Abstract
The impact of exosomes derived from patients with High Altitude Cerebral Edema (HACE) on cognitive function in mice was investigated, along with the underlying mechanisms. Exosomes were extracted from HACE patients and injected into the dentate gyrus (DG) of mice. A series of behavioral tests assessed cognitive abilities. Results indicated that mice injected with HACE patient exosomes exhibited significant declines in exploratory behavior and object recognition, suggesting notable cognitive impairments. Additionally, these exosomes induced oxidative stress responses and abnormal activation of microglia, closely associated with neuronal death. Proteomic analysis revealed that the differentially expressed protein STAMBP, which is closely linked to neurodevelopment, may play a key role. In conclusion, our findings highlight the potential impact of exosomes from HACE patients on cognitive dysfunction in mice, providing new insights into the pathophysiological mechanisms of HACE.
Similar content being viewed by others
Introduction
HACE is a brain condition that occurs when individuals rapidly ascend from low to high altitudes without the ability to adapt to the hypoxic environment. Its primary symptoms include headache, vomiting [1, 2], and gait abnormalities, In severe cases, HACE can also lead to cognitive impairments, such as difficulty concentrating and memory loss [3]. Given the rapid onset of HACE and its potential for serious long-term effects, understanding its mechanisms is crucial for early intervention and improving patient outcomes.
Research indicates that cognitive dysfunction in HACE patients primarily results from pathological changes in brain tissue and functional impairments caused by hypoxia [4]. These changes include cerebral edema, increased blood-brain barrier permeability, and neuronal damage. The pathogenesis of HACE is complex, with vascular edema being one of the most widely accepted mechanisms [5]. In high-altitude hypoxic conditions, damage to endothelial cells disrupts the blood-brain barrier, which in turn leads to vascular edema. Moreover, the cytotoxic edema theory suggests that HACE may result from cell damage caused by cytotoxic factors [6]. This involves direct harm to neurons due to hypoxia and subsequent inflammatory responses. Furthermore, the severity and incidence of HACE may be influenced by physiological processes related to ubiquitination and polymorphisms of key enzymes [7].
Exosomes are extracellular vesicles with diameters ranging from 30–150 nanometers, containing various bioactive components that play a crucial role in intercellular communication. They have been extensively studied for applications in early diagnosis, disease monitoring, and drug delivery [8,9,10,11]. Recently, significant attention has been given to the positive effects of exosomes in treating conditions such as schizophrenia and depression [12, 13].
However, the potential negative impacts of the contents of patient-derived exosomes are often overlooked. Furthermore, research indicates that exosomes can cross the blood-brain barrier, raising the possibility that exosomes from HACE patients may play a role in the pathogenesis of HACE by disrupting oxidative stress responses and activating neuroinflammatory pathways [14, 15].
Based on these findings, we hypothesize that exosomes derived from HACE patients contribute to cognitive dysfunction in mice by altering oxidative stress responses and neuroinflammatory pathways. To test this hypothesis, we aim to: investigate the impact of HACE patient-derived exosomes on cognitive function in mice; and explore the molecular mechanisms underlying exosome-induced cognitive dysfunction, focusing on oxidative stress and neuroinflammation. Understanding the role of exosomes in the occurrence and progression of diseases is essential for developing effective therapeutic approaches.
Materials and methods
Animal preparation
Male C57BL/6 mice, aged 11 weeks and weighing 22 ± 1.5 g, were obtained from the Fang Yuanyuan Breeding Farm in Beijing, China. The mice were kept under controlled environmental conditions, with a temperature of 23 ± 1 °C and relative humidity of 50 ± 1%. They were housed on a 12-h light/dark cycle (lights on from 8 a.m.–8 p.m.) and given free access to a standard diet and water.
Ethics approval and consent to participate
All animal experiments within this investigation adhered strongly to the guidelines outlined by the National Institutes of Health for the Care and Use of Laboratory Animals (NIH Publication No. 80-23), receiving full endorsement from the Animal Care and Use Committee at Minzu University of China.
Participants
Eleven patients diagnosed with HACE by physicians at the General Hospital of Tibet Military Region were selected. An additional eleven healthy individuals, matched for age and gender with the HACE patients, were recruited as healthy controls (Ctr). The main characteristics of all participants are presented in Supplementary Table S1.
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of Minzu University of China and followed the guidelines of the Declaration of Helsinki. The samples had been previously used and published by Quan Tang et al. in another article [16].
Blood sample and exosome collection
In our experiment, blood samples were collected using BD Vacutainer™ Serum Tubes (Thermo Fisher Scientific, BD 367895), which do not contain any anticoagulant, allowing the blood to clot naturally. After collection, the samples were left at room temperature for 60 min to complete the coagulation process, followed by centrifugation at 1300 × g for 10 min to separate the serum. The separated serum was transferred to a new EP tube and immediately stored at −80 °C. Exosomes were extracted from the serum according to the manufacturer’s instructions, using the qEVoriginal 70 nm Gen 2 system (IZON, ICO70-13030), and concentrated with the Vivaspin® 20 Ultrafiltration Unit (Sartorius, 30,000 MWCO PES) [13]. The size distribution of the exosomes was measured through nanoparticle tracking analysis using the NanoSight system (NanoSight, London, UK).
Exosome injection and animal grouping
To evaluate whether exosomes from patients with HACE impair learning, memory, and cognitive function, we administered continuous exosome injections to the bilateral dentate DG of mice. To reduce discomfort during the experiment, a cranial implant device was developed, enabling multiple exosome injections following a single surgical procedure [12]. The injection coordinates for the bilateral DG were: AP ± 2.1, ML + 1.7, DV −2.1. After a five-day recovery period, 1 μL of exosomes or saline was injected into each bilateral brain region every four days until euthanasia [17]. Specifically, for the HACE group, mice received 2.8 × 108 particles of exosomes per injection. In our study, human samples within the same group (HACE or healthy control) were pooled in sets of 2–4 samples to reduce individual variability and ensure group representativeness. In particular, we combined the serum from 2–4 patients and then extracted exosomes. The pooled exosomes were then evenly distributed among the corresponding group of mice, ensuring that the observed effects more accurately reflect the overall group characteristics rather than variability from individual human samples. The mice were randomly assigned to one of three groups, with eleven mice in each group:
-
1.
0.9% NaCl: Control group;
-
2.
HACE: Exosomes from HACE patients (1.4E + 11 particles/mL);
-
3.
Ctr: Exosomes from healthy individuals (1.7E + 11 particles/mL).
Behavioral assessments
Sixteen days after the initial exosome injection, the mice underwent a series of behavioral tests. Exosome injections continued every four days until euthanasia. All assessments were carried out in a quiet environment to minimize external stressors. To ensure accurate behavior readings, the mice were relocated to the testing area at least three hours prior to the assessments, allowing for sufficient acclimatization to the new environment.
