol status are relevant covariates in studies on cognitive impairment and dementia, while hypertension and stroke are established vascular risk factors that may accelerate neurodegeneration and cerebral small vessel disease, potentially affecting plasma NFL levels and cognitive function. Adjusting for these variables helps mitigate confounding by vascular factors, enhancing the robustness of our conclusions. However, introducing these additional covariates too early may lead to overadjustment, potentially attenuating or masking key associations. Therefore, Model 1 served as the primary focus of this study.
Statistical analysis
In this study, the normality of continuous variables was assessed using the Shapiro-Wilk test. Non-normally distributed variables were transformed using the Box-Cox method from the R “car” package, and all variables were standardized to z-scores using the R scale function. Plasma biomarker outliers beyond three standard deviations were excluded. Continuous variables are presented as mean ± SD, and categorical variables as frequency and percentage [N (%)]. Baseline characteristics between the CN and MCI groups were compared using the two-sample t-test for continuous variables and the chi-square test for categorical variables.
Multiple linear regression models were employed to investigate the cross-sectional relationships between baseline sleep quality, baseline NFL biomarkers, and cognition in the two aforementioned models. Estimated slopes for cognitive changes were calculated for each individual using linear mixed-effect models. Subsequently, multiple linear regression models were utilized to explore the longitudinal associations between baseline sleep quality and cognitive changes in the two models. Finally, given that axonal injury may be a potential moderator of the relationship between sleep quality and cognition, we conducted mediation analysis after adjusting for the covariates in Model 1 to assess whether plasma NFL concentrations mediate the cross-sectional and longitudinal relationship between sleep quality and cognition. In this study, we are more interested in the results related to the TOTAL score, as it serves as a more comprehensive measure of sleep disturbances compared to FR and SR. The results related to FR and SR are considered sensitivity analyses to strengthen our conclusions. False Discovery Rate (FDR) correction was separately applied to the results of FR, SR, and TOTAL, with significant findings reported as Q-values.
A significance level of P < 0.05 (two-tailed) was considered statistically significant. Data management and analyses were performed using R packages including data.table version 1.14.0, dplyr version 1.0.2, lme4 version 1.1.29, lmerTest version 3.1.3, and car version 3.0.10, in R version 4.0.1. Figures were generated using ggplot2 version 3.2.1, with additional support from stringr version 1.4.0 and fst version 0.9.4 for data management.
Results
Demographical and clinical characteristics
Table 1 presents the demographic and clinical characteristics of the study population. A total of 640 participants were included, comprising 293 in the CN group and 347 in the MCI group. The overall mean age was 72.69 years (SD = 6.81), with 45.31% being female. The mean years of education was 16.29 (SD = 2.65), and 38.59% were APOE ε4 carriers. Compared to the CN group, the MCI group was younger on average, had a higher prevalence of APOE ε4 carriers, and exhibited greater frequency and severity of sleep/nighttime behavioral disturbances. Additionally, the log-transformed TOTAL score for sleep/nighttime behavioral disturbances and the log-transformed plasma NFL levels were significantly higher in the MCI group.
Among the included participants, 607 had longitudinal follow-up data, with a median follow-up time of 4.0 years (Interquartile Range: 3.0, 7.5), including 258 in the CN group and 349 in the MCI group. The MCI group had a shorter follow-up duration compared to the CN group. All p-values in the table were adjusted for multiple comparisons using the FDR method and are presented as Q-values, with Q < 0.05 indicating statistical significance.
Cross-sectional associations between baseline sleep quality and plasma NFL
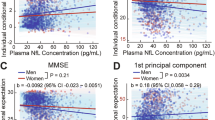
Linear regression analysis revealed a cross-sectional positive association between baseline TOTAL scores and baseline plasma NFL concentrations. In Model 1, higher baseline TOTAL score was associated with increased baseline plasma NFL levels (β = 0.055, P = 0.002). This association remained significant in Model 2 (β = 0.997, P = 0.012) (Fig. 1 and Additional Table 2). Sensitivity analyses further indicated that higher baseline FR and SR scores were also positively associated with baseline plasma NFL levels. In Model 1, higher baseline FR (β = 0.071, P = 0.023) and SR (β = 0.144, P = 0.011) scores were linked to elevated plasma NFL concentrations. These associations persisted in Model 2 (FR: β = 1.423, P = 0.039; SR: β = 2.645, P = 0.035) (Additional Table 2).
