Abstract
Major depressive disorder (MDD) ranks among the leading causes of disability worldwide. An additional burden arises from treatment-resistance, defined by a lack of response to two or more adequate pharmacotherapeutic treatment trials. Unlike in MDD, where the serotonin 1A receptor subtype (5-HT1A) has commonly been used to study pathophysiological alterations, treatment-resistant depression (TRD) subjects represent a less investigated cohort. In this cross-sectional study, 5-HT1A receptor binding was assessed in 33 subjects with TRD with stable medication and 44 healthy control (HC) subjects. Positron emission tomography scans with the radioligand [carbonyl-11C]WAY-100635 were acquired and 5-HT1A receptor nondisplaceable binding potential (BPND) was quantified using the multilinear reference tissue model 2. Regional BPND in amygdala, anterior cingulate cortex, hippocampus, insula, orbitofrontal cortex, dorsal raphe nucleus and median raphe nucleus was assessed using a multivariate analysis of covariance (MANCOVA). The MANCOVA showed a significant effect of group (F = 3.349, p < 0.05) and sex (F = 2.428, p < 0.05). The subsequent pairwise comparison revealed a lower BPND by 17.45% in the TRD group in the dorsal raphe nucleus (mean difference ± SE = −0.59 ± 0.24, p < 0.05) and by 18.39% in the median raphe nucleus (mean difference ± SE = −0.71 ± 0.30, p < 0.05). Our results extend previously reported alterations of 5-HT1A receptor distribution in non-resistant depression to TRD. Ultimately, this knowledge may contribute to clarifying the role of serotonin and help to address the urgent issue of treatment resistance in depression.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) is one of the leading causes of disability worldwide [1]. A particular challenge arises from depressive episodes that show insufficient response to first-line treatment. The lack of response to two or more adequate psychopharmacological treatment trials is commonly referred to as treatment-resistant depression (TRD). As approved treatment options appear to be ineffective in TRD, one might also expect to find differences on a pharmacological level. With regard to the considerable economic and personal consequences of treatment-resistance, understanding the biological characteristics of this form of depression is of particular relevance.
Current models for the pathophysiology of depression consider various biopsychosocial factors [2], including alterations within the serotonergic system [3]. The serotonin 1A (5-HT1A) receptor is one of the most abundant serotonin receptors in the human brain and, thus, among the most researched subtypes. In the past, pathophysiological states and changes to its distribution in the cortex were connected to different psychiatric disorders, including MDD [4, 5]. Positron emission tomography (PET) has become a valuable modality for investigating pathophysiological molecular processes in psychiatric disorders in vivo. Previous studies have shown alterations of 5-HT1A receptor binding potential in MDD patients across various brain regions, including the raphe nuclei [5, 6]. However, there have been inconsistencies regarding the direction of these alterations [7]. On the one hand, a reduction in 5-HT1A receptor availability across different limbic and cortical regions, mesiotemporal cortex and raphe nuclei was demonstrated in various cohorts of MDD patients (including in non-remitters) [6, 8,9,10]. However, on the other hand, convincing publications report increases in cortical and subcortical regions when comparing MDD patients with healthy controls [7, 11]. These discrepancies were reconciled when replication studies concluded that heterogeneous outcome measures and quantification methods were the most plausible origin of the divergent findings [11, 12]. Still, care needs to be taken when comparing and interpreting these results.
Despite the interest in various different MDD cohorts, only a few studies have already described treatment effects on 5-HT1A receptor binding potential in treatment-resistant depression. Previous work from our group suggests that electroconvulsive therapy (ECT) leads to decreased binding in 5-HT1A receptor-rich regions of the amygdala, anterior cingulate cortex, hippocampus, orbitofrontal cortex and insula [13]. Moreover, in a recent study, we report that increased 5-HT1A receptor availability in the dorsolateral prefrontal cortex, induced by transcranial magnetic stimulation (TMS), may lead to a decrease in depression severity in patients with TRD [14]. However, a comparison of 5-HT1A receptor binding between healthy individuals and patients with TRD prior to ECT or TMS has not been published yet.
In this work, we assess differences in 5-HT1A receptor binding potential between treatment-resistant depressive patients at stable medication and healthy control subjects. In particular, we aim to investigate previously implicated high-binding regions (including the anterior cingulate cortex, amygdala, hippocampus, insula, orbitofrontal cortex and dorsal and median raphe nuclei) using in vivo positron emission tomography with the high-affinity radioligand [carbonyl-11C]WAY-100635. In line with previous work on major depressive disorder and associated changes to the serotonergic system, we hypothesize that there are also alterations in serotonin 1A receptor binding potential in patients with TRD.