Open field test
The open field test (OFT) is a commonly used method for evaluating the locomotor activity and exploratory behavior of mice [18, 19]. The apparatus is divided into 16 equal squares, and mice are initially placed in the center to acclimate for 2 min. This period allows the mice to adjust to their new environment, ensuring natural responses. After acclimation, the following metrics are recorded for 3 min: total distance traveled, time spent in the central zone, and the number of center entries. To maintain the objectivity of the analysis, the test is conducted by an investigator blinded to the treatment groups.
Novel object recognition test
The novel object recognition (NOR) test assesses memory based on the rodents’ natural tendency to explore unfamiliar objects [20, 21]. During the experiment, mice are allowed to explore two identical objects for 10 min. Following a one-hour rest period, one of the objects is replaced with a novel object, and the mice are reintroduced to the arena for an additional 10 min. The exploration times and interaction frequencies with both the new and familiar objects are recorded to evaluate the mice’s recognition memory.
Y-maze test
The Y-maze test measures spatial learning and memory by tracking the exploratory behavior of animals within the maze [22, 23]. Mice are placed in the starting arm of the maze and allowed to explore freely. Metrics such as time spent in each arm and the sequence of arm entries are recorded. The spontaneous alternation percentage, an indicator of working memory, is calculated using the following formula: [(number of alternations)/(total arm entries - 2)] × 100.
Novelty-suppressed feeding
The novelty-suppressed feeding (NSF) test evaluates the motivation and anxiety levels of mice when exposed to a novel environment following a 24-h fasting period [24]. n this test, food is placed on a piece of white filter paper in the center of a 50 × 50 × 45 cm apparatus. Each mouse is individually placed in a corner of the arena and allowed to explore. The latency to begin feeding is recorded, providing insights into the animal’s anxiety and motivation in response to the novel surroundings.
Fear conditioning
The fear conditioning reflex involves two chambers: a freeze monitor box (23 × 23 × 30 cm) within a larger soundproof chamber (30 × 30 × 37 cm). The freeze monitor box is equipped with a metal grid for foot shocks and records the animals’ vertical and horizontal movements. The first day focuses on conditioning training, following a specific protocol: the animals remain still for 60 s, after which they receive 12 stimulations. Each stimulation consists of a 30-s conditional stimulus at 75 dB, followed by a 30-s inter-stimulation interval, and concludes with a 2-s foot shock (30 mA) and 15 s of inactivity. Six hours later, a short-term memory test is conducted, reducing the number of stimulations to six. Each trial during this test includes the conditional stimulus and a 30-s tracking interval, concluding without foot shocks. The long-term memory test occurs on the second day, one day after the short-term test, using the same methodology to evaluate long-term memory quality [25].
Detection of oxidative markers
The animals were first anesthetized with intraperitoneal pentobarbital (100 mg/kg), which was administered using a solution prepared with a 10% pentobarbital sodium (Sigma-Aldrich) solution dissolved in sterile saline. Once the animals were fully anesthetized and unresponsive to external stimuli, we proceeded with the procedure of eyeball blood collection. After blood collection, the intracardiac perfusion was performed with saline to ensure the removal of blood from the circulatory system. No heparin was used in this procedure. Once perfusion was completed, the animals were euthanized, and tissues were collected for further analysis.
Oxidative markers were detected using enzymatic colorimetric tests according to the manufacturer’s instructions. Peripheral serum samples (0.8 mL) were collected from the retroorbital vessels of the mice and centrifuged for 20 min at 4 °C and 4000 rpm to obtain 300 μL of serum [26]. To assess oxidative stress levels, hippocampal tissue samples were also collected. The levels of malondialdehyde (MDA) (A003-1-2), glutathione peroxidase (GSH-Px) (A005-1-2), catalase (CAT) (A007-1-1), nitric oxide (NO) (A012-1-2), superoxide dismutase (SOD) (A001-3-2), corticosterone, and total antioxidant capacity (T-AOC) (A015-2-1) were measured using a kit from the Nanjing Jiancheng Bioengineering Institute.
Immunofluorescence analysis
Mice were first anesthetized with intraperitoneal pentobarbital (100 mg/kg). Once the animals were deeply anesthetized and unresponsive to stimuli, intracardiac perfusion with saline was performed to euthanize the animals and fix tissues. The fixed specimens were dehydrated in sucrose solutions (20% followed by 30%) prepared in phosphate-buffered saline (PBS). Coronal brain sections, 35 μm thick, were then prepared and washed with PBS. To reduce nonspecific binding, the sections were blocked for one hour at room temperature using a solution of 1% bovine serum albumin, 0.3% Triton X-100, and 10% goat serum in PBS, followed by three PBS washes. The sections were incubated overnight at 4 °C with the following primary antibodies: mouse anti-ionized calcium-binding adapter molecule 1 (IBA-1, Cell Signaling Technology, #14082, 1:400) and rabbit anti-microtubule-associated protein 2 (MAP2, Cell Signaling Technology, #14082, 1:400). Afterward, the sections were incubated for an additional two hours at room temperature with fluorophore-conjugated secondary antibodies: goat anti-rabbit IgG (Alexa Fluor® 594 conjugate, Invitrogen, #A11008, 1:1000) and goat anti-mouse IgG (Alexa Fluor® 594 conjugate, Invitrogen, #A21422, 1:1000). Following three final washes, the sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Imaging was conducted using a Leica TCS SP8 confocal microscope (Leica Microsystems, Germany), facilitating detailed observation and documentation of labeled cellular and subcellular structures [27].
Golgi-cox impregnation
Golgi-Cox impregnation, a classic histological technique, was employed to investigate neuronal structures and dendritic trees in the brain [28, 29]. Following euthanasia, brains were submerged in Golgi-Cox solution for a 14-day impregnation phase in darkness. Specimens were then dehydrated in 30% sucrose, and 100 μm coronal sections were prepared using a vibratome. After mounting on gelatin-coated slides and rinsing to remove excess reagents, the sections were dehydrated, cleared in xylene, and sealed with a coverslip. Bright-field microscopy visualized the Golgi-stained sections, revealing detailed images of neuronal and synaptic formations, which enhanced our understanding of neural functionality and pathology.
Olink
Protein concentrations were quantified using a multiplex immunoassay developed by Olink Proteomics (Uppsala, Sweden), based on PEA technology. This assay converts protein concentration measurements into Ct values via qPCR, subsequently reporting concentrations as normalized protein expression (NPX) on a log2 scale through a normalization procedure [30]. OLINK technology was utilized to assess proteomic indicators in hippocampal tissue, with detailed information available in Supplementary Table S2.
Functional enrichment analysis
The Database for Annotation, Visualization, and Integrated Discovery function annotation tool (https://david.ncifcrf.gov) was used for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis on differentially expressed proteins (DEPs) [31]. GO terms and KEGG pathways with Benjamini-corrected p-values < 0.05 and false discovery rates <0.01 were considered significant.