Model 1: adjusting for age, sex, years of education, and APOE ε4 status; Model 2: Further adjusted for smoking status, alcohol status, and history of hypertension and stroke, in addition to the covariates in Model 1. FR, SR, and TOTAL scores are derived from the K-th item of the NPI scale, specifically the sleep subscale.*P < 0.05, **P < 0.01, ***P < 0.001. APOE ε4 apolipoprotein ε4, FR Frequency ratings, NFL Neurofilament Light chain, NPI Neuropsychiatric Inventory, SR Severity ratings, TOTAL total (FR×SR).
Cross-sectional associations between baseline sleep quality and cognition
Linear regression analysis revealed a cross-sectional negative association between baseline TOTAL scores and cognitive performance. In Model 1, higher baseline TOTAL scores were associated with higher baseline ADAS13 scores (β = 0.059, Q = 0.009) and CDRSB scores (β = 0.096, Q < 0.001), as well as lower baseline EF scores (β = −0.053, Q = 0.012), LAN (β = −0.050, Q = 0.015), and MEM (β = −0.060, Q = 0.007). These associations remained significant in Model 2, with higher baseline TOTAL scores linked to increased ADAS13 scores (β = 0.416, Q = 0.007) and CDRSB (β = 0.117, Q < 0.001), along with decreased EF scores (β = −0.029, Q = 0.018), LAN (β = −0.025, Q = 0.022), and MEM (β = −0.035, Q = 0.007) (Fig. 2 and Additional Table 3). Sensitivity analyses showed similar cross-sectional negative associations between baseline FR and SR scores and cognitive performance. In Model 1, higher baseline FR scores were positively associated with CDRSB scores (β = 0.148, Q < 0.001), while higher SR scores were positively associated with ADAS13 (β = 0.186, Q = 0.007) and CDRSB scores (β = 0.310, Q < 0.001), and negatively associated with EF (β = −0.181, Q = 0.005), LAN (β = −0.162, Q = 0.013), and MEM scores (β = −0.188, Q = 0.007). In Model 2, higher baseline FR scores remained positively associated with ADAS13 (β = 0.538, Q = 0.047) and CDRSB scores (β = 0.187, Q < 0.001) and negatively associated with MEM scores (β = −0.045, Q = 0.046). Similarly, higher SR scores were positively correlated with ADAS13 (β = 1.279, Q = 0.007) and CDRSB scores (β = 0.359, Q < 0.001) and negatively correlated with EF (β = −0.099, Q = 0.009), LAN (β = −0.083, Q = 0.018), and MEM scores (β = −0.110, Q = 0.007) (Additional Table 3).
Model 1: Adjusted for age, sex, education, and APOE ε4 status; Model 2: Further adjusted for smoking status, alcohol status, and history of hypertension and stroke, in addition to the covariates in Model 1. NPI (K. Sleep) refers to the K-th item in the NPI scale, specifically the sleep subscale. Bolded Q values indicate statistically significant results. *P < 0.05, **P < 0.01, ***P < 0.001. ADAS13 Alzheimer’s Disease Assessment Scale -Cognitive Subscale 13, APOE ε4 apolipoprotein ε4, CDRSB Clinical Dementia Rating sum of boxes, EF executive function, FR Frequency ratings, LAN language function, MEM memory function, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment Scale, NPI Neuropsychiatric Inventory, SR Severity ratings, TOTAL total (FR×SR).
Longitudinal associations between baseline sleep quality and cognition
The results showed a negative longitudinal association between baseline TOTAL score and cognitive decline. In Model 1, the baseline TOTAL score was negatively associated with the annual rate of change in MoCA score (β = −0.005, Q = 0.035). A similar negative association was observed in Model 2 (β = −0.005, Q = 0.035) (Fig. 3 and Additional Table 4). Longitudinal sensitivity analyses indicated that after multiple corrections, the baseline FR and SR scores were no longer significantly associated with cognitive decline in both Model 1 and Model 2 (Additional Table 4).