Materials and methods
Subjects and study design
The dataset represents a collection of published studies alongside previously unpublished data. The data of healthy control subjects (HC) were taken from Lanzenberger et al. [15] and Baldinger et al. [16] (registered at the International Standard Randomized Controlled Trial Number Register as ISRCTN30885829). The treatment-resistant depression group data was adopted from Lanzenberger et al. [13], Murgaš et al. [14] and complemented by an additional set of unpublished data collected as a part of the latter study (registered at ClinicalTrials.gov as NCT02810717). For details on demographic information, see Table 1 in the results section and Supplementary Table 1.
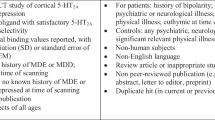
Routine medical examinations, including laboratory measurements, general physical and neurological status and an electrocardiogram, were performed to screen subjects for physical abnormalities. Moreover, clinical interviews by experienced psychiatrists ensured the mental health of the HC. Each TRD subject was carefully screened by a trained psychiatrist using SCID IV (Structural Clinical Interview for DSM IV Diagnosis) and included only if they had already undergone at least two adequate treatment trials with antidepressants and were currently on antidepressant medication. The TRD subjects from Murgaš et al. [14], as well as the newly included subjects, fulfilled the criteria for a single or recurrent major depressive episode having a score ≥ 18 on the 17-item Hamilton Rating Scale for Depression (HAMD) at the screening visit. Lanzenberger et al. [13] included TRD subjects with HAMD ≥ 23 at the screening visit. Subjects with current or past symptoms of mania, schizophrenia or schizoaffective disorder were not included in the studies. TRD subjects currently receiving medication targeting the 5-HT1A directly, in particular aripiprazol, amitriptyline, buspirone, chlorpromazine, clozapine, nebivolol, nefazodone, pindolol, propranolol, quetiapine (>100 mg), risperidone, trazodone, triptans and ziprasidone, were excluded. Additionally, TRD subjects receiving mirtazapine from clinical trial NCT02810717 were not included. Additionally, the healthy volunteers in Baldinger et al. [16] did not exceed a daily consumption of the equivalent quantity of 20 g of alcohol or 10 cigarettes, while Lanzenberger et al. [15] included only male subjects. Only subjects with the available medication history were included in this study. Solely baseline PET measurements of the TRD subjects with no additional challenge or treatment were included. Subjects gave written informed consent at the screening visit. All procedures were performed according to the Declaration of Helsinki. All studies were approved by the Ethics Committee at the Medical University of Vienna (318/2002; 475/2011; 1761/2015).
Neuroimaging
Imaging procedures for HC and TRD groups were similar, although details may vary. For each subject, a 90-min PET scan was acquired using a GE Advance PET scanner (General Electric Medical Systems, Milwaukee, Wisconsin) at the Department of Biomedical Imaging and Image-guided Therapy, Division of Nuclear Medicine, Medical University of Vienna, Austria. Acquisition in 3-D mode started with bolus infusion of the radioligand [carbonyl-11C]WAY-100635 with a dose of 4.6–5.4 MBq/kg. The radioligand [carbonyl-11C]WAY-100635 was prepared at the cyclotron unit of the PET Centre according to the previously described method [17]. A 5-min transmission scan in 2-dimensional mode with retractable 68Ge sources was used for attenuation correction. Images were reconstructed as a 128 × 128 matrix (35 slices) utilizing an iterative filtered back-projection algorithm with a spatial resolution of 4.36 mm full-width at a half-maximum 1 cm next to the centre of the field of view. Here, the number and the length of frames in the reconstructed dynamic PET images vary across the studies: Lanzenberger et al. [15] used 30 frames (15 × 60 s and 15 × 300 s), Baldinger et al. [16] used 50 frames (12 × 5 s, 6 × 10 s, 3 × 20 s, 6 × 30 s, 4 × 60 s, 5 × 120 s and 14 × 300 s). TRD subject groups from both studies [13, 14] were reconstructed to 51 frames (12 × 5 s, 6 × 10 s, 3 × 20 s, 6 × 30 s, 9 × 60 s and 15 × 300 s).
The following section refers to data acquisition and processing of unpublished baseline measurements of TRD subjects collected as a part of the study presented in Murgaš et al. [14]. For more details on the remaining data sets, please refer to the respective publications [13, 15, 16]. In addition to the PET scan, a structural T1-weighted magnetic resonance (MR) image was acquired for each subject. Subjects with TRD were scanned at a 3 T PRISMA MR Scanner (Siemens Medical, Erlangen, Germany) using the magnetization-prepared rapid gradient-echo sequence (TE/TR = 4.21/3000 ms, voxel size 1 × 1 × 1.1 mm3).