Data processing and analysis
Statistical analysis was conducted using GraphPad Prism (version 5.0). One-way ANOVA was employed to detect group differences, and Tukey’s multiple comparisons test was performed for post hoc analysis (P < 0.05). Results are presented as mean ± SEM to convey central tendency and variability. The quantification of dendritic spines in the article was performed by counting the number of dendritic spines within the same visual field using ImageJ software.
Results
Cognitive dysfunction induced in mice by exosomes from HACE patients
The potential negative impact of exosomes secreted by patients with HACE on cognitive function needs to be evaluated through behavioral testing. To investigate this, we extracted exosomes from these patients and injected them into the DG of mice. Using a specialized device, we administered continuous injections after an initial injury. Following 16 days of exosome infusion, we conducted a series of behavioral tests.
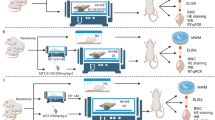
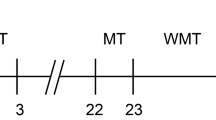
Figure 1A shows the schematic of exosome extraction and injection, while Fig. 1B outlines the timeline for model establishment. Mice injected with exosomes from HACE patients exhibited reduced exploration time in the center area of the OFT (Fig. 1C), indicating impaired exploratory behavior. In contrast, mice injected with exosomes from healthy individuals or with saline demonstrated higher activity levels. Among them, the mice receiving healthy exosomes performed better in exploratory behavior than those injected with saline. In the NOR test, the HACE mice took significantly longer to recognize a new object, clearly demonstrating that exosomes from HACE patients negatively affected their learning, memory, and cognitive functions (Fig. 1D). The Y-maze test, used to assess learning and cognitive abilities, revealed that HACE mice completed fewer successful alternations, further reflecting deficits in cognition and memory (Fig. 1E). In the NSF test, HACE mice took longer to approach food in the center area, further indicating a notable reduction in exploratory ability (Fig. 1F). During the fear conditioning test, HACE mice showed fewer freezing responses to auditory stimuli, while no abnormalities were observed in the other groups (Fig. 1G).
A Experimental Design: Schematic Representation of Exosome Injection. B Flowchart of Animal Experiments. C In the open field test, exosomes from HACE patients reduced the time mice spent exploring the central area. D In the novel object recognition test, HACE group mice spent less time exploring the new object. E In the Y-maze experiment, HACE group mice showed a decrease in the number of entries into different arms. F In the novel environment feeding suppression test, HACE group mice took longer to eat food. G Performance of HACE in Contextual Fear Conditioning Experiments. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 11 per group. *P < 0.05; **P < 0.01; ***P < 0.001. (OFT open field test, NOR novel object recognition, NSF novelty-suppressed feeding test, NFT fear conditioning).
In summary, we identified significant cognitive impairments in HACE mice through various behavioral tests, while no such deficits were observed in mice receiving healthy exosomes. In fact, some cognitive indicators were even better in the healthy exosome group compared to the saline group. This suggests that exosomes from HACE patients may contain specific substances that contribute to cognitive dysfunction. We further conducted physiological assessments on the mice’s brain tissue and blood.
Exosomes from high-altitude cerebral edema patients induce oxidative stress responses in mice
Research has shown that oxidative stress homeostasis imbalance is involved in the pathogenesis of HACE [32]. This imbalance primarily arises from excessive production of reactive oxygen species (ROS) or insufficient antioxidant defenses. These factors contribute to abnormal microglial activation and the disruption of endothelial tight junctions, ultimately compromising the integrity of the neurovascular unit [33]. Such disruption leads to irreversible neuronal death and subsequent damage to the blood-brain barrier, resulting in cerebral edema [34]. To assess whether HACE exosomes induce abnormalities in oxidative capacity and ROS levels, we measured the expression levels of NO, SOD, GSH-Px, MDA, CAT, and T-AOC in the serum and two brain regions—the hippocampus (Hip) and medial prefrontal cortex (mPFC)—of mice [35].
NO is a redox-active molecule that plays a key role in regulating vascular endothelial tone, improving blood flow and oxygen supply, and influencing oxidative stress and inflammatory responses. These functions significantly impact the occurrence and progression of HACE [36]. We measured NO levels in the serum, hippocampus, and prefrontal cortex of the mice. The results showed a marked increase in NO levels in both the serum and hippocampus of mice injected with HACE exosomes (Fig. 2A, G).
A–L Exosomes from HACE patients can induce abnormal expression of oxidative stress-related factors in the blood, hippocampus, and prefrontal cortex of mice. These factors include NO, MDA, GSH, SOD, CAT and T-AOC. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001. (NO nitric oxide, MDA malondialdehyde, GSH glutathione peroxidase, SOD superoxide dismutase, CAT catalase, T-AOC total antioxidant capacity).
SOD is an antioxidant enzyme that catalyzes the conversion of superoxide into hydrogen peroxide, thereby affecting oxidative stress levels. Research has demonstrated significantly elevated SOD expression in the brain tissues of rats exposed to high-altitude cerebral edema [37]. We conducted a detailed examination of SOD levels in mice injected with HACE exosomes, finding abnormal elevations in the hippocampus, prefrontal cortex, and serum of the HACE group (Fig. 2B, H). GSH-Px, an antioxidant that neutralizes excessive ROS and prevents oxidative damage [38, 39], was significantly reduced in the hippocampus of the HACE group mice (Fig. 2C, I).
MDA, a byproduct of lipid peroxidation, is an indirect marker of oxidative stress. Ruzanna A. Shushanyan et al. observed a significant increase in MDA levels in the hippocampus and cortex of rats with HACE [40]. Similarly, we noted a significant rise in MDA levels in the blood, prefrontal cortex, and hippocampal tissues of the HACE group mice (Fig. 2D, J).
CAT primarily decomposes hydrogen peroxide into water and oxygen, protecting cells from oxidative stress. A decrease in CAT levels leads to heightened oxidative stress [41, 42]. In the HACE group, we observed a slight increase in CAT expression (Fig. 2E, K).
T-AOC reflects the overall antioxidant status of various substances and enzymes, serving as a critical indicator of oxidative stress [43]. The T-AOC levels in the hippocampus of the HACE group showed significant changes. Overall, the HACE group displayed active lipid peroxidation and redox imbalance in both serum and brain tissues (Fig. 2F, L).
Exosomes from HACE patients cause abnormal death of mouse hippocampal neurons
Neuroinflammatory responses are closely linked to oxidative stress, and excessive oxidative stress can inhibit neurotransmission and synaptic plasticity, ultimately leading to neuronal damage [44, 45]. To assess whether exosomes from HACE patients cause such damage, we performed Golgi staining on the DG region of the mouse hippocampus. The results showed a significant reduction in dendritic spine density in the neurons of the HACE group (Fig. 3A, B). Furthermore, MAP2 immunofluorescence staining, which labels neurons in the hippocampal DG region, indicated a substantial decrease in the number of neurons in the HACE group (Fig. 3C, D). These findings suggest that exosomes from HACE patients severely impair hippocampal neurons in mice.