Model 1: Adjusted for age, sex, education, and APOE ε4 status; Model 2: Further adjusted for smoking status, alcohol status, and history of hypertension and stroke, in addition to the covariates in Model 1. NPI (K. Sleep) refers to the K-th item in the NPI scale, specifically the sleep subscale. Bolded Q values indicate statistically significant results. *P < 0.05, **P < 0.01, ***P < 0.001. ADAS13 Alzheimer’s Disease Assessment Scale -Cognitive Subscale 13, APOE ε4 apolipoprotein ε4, CDRSB Clinical Dementia Rating sum of boxes, EF executive function, FR Frequency ratings, LAN language function, MEM memory function, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment Scale, NPI Neuropsychiatric Inventory, SR Severity ratings, TOTAL total (FR×SR).
Mediation analysis
The results indicated that plasma NFL levels mediated the association between baseline sleep/nighttime behavior disturbances (TOTAL score) and baseline cognitive performance, accounting for 21.9% of the effect when measured by ADAS13 (P = 0.008), 11.2% when measured by CDRSB (P = 0.008), 13.8% when measured by LAN (P = 0.016), and 18.7% when measured by MEM (P = 0.007). Similarly, plasma NFL levels mediated the relationship between baseline TOTAL score and longitudinal cognitive decline, explaining 26.7% of the effect when assessed by MoCA (P = 0.007) (Fig. 4). These findings suggest a significant mediating role of plasma NFL in the effects of baseline sleep/nighttime behavior disturbances on both baseline cognitive performance and longitudinal cognitive changes.
The dark blue mediation model represents the mediating effect of plasma NFL on the cross-sectional relationship between sleep and cognitive performance. The dark red mediation model illustrates the mediating effect of plasma NFL on the longitudinal association between sleep and the annual rate of cognitive decline. The mediation analysis was adjusted for covariates included in Model 1, including age, sex, education, and APOE ε4 status. *P < 0.05, **P < 0.01, ***P < 0.001. ADAS13 Alzheimer’s Disease Assessment Scale -Cognitive Subscale 13, APOE ε4 apolipoprotein ε4, CDRSB Clinical Dementia Rating sum of boxes, EF executive function, FR Frequency ratings, LAN language function, MEM memory function, MoCA Montreal Cognitive Assessment Scale, NPI Neuropsychiatric Inventory, SR Severity ratings, TOTAL total (FR×SR).
To enhance the comprehensiveness of the study and further validate the mediating role of plasma NFL in the relationship between baseline sleep disturbances and cognitive function, we conducted sensitivity analyses using mediation models for FR and SR scores. The results confirmed that plasma NFL mediated the association between baseline sleep/nighttime behavioral FR and SR scores and baseline cognitive function. In the relationship between FR score and CDRSB, the mediation proportion of plasma NFL was 10.2%. In the relationship between SR and cognitive function, the mediation proportions were 18.3% for ADAS13, 9.2% for CDRSB, 11.3% for LAN, and 15.9% for MEM (Fig. 4).
Discussion
This study yielded three key findings: (1) Higher frequency, greater severity, and higher total scores of baseline sleep/nighttime behavioral disturbances were associated with higher baseline plasma NFL levels and poorer baseline cognitive function. (2) Greater baseline sleep/nighttime behavioral disturbances were linked to more pronounced longitudinal cognitive decline. (3) Axonal injury, reflected by plasma NFL levels, mediated the association between baseline sleep/nighttime behavioral disturbances and both baseline cognition and longitudinal cognitive decline. These findings support the hypothesis that the relationship between sleep/nighttime behavioral disturbances and cognition may be partially mediated by neuronal damage. Our results suggest that sleep/nighttime behavioral disturbances are associated with poorer baseline cognition and accelerated cognitive decline.