Data processing and quantification
Each PET scan was corrected for tissue attenuation and scatter. Image pre-processing was performed using SPM12 (The Wellcome Centre for Neuroimaging, www.fil.ion.ucl.ac.uk) and MATLAB R2018b (The Mathworks Inc., Natick, MA, USA). Correction for head motion was applied, followed by the co-registration of dynamic PET images to the T1-weighted structural MR image. The structural image was then normalized to the Montreal Neurological Institute (MNI) space, generating a transformation matrix that was applied to the co-registered dynamic PET images as well.
Time activity curves (TACs) were extracted for selected regions of interest (ROIs) as described below and cerebellar white matter (CWM). The selected regions of interest have a high abundance of 5-HT1A receptors and previously exhibited changes in 5-HT1A receptor binding potential in TRD subjects suffering from major depressive disorder [13, 18]. These regions are the amygdala (AMY), anterior cingulate cortex (ACC), hippocampus (HIP), orbitofrontal cortex (OFC), insula (INS), dorsal raphe nucleus (DRN) and median raphe nucleus (MRN). Region-wise nondisplaceable binding potential (BPND) was quantified with the multilinear reference tissue model 2 (MRTM2) with an individually fixed clearance rate of radioligand from reference tissue (k2’) [19]. Multilinear reference tissue model (MRTM) was utilized to estimate k2’ with insula as a receptor-rich region. In this paper, binding potential refers to BPND as it is described in the consensus on nomenclature [20]. Based on previous research [21], CWM was selected as the reference region, in contrast to earlier studies that used cerebellar gray matter [12]. The validity of CWM as a suitable reference region for [carbonyl-11C]WAY-100635 was also confirmed in a blocking experiment [11] as well as a post-mortem autoradiography study [22] showing the lowest 5-HT1A receptor concentration in the cerebellar white matter. All quantification steps were done in PMOD, version 4.4 (PMOD Technologies LLC, https://www.pmod.com/web/). TACs were extracted using the Harvard-Oxford atlas as provided with FSL for AMY, ACC, HIP, OFC and INS. Regional values in MRN and DRN were extracted by manually placing a volume of interest for each subject around the voxel with local peak intensity in the summed normalized dynamic PET image. The volumes of interest for MRN and DRN were defined as a sphere with a radius of 3 mm comprising 14 voxels [23]. An in-house atlas [24] was used for CWM.
Statistical analysis
Data harmonization was applied to correct possible bias introduced by data originating from different studies (e.g., different frame lengths). Here, we used the Matlab implementation of the ComBat toolbox [25,26,27] with age and sex as covariates for the healthy control group and using age, sex and HAMD for the TRD group, respectively. ComBat builds upon an empirical Bayes framework to reduce technical variability while at the same time preserving biological variability. This harmonization approach was shown to perform well with small sample sizes and unbalanced groups with regard to the biological covariates. Its design does not make any assumption on the nature of the imaging parameter of interest and is applicable to ROI-based as well as voxel-wise analyses. Before further analyses, the dataset was checked for univariate as well as multivariate outliers. Harmonized BPND values were assessed for univariate outliers for each combination of sex, group and region. For identification of multivariate outliers Mahalanobis distance was calculated for each subject, where one observation consists of the seven regional BPND values. Distances exceeding a critical χ2 value were excluded. To investigate the possible association between 5-HT1A receptor binding potential and depression symptom severity, represented by HAMD scores, we used a multivariate analysis of covariance (MANCOVA). Here, only the TRD group was included, with the HAMD score taken close to the PET measurement and with age as an independent covariate and sex as a fixed factor. Next, the differences in 5-HT1A receptor distribution between healthy control and TRD subjects were investigated. We utilized MANCOVA with the regional BPND values as dependent variables, group and sex as fixed factors, age as a covariate and interaction between group and sex. The exploratory post-hoc (not corrected for multiple comparisons) analyses were performed using pairwise comparison between group BPND corrected for age and sex or post-hoc Pearson correlation analysis between the significant covariate and BPND corrected for age and sex. Estimates of effect size are reported as partial eta squared (η2). Results with p-value < 0.05 were considered significant. Unless otherwise stated, all statistical analyses were performed in SPSS version 26 for Windows (SPSS Inc., Chicago, Illinois, USA; www.spss.com).