A Schematic representation of Golgi staining in the DG region. B Significant reduction in the number of dendritic spines in the DG region of HACE group mice. C Noticeable decrease in the number of MAP2-positive cells in the DG region of HACE group mice. D Schematic representation of MAP2 immunofluorescence in the DG region. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. Immunofluorescence images were analyzed using ImageJ. n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars in A and D = 50 μm. (DAPI 2-(4-amidinophenyl)-6-indoleca rbamidine dihydrochloride, DG dentate gyrus, MAP2 Microtubule-Associated Protein 2).
Exosomes from HACE induce proliferation and activation of microglia in mouse hip and mPFC
Zhao et al. found that the activation of microglia increases inflammation levels and exacerbates brain edema in rats [46]. This abnormal activation releases inflammatory factors that impact surrounding neurons, resulting in inflammatory damage to them [47]. To further investigate the quantity and activation status of microglia in the hippocampus, we labeled them using IBA1 and CD68. The results indicated an increase in the number of microglia in the DG (Fig. 4A), CA1 (Fig. 4C), CA2 (Fig. 4D), CA3 (Fig. 4E), and mPFC (Fig. 4F) regions of the HACE group mice, with a significant rise in the number of activated microglia (Fig. 4B). Additionally, these microglia displayed abnormal characteristics, such as enlarged cell bodies and shortened processes.
A Schematic representation of IBA1 immunofluorescence in the DG region. B Increased number of microglial cells in the hippocampus and prefrontal cortex of HACE group mice. C–F Schematic representations of IBA1 immunofluorescence in the CA1, CA2, CA3, and mPFC regions. All values are presented as the means ± SEMs. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. The normality of the data was assessed using the Shapiro-Wilk test, and F test was used to compare variances between groups. Immunofluorescence images were analyzed using ImageJ. n = 6 per group. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars in A, C, D, E and F = 50 μm. (DAPI 2 - (4 - amidinophenyl) – 6 - indolecarbamidine dihydrochloride, IBA1 ionized calcium-binding adaptor molecule 1, DG dentate gyrus, CA1 cornu ammonis 1 of the hippocampus, CA2 cornu ammonis 2 of the hippocampus, CA3 cornu ammonis 3 of the hippocampus, mPFC medial prefrontal cortex).
Exosomes from HACE patients affect mouse cognitive function by depleting neuronal development proteins
To investigate the mechanisms underlying cognitive dysfunction in HACE, we examined the impact of exosomes from HACE patients on protein expression in the hippocampus of mice. We performed Olink proteomic sequencing on the hippocampal tissues from both HACE and control groups, each consisting of six independent samples, totaling twelve mice. We utilized Uniform Manifold Approximation and Projection (UMAP) for dimensionality reduction, which presented the overall protein group characteristics (Fig. 5A). Next, we quantified the NPX alues of the differentially expressed proteins (Fig. 5B). The volcano plot revealed a total of 25 genes with significant differences in expression (Fig. 5C). Heatmap analysis further demonstrated substantial differences between the HACE and control groups (Fig. 5D). Specifically, 15 proteins were upregulated in the HACE group, including Ribokinase (RBKS), Protein Transport Factor (PRTFDC1), Fc Receptor-Like Protein 6 (FCRL6), C-C Motif Chemokine Ligand 27 (CCL27), Cathepsin S (CTSS), Defensin Beta 4 A (DEFB4A), Interleukin-1 Receptor-Associated Kinase 4 (IRAK4), Tenascin-R (TN-R), Corneodesmosin (CDSN), SPARC-related Modular Calcium-Binding Protein 2 (SMOC2), Ezrin (EZR), Galectin-8 (gal-8), A Disintegrin and Metalloproteinase 15 (ADAM15), and FK506 Binding Protein 7 (FKBP7). Conversely, 10 proteins were downregulated, including STAM Binding Protein (STAMBP), Neurotrophic Receptor Tyrosine Kinase 2 (NTRK2), Axin Protein 1 (AXIN1), Delta/Notch EGF Repeat-Containing (DNER), Ephrin Type-B Receptor 6 (EPHB6), Neutral Endopeptidase (NEP), Fibroblast Growth Factor 5 (FGF-5), Neuronal Cell Adhesion Molecule (Nr-CAM), Spondin 1 (SPOCK1) and S SH2 Domain Containing 1 A (SH2D1A).
A Dimensionality reduction and overall proteomic feature visualization using UMAP for 12 samples. B Quantification of NPX values for differential proteins. C Volcano plot of differentially expressed proteins. D Heatmap of significantly differentially expressed proteins between the HACE and CTR groups. E GO functional clustering analysis of differential proteins. n = 6 per group.
These differentially expressed proteins may collectively contribute to cognitive dysfunction through multi-pathway interactions. In the neuroinflammatory response induced by HACE exosomes, pro-inflammatory factors including CTSS, IRAK4, and CCL27 were significantly upregulated, potentially driving microglial hyperactivation via NF-κB signaling pathway [48,49,50,51]. Concurrently, downregulation of STAMBP and NEP may impair protein clearance mechanisms, exacerbating proteotoxic stress and β-amyloid (Aβ) deposition [52], ultimately triggering neuronal apoptosis, which aligns with our previous observation of reduced MAP2-positive neurons. Regarding neuroplasticity regulation, suppressed Notch/DNER signaling and dysregulated Wnt/AXIN1 pathway may compromise hippocampal neurogenesis [26, 53, 54], while disrupted NTRK2-BDNF signaling and impaired Nr-CAM-mediated cell adhesion could lead to decreased synaptophysin synthesis and dendritic complexity [55]. These alterations resulted in significant exploratory behavior deficits in novel object recognition and open field tests, corroborated by reduced synaptic density in Golgi-stained HACE model mice. Furthermore, aberrant expression of FCRL6 and DEFB4A may induce autoimmune-like damage [56, 57], whereas SMOC2 and EZR overexpression could disrupt blood-brain barrier integrity, promoting vascular leakage and cerebral edema [58, 59]. Metabolic disturbances involving RBKS and PRTFDC1 may further aggravate cerebral energy crisis [60, 61]. These multi-omics findings demonstrate that HACE-associated cognitive impairment essentially stems from synergistic effects of neuroinflammatory storms, synaptic remodeling dysfunction, and proteostasis imbalance.
GO enrichment analysis further elucidated functional pathway dysregulation in hippocampal tissues following HACE exosome intervention. Significant downregulation of interleukin-1 receptor binding activity correlated with low STAMBP expression (a deubiquitinase regulating IL-1R signaling), suggesting compromised anti-inflammatory feedback that exacerbates IL-1β-mediated neural damage [62]. Impaired magnesium ion binding and carbohydrate kinase activities corresponded with dysfunctional RBKS and PRTFDC1, indicating TCA cycle disruption and mitochondrial oxidative phosphorylation inefficiency [63, 64]. Notably, aberrant activation of transmembrane receptor protein kinase and protein tyrosine kinase activities reflected EGFR/VEGFR signaling dysregulation, forming a pro-inflammatory/vascular leakage loop with IRAK4 (IL-1R-associated kinase) and ADAM15 (extracellular matrix protease) [65, 66]. Additionally, upregulated Notch binding and metallopeptidase activities matched expression changes of TN-R (Notch-regulated ECM protein) and CTSS, potentially aggravating blood-brain barrier disruption via Notch-dependent microglial activation [67, 68] (Fig. 5E) .