Recent studies have emphasized the critical role of sleep in maintaining cognitive function, consistent with our findings [21]. Indeed, prospective studies have identified poor sleep quality as a risk factor for cognitive impairment and AD [22, 23]. A meta-analysis highlighted the significant relationship between various sleep disturbances—including insomnia, altered total sleep duration, poor sleep quality, excessive daytime sleepiness, sleep-disordered breathing, and circadian rhythm disruptions—and cognitive decline [5]. A retrospective cohort study reported that individuals clinically diagnosed with insomnia and using hypnotic medications over an extended period had twice the risk of developing dementia compared to those without insomnia [24]. Furthermore, decreased sleep quality has been linked to a higher risk of cognitive decline [25], with other studies reporting an increased risk of cognitive impairment and dementia [26, 27]. Prospective studies have also demonstrated a U-shaped relationship between sleep duration and cognitive outcomes, where both reduced and prolonged sleep durations are associated with poorer cognitive performance [28, 29]. Additionally, excessive daytime sleepiness has been consistently linked to cognitive decline and an increased risk of dementia [30, 31]. Recent findings suggest that sleep-disordered breathing is associated with a higher risk of mild cognitive impairment or dementia in older women [32]. A five-year prospective study reported that circadian rhythm alterations, such as reduced activity amplitude, lower rhythm stability, and delayed activity peaks, were associated with an increased risk of dementia and mild cognitive impairment [33]. These studies collectively support the strong relationship between various sleep disturbances and cognitive decline, providing a theoretical foundation for investigating the association between sleep/nighttime behavioral disturbances and cognition. However, cross-sectional and longitudinal studies specifically examining the frequency and severity of sleep/nighttime behavioral disturbances in relation to cognitive function remain scarce, and the underlying neuroinflammatory mechanisms linking sleep disturbances to cognitive decline require further exploration.
The relationship between sleep and cognition is complex. Some evidence suggests that sleep fragmentation, such as obstructive sleep apnea, may not necessarily increase the risk of cognitive impairment [34]. However, other sstudies indicate that AD has been reported to be associated with sleep disturbances, subjective sleep impairment, and chronic sleep deprivation, as well as reduced sleep duration, quality, and efficiency, suggesting a bidirectional association [35,36,37]. Furthermore, individuals with AD or MCI frequently experience sleep problems [38, 39]. These findings highlight the bidirectional relationship between sleep and cognitive impairment or AD. Therefore, future research should utilize methods such as Mendelian randomization to further explore the complex interactions between specific sleep characteristics and cognition.
Our study demonstrated that poorer sleep was associated with higher baseline plasma NFL levels. Consistent with our findings, a study in middle-aged individuals found that patients with insomnia had higher NFL levels than non-insomniac individuals, with a subsequent decrease following insomnia treatment [40]. A cross-sectional study also reported an association between increased plasma NFL levels and sleep disturbances, suggesting that NFL may serve as a potential biomarker for sleep disturbances [41]. Furthermore, poor sleep—particularly acute sleep deprivation—has been linked to elevated blood levels of neuronal and astrocytic injury markers [42, 43]. These studies reinforce the significant association between sleep disturbances and NFL levels, further supporting our findings. However, some prior studies have reported conflicting results. One study found no association between plasma NFL levels and subjective baseline sleep disturbances, actigraphy-estimated sleep parameters, or 24-hour activity rhythms in non-demented individuals [44]. This discrepancy may be explained by the fact that while sleep disturbances and circadian rhythm alterations can affect neuronal health, they may not necessarily lead to neuronal apoptosis. At the cellular level, NFL, primarily localized in axons, is released following neuronal apoptosis or axonal-specific damage [45]. Thus, sleep disturbances, NFL levels, and cognitive decline may be closely interrelated. A recent study aligned with our findings, demonstrating that the association between a composite sleep index derived from the Pittsburgh Sleep Quality Index and cognitive decline was partially mediated by plasma NFL [46]. Both our study and this research highlight the critical mediating role of NFL in the sleep-cognition relationship. However, while the previous study examined multiple sleep disturbances—including sleep quality, sleep disturbances, rapid eye movement sleep behavior disturbances, and daytime sleepiness—our study specifically focused on the frequency, severity, and total composite score of sleep/nighttime behavioral disturbances.