Results
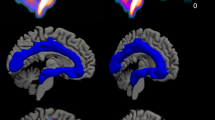
Data from 80 subjects were initially available for the comparison of serotonin 1A receptor distribution in selected regions. One healthy control subject was identified as a multivariate outlier based on Mahalanobis distance and one healthy control subject was identified as a univariate outlier by deviating more than three standard deviations from the mean. Both were excluded from all subsequent analyses. Ultimately, 44 HC subjects (mean age ± SD = 25.2 ± 3.5; 15 female) and 33 subjects with TRD (mean age ± SD = 40.5 ± 11.4; 22 female) were analyzed for this study (see Table 1). The BPND representing 5-HT1A receptor distribution in both groups is shown in Fig. 1, complemented with estimated marginal means in Table 2 and BPND corrected for age and sex in Fig. 2.
The tri-planar visualization (MNI coordinates x = 0 mm, y = 0 mm, z = 12 mm) of median serotonin 1A receptor distribution measured by PET with [carbonyl-11C]WAY-100635 radioligand. The comparison of nondisplaceable binding potential (BPND) in the healthy subjects group (top row) and Patients with TRD group (bottom row) shows the highest difference in the regions of raphe nuclei (dorsal and median).
The boxplot comparing 5-HT1A BPND values between treatment-resistant depressive patients (PAT) and healthy control (HC) group regional An asterisk indicates significant differences between groups (p < 0.05). Regional BPND was corrected for sex and age. Middle lines represent median values. Values 1.5 times the inter-quartile range above/below the 75th/25th percentile are drawn as separate data points (outliers). Anterior cingulate cortex (ACC), amygdala (AMY), hippocampus (HIP), insula (INS), orbitofrontal cortex (OFC), dorsal raphe nucleus (DRN) and median raphe nucleus (MRN).
The analysis of the effect of symptom severity in the TRD subject group showed a main effect of HAMD score on binding (F = 2.557, p < 0.05, η2 = 0.438) and sex (F = 2.830, p < 0.05, η2 = 0.463). The effect of age (F = 1.807, p = 0.13) was not significant. However, region-wise post-hoc correlation analysis between HAMD and BPND corrected for the sex and age did not show significant correlations (Supplementary Fig. 1) between 5-HT1A BPND and HAMD in any investigated region. Similarly, the post-hoc pairwise comparison of male and female TRD subjects did not show significant differences in any particular region.
Using MANCOVA, with regional 5-HT1A receptor BPND values as dependent variables to compare the TRD subjects and control group, showed a significant difference in receptor binding between the TRD group and the control group (F = 4.349, p < 0.05, η2 = 0.316, Fig. 2). Moreover, the effect of sex was significant as well (F = 2.428, p < 0.05, η2 = 0.205), while no effects of age (F = 1.776, p = 0.11) or interaction between group and sex (F = 1.440, p = 0.21) were unveiled. Exploratory tests of between-subject effects revealed a significant difference of BPND in the dorsal (F = 5.898, p < 0.05, η2 = 0.076) and median raphe nucleus (F = 4.508, p = < 0.05, η2 = 0.073) between TRD subjects and the control group but not in the remaining regions of interest. The estimated marginal means showed lower BPND in the TRD group (mean ± SD, DRN: 2.78 ± 0.94, MRN: 3.13 ± 1.15) than in the HC group (mean ± SD, DRN: 3.36 ± 0.91, MRN: 3.83 ± 1.11) in the raphe nuclei (see Table 2). In contrast, sex did not affect BPND significantly in any specific region (see Supplementary Table 2).
Discussion
In this study, we investigated the differences in the 5-HT1A receptor nondisplaceable binding potential between medicated subjects with TRD and healthy controls. A decrease in the presynaptic 5-HT1A BPND in the dorsal and median raphe nuclei were found between TRD and control subjects. In addition, we unveiled an association between symptom severity assessed by HAMD and 5-HT1A BPND in the TRD subject’s group. However, post-hoc analyses showed no significant region-wise effects of sex, age or symptom severity on receptor binding.