Discussion
Exosomes derived from patients with HACE significantly impact cognitive function in mice. Experimental results showed that mice injected with exosomes from HACE patients exhibited notable cognitive impairments in various behavioral tests, particularly in the Y-maze, NOR and NSF tests. These findings align with recent research on the role of exosomes in neurodegenerative diseases, where patient-derived exosomes have been shown to contain pathological components affecting neuronal function, such as tau protein and α-synuclein [69].
While previous studies have indicated the crucial role of exosomes in disease progression, the potential negative effects of exosomes from HACE patients are seldom reported. Results suggest that these exosomes may increase oxidative stress in neurons, lead to abnormal activation of microglia, and cause neuronal death by releasing specific bioactive molecules. This supports the view that oxidative stress plays a key role in HACE’s pathological processes. Specifically, elevated levels of ROS were observed in mice, accompanied by decreased antioxidant enzyme activity, consistent with oxidative damage and neuronal dysfunction observed in high-altitude environments [70, 71]. Compared to literature on oxidative stress-induced microglial activation and neuroinflammatory responses, these findings emphasize the potential bridging role of exosomes in this process, deepening understanding of the mechanisms underlying HACE and its cognitive dysfunction [72]. Proteomic analysis results corroborate these findings. The differential protein CCL27, an important chemokine from the C-C motif family [73], promotes the migration of leukocytes, especially T cells, to sites of inflammation by binding to its receptors (e.g., CCR10). Its expression in the brain may be closely related to neuroinflammation and injury [74]. GO analysis revealed that proteins extracted from exosomes were enriched in functions related to “interleukin-1 receptor binding,” closely associated with the pathological mechanisms of HACE. Interleukin-1 (IL-1), a key pro-inflammatory cytokine, participates in neuroinflammation and oxidative stress processes [75]. Previous studies indicate that excessive expression of IL-1 can lead to neuronal damage and dysfunction, potentially exacerbating cerebral edema by promoting microglial activation and inflammatory responses [76, 77]. Therefore, exploring intervention strategies targeting exosomes in inflammation and oxidative stress may provide new insights for HACE treatment.
Olink proteomic detection revealed that exosomes from HACE patients may be associated with altered expression of the STAMBP protein, which is related to neurodevelopment, and this alteration may contribute to cognitive dysfunction in mice. STAMBP is involved in deubiquitination, and mutations in this gene have been reported to reduce the proliferation capacity of neural stem cells (NSCs) [78]. The role of STAMBP in maintaining normal NSC proliferation is crucial; its downregulation may potentially influence brain development and function, which could lead to learning and memory deficits. While these findings suggest that cognitive impairments in HACE could be linked to STAMBP downregulation, additional studies are needed to confirm this association [78].
In addition, proteins related to neurodevelopment, such as Nr-CAM, NTRK2, and DNER, also showed significant differences. Nr-CAM, a cell adhesion molecule, is involved in interactions between nerve cells, and its abnormal expression may lead to changes in neuronal adhesion and signaling, affecting cognitive function and learning abilities [79]. NTRK2, a neurotrophic factor receptor, participates in neuronal growth and synaptic plasticity [80]. DNER plays a crucial role in neuronal development; changes in its expression levels may relate to nerve injury and regeneration processes, thereby influencing cognitive function and the formation of neural networks [81]. The abnormal expression of these proteins provides evidence that exosomes from HACE patients may contribute to disruptions in neurodevelopment, thereby affecting cognitive function.
Despite significant findings, some limitations remain. The water maze is an excellent behavioral tool for assessing learning, memory, and cognitive dysfunction; however, relevant data could not be obtained due to the constraints of the head apparatus used on experimental animals. Additionally, establishing HACE mouse models (such as hypoxia-induced models or chronic high-altitude exposure models) would facilitate more robust comparisons with mice injected with patient exosomes.
In summary, exosomes from HACE patients potentially impact cognitive dysfunction by inducing oxidative stress, promoting neuroinflammation, and disrupting neurodevelopment. These findings provide new insights into the pathological mechanisms of HACE and offer novel strategies for future therapeutic approaches.
Conclusion
The findings of this study demonstrate that exosomes derived from patients with HACE significantly impair cognitive function in mice. The depletion of STAMBP protein was observed in association with disrupted oxidative stress responses, which may contribute to the cognitive dysfunction. Additionally, the activation of neuroinflammatory pathways was noted to further exacerbate neuronal damage and cognitive impairments. These insights provide valuable information for understanding the pathophysiological processes associated with HACE and suggest that targeting exosomal components may offer new therapeutic strategies for managing cognitive dysfunction in affected individuals.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Turner RE, Gatterer H, Falla M, Lawley JS. High-altitude cerebral edema: its own entity or end-stage acute mountain sickness? J Appl Physiol. 2021;131:313–25.
DiPasquale DM, Muza SR, Gunn AM, Li Z, Zhang Q, Harris NS, et al. Evidence for cerebral edema, cerebral perfusion, and intracranial pressure elevations in acute mountain sickness. Brain Behav. 2016;6:e00437.
Ng YS, Chua KS. States of severely altered consciousness: clinical characteristics, medical complications and functional outcome after rehabilitation. NeuroRehabilitation. 2005;20:97–105.
Zila-Velasque JP, Grados-Espinoza P, Goicochea-Romero PA, Tapia-Sequeiros G, Pascual-Aguilar JE, Ruiz-Yaringaño AJ, et al. Mountain sickness in altitude inhabitants of Latin America: a systematic review and meta-analysis. PLoS One. 2024;19:e0305651, https://doi.org/10.1371/journal.pone.0305651.
Li Q, Xu Z, Gong Q, Shen X. Identification and validation of STC1 act as a biomarker for high-altitude diseases and its pan-cancer analysis. Int J Mol Sci. 2024;25:9085, https://doi.org/10.3390/ijms25169085.
Gatterer H, Villafuerte FC, Ulrich S, Bhandari SS, Keyes LE, Burtscher M. Altitude illnesses. Nat reviews Dis Primers. 2024;10:43. https://doi.org/10.1038/s41572-024-00526-w.
Kaneko Y, Suzuki M, Ishihara M, Kitamura M, Bando S, Sagawa T, et al. A case of high altitude cerebral edema with a prolonged motivational deficit. Wilderness Environ Med. 2021;32:88–91. https://doi.org/10.1016/j.wem.2020.10.006.
Fan F, Du Y, Chen L, Chen Y, Zhong Z, Li P, et al. Metabolomic and proteomic identification of serum exosome for hypoxic preconditioning participants. Oxid Med Cell Longev. 2023;2023:5509913, https://doi.org/10.1155/2023/5509913.