The etiology of cognitive decline in individuals with poor sleep is complex and may involve multiple pathological mechanisms (Fig. 5). One possibility is that poor sleep quality contributes to increased amyloid-β plaque deposition. In healthy adults, the burden of amyloid β-protein (Aβ) can be mitigated through clearance of the neurotoxic protein. Many studies have suggested that clearance of waste products, such as Aβ, is enhanced during normal sleep [47, 48]. Some studies reported that deposition of Aβ is greater if sleep is disrupted [23, 48,49,50,51]. Notably, this current evidence is based on acute sleep deprivation, whereas the effect of chronic sleep disturbance on Aβ formation is still under investigation [49, 52]. A second potential mechanism is that sleep plays a protective role against oxidative stress, while sleep deprivation exacerbates oxidative damage. Accumulated free radicals can cause mitochondrial dysfunction, ultimately leading to ATP depletion and neuronal death [53]. We speculate that a third possibility is that chronic insufficient sleep activates the inflammatory pathways, which is important in AD development. many studies showed that sleep duration and sleep disturbances are associated with dysregulation of inflammatory markers and immune cell counts [13, 54, 55]. The increased brain inflammation leads to the deposition of AD pathology and damage or death of neurons [56]. A foundational animal study on sleep disturbances demonstrated that poor sleep quality activates hippocampal microglia, inducing the release of pro-inflammatory cytokines such as IL-6, which trigger neuroinflammation. However, these findings have yet to be confirmed in human studies [57]. Our findings fill a critical gap by demonstrating that neuroinflammation, as indicated by NFL, mediates the relationship between sleep disturbances and cognitive decline in a human population. In summary, our findings showed sleep quality was associated with plasma NFL level, cognitive impairment, and rapid cognitive decline. These novel findings provide insight into the mechanisms linking sleep quality to cognitive impairment and AD development and could inform priorities for preventing cognitive impairment and AD by alleviating neuro-injury. Future studies should validate our results in diverse populations.
Our study had two key strengths. Firstly, we were the first to demonstrate a significant association between sleep quality and cognitive impairment in both cross-sectional and longitudinal analyses. Secondly, we revealed, for the first time, that plasma NFL levels mediated the relationship between sleep quality and cognitive performance, offering new insights into potential mechanisms. However, it also had several limitations. Firstly, sleep characteristics were assessed based on self-reported information rather than objective measurements, which could introduce random bias. Secondly, the NPI questionnaire used was not specifically designed to assess sleep quality, potentially limiting the accuracy of our sleep-related findings. Thirdly, This study included participants from the ADNI cohort, which has a higher average education level. This population is typically associated with higher socioeconomic status, which may contribute to greater cognitive reserve, enabling individuals to better cope with cognitive decline or neurodegenerative diseases. The high education level in this study could lead to participants exhibiting better cognitive function or slower cognitive decline, potentially attenuating the significance of the observed associations between sleep disturbances, cognitive decline, and plasma NFL. Therefore, the findings may not be fully applicable to populations with lower education levels or socioeconomic backgrounds. Further validation of these results in populations with varying levels of education is warranted. Fouthly, this study investigates the impact of sleep on cognition but does not exclude the possibility of a bidirectional relationship between them. Future research should employ methods such as Mendelian randomization to further validate this bidirectional association.
In conclusion, our findings indicate that poor baseline sleep quality is linked to cognitive impairment and accelerated cognitive decline. This association may be partly mediated by neuro-injury, as indicated by plasma NFL levels. Future studies incorporating objective measures of sleep quality are necessary to determine the correlation between sleep quality and neuronal damage assessed through plasma NFL levels.
Data availability
Data used in the presented study were originally from the online repository of Alzheimer’s Disease Neuroimaging Initiative (ADNI) (http://adni.loni.usc.edu/). The data generated during processing and analyzing are available from the authors upon request.
Code availability
All scripts used in analyses are available from the authors upon request.