Post-hoc analysis of the difference in 5-HT1A receptor binding between TRD and HC subjects showed a significant difference in some of the investigated regions. While both the dorsal and median raphe nuclei showed significantly lower BPND, other regions exhibited no alterations to the binding. Similar to our results, a meta-analysis [6] has shown a significant reduction in 5-HT1A receptor availability in the raphe nuclei. Studies [9, 28] using the whole cerebellum as a reference region reported these changes in MDD cohorts that were previously exposed to medication (yet medication free at least two weeks before the study). On the other hand, investigating discrepancies in BPND between antidepressant-naïve MDD and HC in the raphe nuclei using CWM as a reference region showed no changes at all [29]. Similarly, no statistically significant differences between antidepressant-naïve MDD, antidepressant-exposed MDD and HC were reported [30]. In line with our results, a post-mortem study suggested reduced 5-HT1A binding capacity, representing a decrease in the number of receptors in DRN and MRN of depressed suicide victims when compared to healthy controls [31]. The lower 5-HT1A receptor abundance in the raphe nuclei was suggested to be a result of the homeostatic response of the serotonin system [32]. In this context, the observed changes in raphe 5-HT1A receptor BPND might indicate an imbalance in the serotonergic system. More specifically, these alterations lead to a decreased feedback inhibition mediated by the inhibitory 5-HT1A autoreceptors in the raphe nuclei. The proposed mechanisms for reduced receptor binding are lower baseline expression, cell loss in the raphe nuclei or decreases in arborization and neuron size [8, 33]. Furthermore, concerning differences in localization and function of 5-HT1A receptors, our results may indicate an imbalance between the raphe nuclei and the remaining ROIs. While inhibitory presynaptic 5-HT1A autoreceptors with a regulatory role [23] are found in the raphe nuclei [8, 34], the other regions are rich in postsynaptic 5-HT1A heteroreceptors [35]. However, previous research demonstrated a positive association between raphe binding and heteroreceptor binding [36].
Although the current understanding of the pathophysiology of depression certainly goes beyond serotonergic mechanisms alone [37, 38], antidepressant treatments targeting the serotonergic system have demonstrated clinical efficacy over decades [39]. With the raphe nuclei being a major source of serotonergic neurons, its pathophysiology was previously linked with depressive disorders [40, 41] but not specifically with TRD. Our results, therefore, substantiate the notion that perturbations in serotonergic neuromodulation indeed play a role in depression [42]. Specifically, regarding the question of whether TRD can be seen as a discrete biological entity from non-resistant depression [43], our findings point towards a similarity of both, at least regarding the lower 5-HT1A autoreceptor BPND in the raphe nuclei [6]. However, a direct comparison between TRD and MDD cannot be readily drawn from our data, as these two groups were not compared directly in this work.
A valid question arising from our data concerns the potential influence of serotonergic medication on the results. In contrast to studies that include drug-naïve or not recently medicated MDD patients, here, we investigate a TRD subject cohort with constant concomitant medication. As per the definition of TRD, all TRD subjects underwent at least two different antidepressant treatments during the current depressive episode [44, 45] and were still under medication affecting the serotonergic system at the time of the PET scan. Although the effects of medication on TRD may theoretically manifest differently from MDD, we assume it to have a similar effect on 5-HT1A receptor binding potential based on the congruent findings between the two conditions reported above and previous comparisons between treatment responders and nonresponders [7]. Moreover, prior research has shown that such treatment might only have region and substance-specific effects [46]. For example, long-term antidepressant treatment was suggested to reduce 5-HT1A receptor density in limbic regions [47], but no significant effect was found in the DRN. Conversely, comparing medicated and drug-naïve MDD patients showed no statistically significant differences in BPND between these two groups, while both showed a lower abundance compared to a healthy control group [28, 48]. Moreover, a comparison of the BPND before and after the treatment with SSRI showed no difference 5-HT1A receptor binding in the MDD subjects group [49]. In addition, in the data from Nash et al. [50], SSRI treatment, albeit in anxiety disorder, did not affect the reduced BPND in the raphe nuclei observed in both unmedicated and medicated participants. Furthermore, Parsey et al. [30] did not find statistically significant alterations in BPND between antidepressant naïve and medicated volunteers after two weeks of washout period. Ultimately, this does not contradict the idea of desensitization of 5-HT1A autoreceptors after sustained SSRI treatment occurring through either internalization [8] or shifts from high- to low-affinity states. This is because 1) [carbonyl-11C]WAY-100635 as a 5-HT1A antagonist binds to high as well as low affinity binding sites while being insensitive to competition from endogenous 5-HT levels [51] and 2) it is still assumed to bind to internalized receptors due to its lipophilic properties [52, 53]. However, this is not to say, that receptor binding is not changed, but potentially not affected to such an extent that would be detectable with the methodology presented in this work. The distinction between outcome measures is especially relevant in light of studies showing alterations in 5-HT1A binding using BPF (ratio of specifically bound radioligand in tissue and free radioligand in plasma) or BPP (ratio of specifically bound radioligand in tissue and total radioligand in plasma) after antidepressant treatment [30, 54]. Hence, even though a possible treatment effect acting on 5-HT1A BPND cannot be ruled out, we are not convinced that the herein-reported alterations in BPND, specifically in MRN and DRN, primarily reflect a medication effect. However, if contrary to our assumption the signal was mainly driven by a drug effect, then our results would indicate a measurable dissociation between a molecular treatment effect on the one hand and the absence of symptom relief on the other.