Wei Z-X, Xie G-J, Mao X, Zou X-P, Liao Y-J, Liu Q-S, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. 2020;45:1050–8. https://doi.org/10.1038/s41386-020-0622-2.
Fox AS, Duggleby WF, Gelbart WM, Yoon SB. DNA-induced transformation in Drosophila: evidence for transmission without integration. Proc Natl Acad Sci USA. 1970;67:1834–8. https://doi.org/10.1073/pnas.67.4.1834.
Mishra NC, Tatum EL. Non-Mendelian inheritance of DNA-induced inositol independence in Neurospora. Proc Natl Acad Sci USA. 1973;70:3875–9. https://doi.org/10.1073/pnas.70.12.3875.
Zhong XL, Huang Y, Du Y, He LZ, Chen YW, Cheng Y, et al. Unlocking the therapeutic potential of exosomes derived from nasal olfactory mucosal mesenchymal stem cells: restoring synaptic plasticity, neurogenesis, and neuroinflammation in schizophrenia. Schizophrenia Bull. 2024;50:600–14. https://doi.org/10.1093/schbul/sbad172.
Du Y, Dong JH, Chen L, Liu H, Zheng GE, Chen GY, et al. Metabolomic identification of serum exosome-derived biomarkers for bipolar disorder. Oxid Med Cell Longev. 2022;2022:5717445 https://doi.org/10.1155/2022/5717445.
Tao B, Du R, Zhang X, Jia B, Gao Y, Zhao Y, et al. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J Controlled Rel. 2023;363:692–706. https://doi.org/10.1016/j.jconrel.2023.10.007.
Gao P, Yi J, Chen W, Gu J, Miao S, Wang X, et al. Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway. J Nanobiotechnol. 2023;21:452, https://doi.org/10.1186/s12951-023-02110-y.
Tang Q, Fan F, Chen L, Chen Y, Yuan L, Wang L, et al. Identification of blood exosomal metabolomic profiling for high-altitude cerebral edema. Sci Rep. 2024;14:11585, https://doi.org/10.1038/s41598-024-62360-0.
Liu S, Chen L, Guo M, Li Y, Liu Q, Cheng Y. Targeted delivery of engineered RVG-BDNF-Exosomes: a novel neurobiological approach for ameliorating depression and regulating neurogenesis. Research. 2024;7:0402, https://doi.org/10.34133/research.0402.
Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–60. https://doi.org/10.1016/s0149-7634(01)00011-2.
Zhang J, Gao T, Chen G, Liang Y, Nie X, Gu W, et al. Vinegar-processed Schisandra Chinensis enhanced therapeutic effects on colitis-induced depression through tryptophan metabolism. Phytomedicine. 2024;135:156057, https://doi.org/10.1016/j.phymed.2024.156057.
Lee H, Park H, Ryu DY, Jang W-D. Porphyrin-based supramolecular polymers. Chem Soc Rev. 2023;52:1947–74. https://doi.org/10.1039/d2cs01066f.
Karimani F, Taei AA, Kaveh N, Ghahfarokhi MR, Abolghasemi-Dehaghani M-R, Dargahi L. Hippocampal sharp-wave ripples and hippocampal-prefrontal synchrony re gulate memory-enhancing effects of intranasal insulin in an STZ-induce d Alzheimer’s model. Life Sci. 2024;357:123094, https://doi.org/10.1016/j.lfs.2024.123094.
Mohammadpour-Haratbar A, Zare Y, Rhee KY. Electrochemical biosensors based on polymer nanocomposites for detecti ng breast cancer: recent progress and future prospects. Adv Colloid Interface Sci. 2022;309:102795, https://doi.org/10.1016/j.cis.2022.102795.
Liu Q, Yan R, Wang L, Li R, Zhang D, Liao C, et al. Alpha-asarone alleviates cutaneous hyperalgesia by inhibiting hyperexcitability and neurogenic inflammation via TLR4/NF-κB/NLRP3 signaling pathway in a female chronic migraine rat model. Neuropharmacology. 2024;261:110158, https://doi.org/10.1016/j.neuropharm.2024.110158.
Ramaker MJ, Dulawa SC. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry. 2017;22:656–65. https://doi.org/10.1038/mp.2017.36.
Fan F-C, Du Y, Zheng W-H, Loh YP, Cheng Y. Carboxypeptidase E conditional knockout mice exhibit learning and memory deficits and neurodegeneration. Transl Psychiatry. 2023;13:135, https://doi.org/10.1038/s41398-023-02429-y.
Fu Q, Qiu R, Chen L, Chen Y, Qi W, Cheng Y. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl Psychiatry. 2023;13:317, https://doi.org/10.1038/s41398-023-02606-z.
Im K, Mareninov S, Diaz MFP, Yong WH. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299–311. https://doi.org/10.1007/978-1-4939-8935-5_26.
Boros BD, Greathouse KM, Gentry EG, Curtis KA, Birchall EL, Gearing M, et al. Dendritic spines provide cognitive resilience against Alzheimer’s dise ase. Ann Neurol. 2017;82:602–14. https://doi.org/10.1002/ana.25049.
Li Y, Nie Z, Du Y, Chen L, Liu Q, Wu X, et al. “RNSP (Rannasangpei)” rescued MK-801-induced Schizophrenia-like behaviors in mice via oxidative stress and BDNF-TrkB/Akt pathway. Mol Neurobiol. 2024;61:10538–50. https://doi.org/10.1007/s12035-024-04213-5.
Wu S, Li Y, Zhao X, Shi F-D, Chen J. Multiplex proteomics identifies inflammation-related plasma biomarkers for aging and cardio-metabolic disorders. Clin Proteom. 2024;21:30, https://doi.org/10.1186/s12014-024-09480-x.
Esfahani SA, Ma H, Krishna S, Shuvaev S, Sabbagh M, Deffler C, et al. Collagen type I PET/MRI enables evaluation of treatment response in pancreatic cancer in pre-clinical and first-in-human translational studies. Theranostics. 2024;14:5745–61. https://doi.org/10.7150/thno.100116.
Li Y, Li C, Luo T, Yue T, Xiao W, Yang L, et al. Progress in the treatment of high altitude cerebral edema: targeting REDOX homeostasis. J Inflamm Res. 2023;16:2645–60. https://doi.org/10.2147/jir.S415695.
Klawonn AM, Fritz M, Castany S, Pignatelli M, Canal C, Similä F, et al. Microglial activation elicits a negative affective state through prostaglandin-mediated modulation of striatal neurons. Immunity. 2021;54:225–23.e226. https://doi.org/10.1016/j.immuni.2020.12.016.
Cheng Y, Chen B, Xie W, Chen Z, Yang G, Cai Y, et al. Ghrelin attenuates secondary brain injury following intracerebral hemorrhage by inhibiting NLRP3 inflammasome activation and promoting Nrf2/ARE signaling pathway in mice. Int Immunopharmacol. 2020;79:106180, https://doi.org/10.1016/j.intimp.2019.106180.
Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–52. https://doi.org/10.1016/j.arcmed.2006.10.005.
Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797, https://doi.org/10.1155/2016/7432797.
Dong YS, Wang JL, Feng DY, Qin HZ, Wen H, Yin ZM, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 2014;11:282–90. https://doi.org/10.7150/ijms.7634.
Mohammed AM, Khardali IA, Oraiby ME, Hakami AF, Shaheen ES, Ageel IM, et al. Anxiety, depression-like behaviors and biochemistry disorders induced by cannabis extract in female mice. Saudi J Biol Sci. 2021;28:6097–111. https://doi.org/10.1016/j.sjbs.2021.08.085.
Murrough JW, Huryk KM, Mao X, Iacoviello B, Collins K, Nierenberg AA, et al. A pilot study of minocycline for the treatment of bipolar depression: effects on cortical glutathione and oxidative stress in vivo. J Affect Disord. 2018;230:56–64. https://doi.org/10.1016/j.jad.2017.12.067.
Shushanyan RA, Avtandilyan NV, Grigoryan AV, Karapetyan AF. The role of oxidative stress and neuroinflammatory mediators in the pathogenesis of high-altitude cerebral edema in rats. Respiratory Physiol Neurobiol. 2024;327:104286, https://doi.org/10.1016/j.resp.2024.104286.
Chen Z, Zhao X, Lin L, Cui Y, Cao D, Chen X-L, et al. CaGA nanozymes with multienzyme activity realize multifunctional repai r of acute wounds by alleviating oxidative stress and inhibiting cell apoptosis. Biomater Sci. 2025;13:422–33. https://doi.org/10.1039/d4bm01155d.
Liu K, Liu E, Lin L, Hu Y, Yuan Y, Xiao W. L-Theanine mediates the p38MAPK signaling pathway to alleviate heat-induced oxidative stress and inflammation in mice. Food Funct. 2022;13:2120–30. https://doi.org/10.1039/d1fo03077a.
Steenkamp LR, Hough CM, Reus VI, Jain FA, Epel ES, James SJ, et al. Severity of anxiety- but not depression- is associated with oxidative stress in major depressive disorder. J Affect Disord. 2017;219:193–200. https://doi.org/10.1016/j.jad.2017.04.042.
Akang EN. Combination antiretroviral therapy (cART)-induced hippocampal disorders: highlights on therapeutic potential of Naringenin and Quercetin. IBRO Rep. 2019;6:137–46. https://doi.org/10.1016/j.ibror.2019.04.002.
Saramowicz K, Siwecka N, Galita G, Kucharska-Lusina A, Rozpędek-Kamińska W, Majsterek I. Alpha-synuclein contribution to neuronal and glial damage in Parkinson’s disease. Int J Mol Sci. 2023;25:360, https://doi.org/10.3390/ijms25010360.
Zhao B, Peng Q, Wang D, Zhou R, Wang R, Zhu Y, et al. Leonurine protects bone mesenchymal stem cells from oxidative stress by activating mitophagy through PI3K/Akt/mTOR pathway. Cells. 2022;11:1724, https://doi.org/10.3390/cells11111724.
Gern OL, Pavlou A, Mulenge F, Busker LM, Ghita L, Aringo A, et al. MAVS signaling shapes microglia responses to neurotropic virus infection. J Neuroinflammation. 2024;21:264, https://doi.org/10.1186/s12974-024-03258-6.
Lee H, Lee DH, Oh JH, Chung JH. Skullcapflavone II suppresses TNF-α/IFN-γ-induced TARC, MDC, and CTSS production in HaCaT cells. Int J Mol Sci. 2021;22:6428, https://doi.org/10.3390/ijms22126428.
Kang C, Li X, Liu P, Liu Y, Niu Y, Zeng X, et al. Tolerogenic dendritic cells and TLR4/IRAK4/NF-κB signaling pathway in allergic rhinitis. Front Immunol. 2023;14:1276512, https://doi.org/10.3389/fimmu.2023.1276512.
Tao R, Mao Y, Li Y, Sun M, Cao X, Chen N, et al. Connexin26 modulates radiation-induced skin damage by regulating chemokine CCL27 through MAPK signaling. Radiat Res. 2023;200:281–8. https://doi.org/10.1667/rade-20-00085.1.
Fu Q, Qiu R, Liang J, Wu S, Huang D, Qin Y, et al. Sugemule-7 alleviates oxidative stress, neuroinflammation, and cell death, promoting synaptic plasticity recovery in mice with postpartum depression. Sci Rep. 2025;15:1426. https://doi.org/10.1038/s41598-025-85276-9.
Qian C, Yang C, Lu M, Bao J, Shen H, Deng B, et al. Activating AhR alleviates cognitive deficits of Alzheimer’s disease model mice by upregulating endogenous Aβ catabolic enzyme Neprilysin. Theranostics. 2021;11:8797–812. https://doi.org/10.7150/thno.61601.
To HTN, Park JH, Kim JW, Kang D. Delta/Notch-like Epidermal growth factor-related Receptor (DNER), a potential prognostic marker of gastric cancer regulates cell survival and cell cycle progression. Int J Mol Sci. 2023;24:10077. https://doi.org/10.3390/ijms241210077.
Wang Y, Yue J, Xiao M, Lu X, Chin YE. SIRT4-Catalyzed deacetylation of Axin1 modulates the Wnt/β-catenin signaling pathway. Front Oncol. 2022;12:872444. https://doi.org/10.3389/fonc.2022.872444.
Rask-Andersen M, Almén MS, Olausen HR, Olszewski PK, Eriksson J, Chavan RA, et al. Functional coupling analysis suggests link between the obesity gene FTO and the BDNF-NTRK2 signaling pathway. BMC Neurosci. 2011;12:117. https://doi.org/10.1186/1471-2202-12-117.
Alexandersson A, Venäläinen MS, Heikkilä N, Huang X, Taskinen M, Huttunen P, et al. Proteomics screening after pediatric allogenic hematopoietic stem cell transplantation reveals an association between increased expression of inhibitory receptor FCRL6 on γδ T cells and cytomegalovirus reactivation. Immunol Cell Biol. 2024;102:513–25. https://doi.org/10.1111/imcb.12762.
Wu Q, Wang D, Zhang Z, Wang Y, Yu W, Sun K, et al. DEFB4A is a potential prognostic biomarker for colorectal cancer. Oncol Lett. 2020;20:114. https://doi.org/10.3892/ol.2020.11975.
Liu D, Li R, Xu S, Shi M, Kuang Y, Wang J, et al. SMOC2 promotes aggressive behavior of fibroblast-like synoviocytes in rheumatoid arthritis through transcriptional and post-transcriptional regulating MYO1C. Cell Death Dis. 2022;13:1035. https://doi.org/10.1038/s41419-022-05479-0.
Xu J, Zhang W. EZR promotes pancreatic cancer proliferation and metastasis by activating FAK/AKT signaling pathway. Cancer Cell Int. 2021;21:521. https://doi.org/10.1186/s12935-021-02222-1.