References
GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–97.
Saint Martin M, Sforza E, Barthélémy JC, Roche F, Lefèvre P, Liénard G, et al. Long-lasting active lifestyle and successful cognitive aging in a healthy elderly population: the PROOF cohort. Rev Neurol (Paris). 2017;173:637–44.
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–61.
Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–66.
Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–28.
Hokett E, Arunmozhi A, Campbell J, Verhaeghen P, Duarte A. A systematic review and meta-analysis of individual differences in naturalistic sleep quality and episodic memory performance in young and older adults. Neurosci Biobehav Rev. 2021;127:675–88.
Scullin MK, Gao C, Fillmore P, Roberts RL, Pruett N, Bliwise DL. Rapid eye movement sleep mediates age-related decline in prospective memory consolidation. Sleep. 2019;42:zsz055.
Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19:702–15.
Virta JJ, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36:1533–41.
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–89.
Zhang C, Yang Y, Liu H, Zhang J. Relationship between longer sleep and serum neurofilament light chain in american adults: evidence from the 2013-2014 US national health and nutrition examination survey. BMC Public Health. 2024;24:2717.
Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76:791–9.
Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99:1325–80.
Piber D, Cho JH, Lee O, Lamkin DM, Olmstead R, Irwin MR. Sleep disturbance and activation of cellular and transcriptional mechanisms of inflammation in older adults. Brain Behav Immun. 2022;106:67–75.
Weiner MW, Aisen PS, Jack CR Jr., Jagust WJ, Trojanowski JQ, Shaw L, et al. The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–11.e7.
Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement. 2013;9:e111–e94.
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s disease neuroimaging initiative (ADNI). Clinical characterization. Neurology. 2010;74:201–9.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease. Report of the NINCDS‐ADRDA Work Group* under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–44.
Cummings J. The neuropsychiatric inventory: development and applications. J Geriatr Psychiatry Neurol. 2020;33:73–84.
Rohrer JD, Woollacott IOC, Dick KM, Brotherhood E, Gordon E, Fellows A, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329–36.
Lucey BP, Wisch J, Boerwinkle AH, Landsness EC, Toedebusch CD, McLeland JS, et al. Sleep and longitudinal cognitive performance in preclinical and early symptomatic Alzheimer’s disease. Brain. 2021;144:2852–62.
Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS). Int J Geriatr Psychiatry. 2002;17:73–7.
Hossain MF, Wang N, Chen R, Li S, Roy J, Uddin MG, et al. Exploring the multifunctional role of melatonin in regulating autophagy and sleep to mitigate Alzheimer’s disease neuropathology. Ageing Res Rev. 2021;67:101304.
Chen PL, Lee WJ, Sun WZ, Oyang YJ, Fuh JL. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PLoS One. 2012;7:e49113.
Exalto LG, Hendriksen HMA, Barkhof F, van den Bosch KA, Ebenau JL, van Leeuwenstijn-Koopman M, et al. Subjective cognitive decline and self-reported sleep problems: the SCIENCe project. Alzheimers Dement (Amst). 2022;14:e12287.
Elder GJ, Lazar AS, Alfonso-Miller P, Taylor JP Sleep disturbances in Lewy body dementia: a systematic review. Int J Geriatr Psychiatry. 2022;37.
Tadokoro K, Ohta Y, Hishikawa N, Nomura E, Wakutani Y, Takao Y, et al. Discrepancy of subjective and objective sleep problems in Alzheimer’s disease and mild cognitive impairment detected by a home-based sleep analysis. J Clin Neurosci. 2020;74:76–80.
Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, Schernhammer ES. Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc. 2014;62:1073–81.
Huang SY, Li YZ, Zhang YR, Huang YY, Wu BS, Zhang W, et al. Sleep, physical activity, sedentary behavior, and risk of incident dementia: a prospective cohort study of 431,924 UK Biobank participants. Mol Psychiatry. 2022;27:4343–54.
Liu X, Ma Y, Ouyang R, Zeng Z, Zhan Z, Lu H, et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J Neuroinflammation. 2020;17:229.