Besides lower BPND of 5-HT1A receptor in subjects with TRD, we found a significant effect of sex on 5-HT1A receptor BPND. However, we could not show significant differences in any ROI specifically (see Supplementary Table 2). Other neuroimaging studies report lower 5-HT1A receptor binding potential in women than in men. Nevertheless, the results are either borderline significant [55] or lacking significance altogether [30, 56]. In contrast, a post-mortem study [57] reported higher serotonin 1A binding in female suicide victims. These findings again highlight the importance of considering the potential effects of sex in study design and analysis [5, 58].
While sex differences were shown within the combined study cohort of healthy control subjects and subjects with TRD, the effect of depressive symptom severity was only investigated in the TRD subject group. We found an impact of symptom severity on 5-HT1A receptor availability, yet post-hoc analyses did not show a significant association of HAMD in any ROI. The lack of a region-wise correlation might be due to an absence of mild or moderate levels of depression, but it is in line with previous research [6, 29]. Unlike symptom severity, age had no significant effect on BPND. Likewise, a study including a large cohort of 61 healthy male subjects [59] found no significant correlation between age and 5-HT1A receptor BPND. However, investigating the effects of sex and age in healthy volunteers [55] reported a tendency towards an inverse relationship between age and postsynaptic 5-HT1A receptor binding potential in men and a small positive association in women.
Finally, some limitations to the study have to be considered. First, the lack of arterial blood samples or population based input function limited our ability to quantify BPP and BPF, which were found to be a more accurate estimation of 5-HT1A receptor abundance [29, 30]. Similarly to our results, previous work on MDD patients reports changes in raphe nuclei [6, 9, 28]. While publications investigating MDD cohorts using these outcome measures report changes in DRN as well, the direction of the change in 5-HT1A receptor binding depends on the metric [5, 11, 12]. While the alterations to 5-HT1A receptor are evident in most of the studies, the directionality of these changes is not clear. For example, contrary to the findings of reduced BPND in depressed patients, studies using BPF which is not available with the reference tissue modelling, showed increased binding [11, 60]. The contrasting outcomes emphasize the role of careful interpretation of presented results. Moreover, an additional limitation arises from the lack of an other than treatment-resistant MDD cohort in the study, which prevents a direct comparison of TRD and MDD subjects. Second, an imbalance in age and sex between the healthy control group and the TRD group might be a limiting factor. Finally, although TRD subjects were on stable medication, the diversity of the accompanying treatment might affect the final result. Due to the variety of concomitant medications (see Supplementary Table 1), such an effect could not be investigated in the current study. In addition, the potential effect of the heterogeneous medication in the medication group is addressed by the harmonization step.
In conclusion, we show a significantly lower 5-HT1A autoreceptor binding in the dorsal and median raphe nuclei of treatment-resistant depressive subjects. Thus, with regard to the ongoing discussion about the role of serotonin in depression, the presented results support the notion of a serotonergic imbalance and extend previous findings in MDD patients to treatment-resistant depression.
Data availability
Raw data will not be publicly available due to reasons of data protection. Processed data and custom code can be obtained from the corresponding author with a data-sharing agreement, approved by the departments of legal affairs and data clearing of the Medical University of Vienna.
References
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res. 2020;126:134–40.
Marx W, Penninx B, Solmi M, Furukawa TA, Firth J, Carvalho AF, et al. Major depressive disorder. Nat Rev Dis Primers. 2023;9:44.
Handschuh PA, Konadu ME, Spurny-Dworak B, Silberbauer LR, Murgas M, Lanzenberger R Serotonin receptors and antidepressants: neuroimaging findings from preclinical and clinical research. In: Kim Y-K, Amidfar M, (eds). Translational research methods for major depressive disorder. New York, NY: Springer US; 2022. pp. 373–429.
Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, et al. International union of basic and clinical pharmacology. CX. Classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacol Rev. 2021;73:310–520.
Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in major depressive disorder. Eur Neuropsychopharmacol. 2016;26:397–410.
Wang L, Zhou C, Zhu D, Wang X, Fang L, Zhong J, et al. Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry. 2016;16:319.
Miller JM, Hesselgrave N, Ogden RT, Zanderigo F, Oquendo MA, Mann JJ, et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol Psychiatry. 2013;74:760–7.
Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–93.
Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–77.
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–87.
Parsey RV, Ogden RT, Miller JM, Tin A, Hesselgrave N, Goldstein E, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68:170–8.
Hesselgrave N, Parsey RV. Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120004.
Lanzenberger R, Baldinger P, Hahn A, Ungersboeck J, Mitterhauser M, Winkler D, et al. Global decrease of serotonin-1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol Psychiatry. 2013;18:93–100.