Al Hanjori AS, Alshaer W, Al-Anati B, Wehaibi S, Zihlif M. Studying the anti-tumor effects of siRNA gene silencing of some metabolic genes in pancreatic ductal adenocarcinoma. Curr Mol Pharmacol. 2021;14:604–19. https://doi.org/10.2174/1874467213666201012162250.
Araida J, Ohka S, Soeda M, Nishizawa D, Hasegawa J, Nakayama K, et al. rs12411980 single-nucleotide polymorphism related to PRTFDC1 expression is significantly associated with phantom tooth pain. Mol pain. 2024;20:17448069241272215 https://doi.org/10.1177/17448069241272215.
Caronni N, La Terza F, Vittoria FM, Barbiera G, Mezzanzanica L, Cuzzola V, et al. IL-1β(+) macrophages fuel pathogenic inflammation in pancreatic cancer. Nature. 2023;623:415–22. https://doi.org/10.1038/s41586-023-06685-2.
Jain A, Das R, Giri M, Mane P, Shard A. Carbohydrate kinase inhibition: a promising strategy in cancer treatment. Drug Discov Today. 2025;30:104308. https://doi.org/10.1016/j.drudis.2025.104308.
Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat reviews Mol Cell Biol. 2023;24:255–72. https://doi.org/10.1038/s41580-022-00547-x.
Yang Y, Li S, Wang Y, Zhao Y, Li Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective. Signal Transduct Target Ther. 2022;7:329. https://doi.org/10.1038/s41392-022-01168-8.
Xue C, Yao Q, Gu X, Shi Q, Yuan X, Chu Q, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023;8:204. https://doi.org/10.1038/s41392-023-01468-7.
Kang J, Postigo-Fernandez J, Kim K, Zhu C, Yu J, Meroni M, et al. Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis. JCI Insight. 2023;8:e165369. https://doi.org/10.1172/jci.insight.165369.
Purohit G, Ghosh P, Khalimonchuk O. Mitochondrial metallopeptidase OMA1 in cancer. Adv Cancer Res. 2024;162:75–97. https://doi.org/10.1016/bs.acr.2024.05.001.
D’Egidio F, Castelli V, d’Angelo M, Ammannito F, Quintiliani M, Cimini A. Brain incoming call from glia during neuroinflammation: roles of extracellular vesicles. Neurobiol Dis. 2024;201:106663. https://doi.org/10.1016/j.nbd.2024.106663.
Lu Y, Chang P, Ding W, Bian J, Wang D, Wang X, et al. Pharmacological inhibition of mitochondrial division attenuates simulated high-altitude exposure-induced cerebral edema in mice: involvement of inhibition of the NF-κB signaling pathway in glial cells. Eur J Pharmacol. 2022;929:175137. https://doi.org/10.1016/j.ejphar.2022.175137.
Martin A, Millet G, Osredkar D, Mramor M, Faes C, Gouraud E, et al. Effect of pre-term birth on oxidative stress responses to normoxic and hypoxic exercise. Redox Biol. 2020;32:101497. https://doi.org/10.1016/j.redox.2020.101497.
Cheng J, Zhang R, Xu Z, Ke Y, Sun R, Yang H, et al. Early glycolytic reprogramming controls microglial inflammatory activation. J Neuroinflammation. 2021;18:129. https://doi.org/10.1186/s12974-021-02187-y.
D’Erme AM, Fidanzi C, Bevilacqua M, Bieber T, Tuoni C, Paolicchi A, et al. Cord blood serum levels of IL-31 and CCL17, cutaneous markers, and development of atopic dermatitis. JAMA Dermatol. 2024;160:1112–5. https://doi.org/10.1001/jamadermatol.2024.3178.
Elmore AR, Adhikari N, Hartley AE, Aparicio HJ, Posner DC, Hemani G, et al. Protein identification for stroke progression via Mendelian randomization in million veteran program and UK Biobank. Stroke. 2024;55:2045–54. https://doi.org/10.1161/strokeaha.124.047103.
Williamson KA, Samec MJ, Patel JA, Orandi AB, Wang B, Crowson CS, et al. Clinical phenotype, NOD2 genotypes, and treatment observations in Yao syndrome: a retrospective case series. Front Immunol. 2024;15:1304792. https://doi.org/10.3389/fimmu.2024.1304792.
Khan H, Naseem T, Kaushik P, Narang J, Khan R, Panwar S, et al. Decoding paradoxical links of cytokine markers in cognition: cross talk between physiology, inflammaging, and Alzheimer’s disease- related cognitive decline. Ageing Res Rev. 2024;101:102535. https://doi.org/10.1016/j.arr.2024.102535.
Sivandzade F, Cucullo L. Regenerative stem cell therapy for neurodegenerative diseases: an overview. Int J Mol Sci. 2021;22:22. https://doi.org/10.3390/ijms22042153.
Hu M, Li H, Huang Z, Li D, Xu Y, Xu Q, et al. Novel compound heterozygous mutation in STAMBP causes a neurodevelopmental disorder by disrupting cortical proliferation. Front Neurosci. 2022;16:963813. https://doi.org/10.3389/fnins.2022.963813.
Lagging C, Klasson S, Pedersen A, Nilsson S, Jood K, Stanne TM, et al. Investigation of 91 proteins implicated in neurobiological processes identifies multiple candidate plasma biomarkers of stroke outcome. Sci Rep. 2022;12:20080. https://doi.org/10.1038/s41598-022-23288-5.
Brunello CA, Cannarozzo C, Castrén E. Rethinking the role of TRKB in the action of antidepressants and psychedelics. Trends Neurosci. 2024;47:865–74. https://doi.org/10.1016/j.tins.2024.08.011.
Tong Y, Ratnasiri K, Hanif S, Nguyen AT, Roh ME, Dorsey G, et al. Intermittent preventive treatment for malaria in pregnancy and infant growth: a mediation analysis of a randomised trial. EBioMedicine. 2024;109:105397. https://doi.org/10.1016/j.ebiom.2024.105397.
Funding
This work was supported by the Science and Technology Projects of the Xizang Autonomous Region, China (No. XZ202501YD0018), and the National Natural Science Foundation of China (No. 82471560).
Author information
Authors and Affiliations
Contributions
Yong Cheng conceived the study; Zhongyong Jiang and Yong Cheng designed the study; Qiang Fu, Rui Qiu, Quan Tang, Xiaodong Li, Yaobo Li, Yuxiang Qin, Qiaosheng Li, Jia Yao and Huan Xu performed the experiments; All the authors analyzed and interpreted the data; Qiang Fu drafted the manuscript with critical revisions from Yong Cheng.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, Q., Qiu, R., Tang, Q. et al. Exosomes from high-altitude cerebral edema patients induce cognitive dysfunction by altering oxidative stress responses in mice. Transl Psychiatry 15, 253 (2025). https://doi.org/10.1038/s41398-025-03469-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03469-2