Townsend LTJ, Anderson KN, Boeve BF, McKeith I, Taylor JP. Sleep disorders in Lewy body dementia: mechanisms, clinical relevance, and unanswered questions. Alzheimers Dement. 2023;19:5264–83.
Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9.
Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–32.
Lutsey PL, Bengtson LG, Punjabi NM, Shahar E, Mosley TH, Gottesman RF, et al. Obstructive sleep apnea and 15-year cognitive decline: the atherosclerosis risk in communities (ARIC) study. Sleep. 2016;39:309–16.
Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102–11.
Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement. 2015;11:1090–7.
Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93.
Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9.
Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500.
Zhang P, Tan CW, Chen GH, Ge YJ, Xu J, Xia L, et al. Patients with chronic insomnia disorder have increased serum levels of neurofilaments, neuron-specific enolase and S100B: does organic brain damage exist? Sleep Med. 2018;48:163–71.
Huang L, Huang Q, Xie F, Guo Q. Neuropsychiatric symptoms in Alzheimer’s continuum and their association with plasma biomarkers. J Affect Disord. 2024;348:200–6.
Benedict C, Cedernaes J, Giedraitis V, Nilsson EK, Hogenkamp PS, Vagesjo E, et al. Acute sleep deprivation increases serum levels of neuron-specific enolase (NSE) and S100 calcium binding protein B (S-100B) in healthy young men. Sleep. 2014;37:195–8.
Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9:201–10.
Lysen TS, Ikram MA, Ghanbari M, Luik AI. Sleep, 24-h activity rhythms, and plasma markers of neurodegenerative disease. Sci Rep. 2020;10:20691.
Farley MM, Watkins TA. Intrinsic neuronal stress response pathways in injury and disease. Annu Rev Pathol. 2018;13:93–116.
Yu X, Zhou X, He Z, He B, Wan K, Wei M, et al. Sleep and APOE-epsilon4 have a synergistic effect on plasma biomarkers and longitudinal cognitive decline in older adults. CNS Neurosci Ther. 2024;30:e14558.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science (New York, NY). 2013;342:373–7.
Shokri-Kojori E, Wang G-J, Wiers CE, Demiral SB, Guo M, Kim SW, et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA. 2018;115:4483–8.
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science (New York, NY). 2009;326:1005–7.
Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra22.
Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–11.
Jyoti A, Plano A, Riedel G, Platt B. Progressive age-related changes in sleep and EEG profiles in the PLB1Triple mouse model of Alzheimer’s disease. Neurobiol Aging. 2015;36:2768–84.
Chetsawang B, Govitrapong P, Ebadi M. The neuroprotective effect of melatonin against the induction of c-Jun phosphorylation by 6-hydroxydopamine on SK-N-SH cells. Neuroscience letters. 2004;371:205–8.
Targa A, Dakterzada F, Benítez I, López R, Pujol M, Dalmases M, et al. Decrease in sleep depth is associated with higher cerebrospinal fluid neurofilament light levels in patients with Alzheimer’s disease. Sleep. 2021;44:zsaa147.
Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52.
Suárez-Calvet M, Araque Caballero M, Kleinberger G, Bateman RJ, Fagan AM, Morris JC, et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med. 2016;8:369ra178.
Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–55.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hofmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfzer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding
This study was supported by grants from the National Natural Science Foundation of China (32300824).
Author information
Authors and Affiliations
Contributions
ZTW conceptualized the study, interpreted the data, and revised the manuscript. HHG drafted the manuscript and analyzed the data. DXL, QZ, YF, LYH, and ZHS analyzed and interpreted the data, revised the manuscript, and prepared all the figures. LT interpreted the data and revised the manuscript. All authors contributed to the writing and revisions of the paper and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Partners Healthcare Institutional Review Board (IRB), and written informed consent was obtained from all participants prior to initiation of any study procedures in accordance with IRB guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, HH., Liang, DX., Zhang, Q. et al. Associations between sleep quality, plasma neurofilament light, and cognition in older adults without dementia. Transl Psychiatry 15, 169 (2025). https://doi.org/10.1038/s41398-025-03389-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03389-1