Murgaš M, Unterholzner J, Stohrmann P, Philippe C, Godbersen GM, Nics L, et al. Effects of bilateral sequential theta-burst stimulation on 5-HT(1A) receptors in the dorsolateral prefrontal cortex in treatment-resistant depression: a proof-of-concept trial. Transl Psychiatry. 2023;13:33.
Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–9.
Baldinger P, Höflich AS, Mitterhauser M, Hahn A, Rami-Mark C, Spies M, et al. Effects of silexan on the serotonin-1A receptor and microstructure of the human brain: a randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. Int J Neuropsychopharmacol. 2014;18:1–9.
Wadsak W, Mien LK, Ettlinger DE, Eidherr H, Haeusler D, Sindelar KM, et al. 18F fluoroethylations: different strategies for the rapid translation of 11C-methylated radiotracers. Nucl Med Biol. 2007;34:1019–28.
Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42:2039–57.
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–112.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Hirvonen J, Kajander J, Allonen T, Oikonen V, Nagren K, Hietala J. Measurement of serotonin 5-HT1 Areceptor binding using positron emission tomography and [carbonyl-(11)C]WAY-100635-considerations on the validity of cerebellum as a reference region. J Cereb Blood Flow Metab. 2007;27:185–95.
Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–93.
Lanzenberger R, Kranz GS, Haeusler D, Akimova E, Savli M, Hahn A, et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage. 2012;63:874–81.
Savli M, Bauer A, Mitterhauser M, Ding YS, Hahn A, Kroll T, et al. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage. 2012;63:447–59.
Fortin JP, Parker D, Tunc B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics. 2007;8:118–27.
Fortin J-P, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20.
Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80.
Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H. et al. Decreased brainseroton in 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–76.
Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY. et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry.2006;59:106–13.
Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903.
Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–42.
Rajkowska G. Depression: what we can learn from postmortem studies. Neuroscientist. 2003;9:273–84.
Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–54.
Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417.
Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien LK, et al. Escitalopram enhances the association of serotonin-1A autoreceptors to heteroreceptors in anxiety disorders. J Neurosci. 2010;30:14482–9.
Albert PR, Blier P. Does serotonin matter in depression? J Psychiatry Neurosci. 2023;48:E400.
Bartova L, Lanzenberger R, Rujescu D, Kasper S. Reply to: “The serotonin theory of depression: a systematic umbrella review of the evidence” published by Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA in molecular psychiatry. Mol Psychiatry. 2023;28:3153–4.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66.
Michelsen, KA, Prickaerts, J. & Steinbusch, HWM. In Progress in brain research (eds Di Giovann, G., Di Matteo, V. & Esposito, E.) The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease (Elsevier, 2008)
Hornung, J.P. In Handbook of Behavioral Neuroscience (eds Müller C. P. & Jacobs B. L.) CHAPTER 1.3 - The Neuronatomy of the Serotonergic System (Elsevier, 2010).
Jauhar S, Cowen PJ, Browning M. Fifty years on: serotonin and depression. J Psychopharmacol. 2023;37:237–41.
Dodd S, Bauer M, Carvalho AF, Eyre H, Fava M, Kasper S, et al. A clinical approach to treatment resistance in depressed patients: what to do when the usual treatments don’t work well enough? World J Biol Psychiatry. 2021;22:483–94.
McIntyre RS, Alsuwaidan M, Baune BT, Berk M, Demyttenaere K, Goldberg JF, et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. 2023;22:394–412.
Bartova L, Dold M, Kautzky A, Fabbri C, Spies M, Serretti A, et al. Results of the European group for the study of resistant depression (GSRD) — basis for further research and clinical practice. World J Biol Psychiatry. 2019;20:427–48.
Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 2003;72:1665–82.
Spindelegger C, Lanzenberger R, Wadsak W, Mien LK, Stein P, Mitterhauser M, et al. Influence of escitalopram treatment on 5-HT 1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry. 2009;14:1040–50.
Metts AV, Rubin-Falcone H, Ogden RT, Lin X, Wilner DE, Burke AK, et al. Antidepressant medication exposure and 5-HT(1A) autoreceptor binding in major depressive disorder. Synapse. 2019;73:e22089.
Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, et al. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [11C]WAY-100635. Synapse. 2007;61:523–30.
Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–34.
Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, et al. Effect of 5-HT on binding of [11C] WAY 100635 to 5-HT1A receptors in rat brain, assessed using in vivo microdialysis and PET after fenfluramine. Synapse. 2001;41:150–9.
Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51.
Barton AC, Black LE, Sibley DR. Agonist-induced desensitization of D2 dopamine receptors in human Y-79 retinoblastoma cells. Mol Pharmacol. 1991;39:650–8.
Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang Y-Y, Mann JJ, et al. Antidepressant treatment reduces Serotonin-1A autoreceptor binding in major depressive disorder. Biol Psychiatry. 2013;74:26–31.
Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, et al. Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology. 2011;36:2729–40.
Stein P, Savli M, Wadsak W, Mitterhauser M, Fink M, Spindelegger C. et al. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur J Nucl Med Mol Imaging. 2008;35:2159–68.
Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–33.
Kaufman J, Sullivan GM, Yang J, Ogden RT, Miller JM, Oquendo MA, et al. Quantification of the Serotonin 1A receptor using PET: identification of a potential biomarker of major depression in males. Neuropsychopharmacology. 2015;40:1692–9.
Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, et al. A database of [11C]WAY-100635 binding to 5-HT1A receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15:620–32.
Pillai RLI, Zhang M, Yang J, Boldrini M, Mann JJ, Oquendo MA, et al. Will imaging individual raphe nuclei in males with major depressive disorder enhance diagnostic sensitivity and specificity? Depress Anxiety. 2018;35:411–20.
Acknowledgements
This research was funded in whole, or in part, by the Austrian Science Fund (FWF) [grant https://doi.org/10.55776/DOC33, https://doi.org/10.55776/KLI516, https://doi.org/10.55776/KLI1006; PI: R. Lanzenberger], by the Else Kröner-Fresenius-Stiftung (2014_A192, PI: R. Lanzenberger), the Vienna Science and Technology Fund (WWTF) [grant https://doi.org/10.47379/CS18039, Co-PI: R. Lanzenberger] as well as by the Disruptive Innovation - Early Career Seed Money funding programme of the Austrian Academy of Sciences (OeAW) and the Austrian Science Fund (FWF) [DI_2023‐110, PI: G. M. Godbersen]. This research was funded in whole, or in part, by the Austrian Science Fund (FWF) [grant https://doi.org/10.55776/KLI551, PI: S. Kasper]. Christian Milz is a recipient of a DOC Fellowship (27.221) from the Austrian Academy of Sciences at the Department of Psychiatry and Psychotherapy, Medical University of Vienna. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. We want to express our gratitude towards Pia Baldinger-Melich, Gregor Gryglewski, Marius Hienert, Marie Spies, Christoph Kraus, Alexander Kautzky, Arkadiusz Komorowski, Paul Michenthaler and Richard Frey for clinical support and Murray B. Reed, Lucas Rischka and Sebastian Ganger for technical support and all additional staff and students from the Neuroimaging Lab (NIL) involved in the realization of this research. We would like to thank the Department of Biomedical Imaging and Image-guided Therapy, Division of Nuclear Medicine, Medical University of Vienna, especially Cécile Philippe, Chrysoula Vraka and Wolfgang Wadsak, for their support in radiosynthesis, close cooperation and technical support. Moreover, we would like to thank radiotechnologists, Ingrid Leitinger and Harald Ibeschitz, for operating PET.
Author information
Authors and Affiliations
Contributions
Study design: SK, RL, MH. Data acquisition: MM, JU, GMG, GSK, LN. Data analysis: MM, CM, PS. Manuscript preparation: MM, CM, GMG, AH, RL. All authors discussed the implications of the findings and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
In the past three years S. Kasper has received grant/research support from Lundbeck; he has served as a consultant or on advisory boards for Angelini, Biogen, Esai, Janssen, IQVIA, Lundbeck, Mylan, Recordati, Sage and Schwabe; and he has served on speaker bureaus for Abbott, Angelini, Aspen Farmaceutica S.A., Biogen, Janssen, Lundbeck, Recordati, Sage, Sanofi, Schwabe, Servier, Sun Pharma and Vifor. Without any relevance to this work, R. Lanzenberger received investigator-initiated research funding from Siemens Healthcare regarding clinical research using PET/MR and travel grants and/or conference speaker honoraria from Bruker BioSpin, Shire, AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, Heel and Roche Austria GmbH. in the years before 2020. He has been a shareholder of the start-up company BM Health GmbH, Austria since 2019. M. Hacker received consulting fees and/or honoraria from Bayer Healthcare BMS, Eli Lilly, EZAG, GE Healthcare, Ipsen, ITM, Janssen, Roche, Siemens Healthineers. G.S. Kranz declares that he received a conference speaker honorarium from Roche, AOP Orphan and Pfizer. The other authors do not report any conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murgaš, M., Milz, C., Stöhrmann, P. et al. In vivo serotonin 1A receptor distribution in treatment-resistant depression. Transl Psychiatry 15, 186 (2025). https://doi.org/10.1038/s41398-025-03406-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03406-3