Abstract
Depression is associated with reduced functional connectivity within the brain’s salience network and its strengthened interactions with the default mode network (DMN). Modification of this clinical pattern is challenging. Leveraging the direct neural pathways from olfactory processing regions to the salience network, we explored the effects of electrical stimulation of the olfactory mucosa on brain connectivity. In a randomized, blinded within-subject design, 45 healthy individuals received olfactory or trigeminal nerve stimulation followed by resting-state fMRI. Olfactory stimulation resulted in a significant increase in functional connectivity between the salience network and the piriform cortex – a primary olfactory structure. Importantly, this stimulation increased functional connectivity within the salience network and weakened connectivity between the salience network and the DMN. These findings suggest that olfactory stimulation may modulate connectivity patterns implicated in depression, offering a novel potential minimal invasive therapeutic strategy. However, as these results were obtained from a healthy cohort, further studies are required to evaluate the efficacy in individuals with depression.
Similar content being viewed by others
Introduction
Anhedonia, defined as the diminished capacity to experience pleasure from rewarding stimuli, is a core symptom of depression. It is characterized by a reduction in interest and motivation to sustain activities that would normally be enjoyable. In severe cases of depression, individuals are unable to maintain occupational or social functioning or even carry out basic daily activities such as getting out of bed. Anhedonia and lack of motivation are associated to reduced activity in the brain’s salience network [1, 2], a group of interconnected brain regions responsible for detecting and responding to salient stimuli amidst irrelevant background information [3]. The network integrates interoceptive and exteroceptive inputs to evaluate emotional significance in the anterior insula [4], while the anterior cingulate cortex (ACC) coordinates appropriate responses across sensory, motor, and association cortices [4,5,6].
In addition to diminished activity in response to rewarding stimuli, the salience network of depressed individuals exhibits a less orchestrated interplay. Abnormal patterns during resting state, especially within the ACC, have been proposed as a neural classifier for depression [7]. Almost a decade ago, two comprehensive reviews of resting-state functional connectivity in depression highlighted significant alterations within the salience network [8, 9], including decreased connectivity in the bilateral anterior insula which was found to be associated with the severity of depression [10]. This is further supported by a recent large-scale study involving over 600 patients, which demonstrated relative hypoconnectivity within the salience network [11], potentially driven by reduced myelination of salience network tracts in depression [12].
In addition, depression is also marked by hyperconnectivity between the salience network and the default mode network (DMN), both during resting state [10] and when performing tasks related to salience processing, such as responding to smiling faces [13]. This hyperconnectivity was further correlated with severity and decreased glutamate levels in the perigenual ACC [14].
Modifying this clinical pattern using brain stimulation techniques remains challenging. Regional synchronization during resting state was found to increase for theta activity within salience network components such as dorsal ACC while decreasing beta power in anterior DMN structures such as the medial prefrontal cortex (MPFC) [15]. Two studies, involving 91 and 81 patients, respectively, investigated effects of electroconvulsive therapy and found no significant impact on within-salience-network hypoconnectivity or salience-to-DMN hyperconnectivity [8, 9]. However, a study of 23 patients reported an increase in salience-to-DMN connectivity following stimulation [16]. One potential explanation may be the relatively broad effects of an unspecific, brain wide stimulation during electroconvulsive therapy.
An alternative, more regionally confined, approach to targeting salience network function may involve olfaction, given its strong anatomical overlap with the salience network, which likely stems from the co-evolution of these structures [17, 18]. The neural intertwining of olfaction and emotion processing was first reported by McLean, who described the sense of smell as a key part of the “visceral brain” [19], later termed the limbic system [20]. Olfactory processing begins with olfactory receptor neurons located in the upper part of the nasal cavity (olfactory mucosa), which transmit signals to the olfactory bulb, the primary relay station for olfactory information. From the olfactory bulb, signals are sent to the primary olfactory cortex, including the piriform cortex and amygdala [21]. Hence, olfactory stimulation reaches the amygdala within only two neurons. Almost as direct is the connection to the salience network with the anterior insula and the ACC, which both function as secondary olfactory structures and are involved in the hedonic perception of odors [22].
Based on the anatomical connection, McLean already proposed a link between the olfactory system and depressive states [19]. This hypothesis has since been confirmed. Hyposmia – the reduced ability to perceive smells – is associated with impaired emotional perception and processing [23]. Moreover, both acquired and congenital anosmia correlate with increased depressive symptoms [24]. Conversely, major depressive disorder is often characterized by reduced olfactory function across multiple domains, including odor threshold, identification, discrimination, and central processing [24,25,26], and by reduced volume of the olfactory bulb [24, 25, 27, 28]. Causal support for the olfaction-depression link comes from animal studies, showing that bulbectomized rats (those with surgical removal of the olfactory bulb) exhibit depression-like behaviors, neurotransmitter alterations [29], and amygdala fiber degeneration [30].
The close interplay between emotion, salience and olfaction may bear a potential for improving salience network functioning and emotional responses through olfactory system stimulation. We were inspired by previous research, which demonstrated that electrical stimulation of the olfactory nerve can modify resting-state connectivity. In that study, 18 participants received low-voltage stimulation of the upper nasal tract, followed by a resting-state fMRI scan. The results showed increased connectivity within the primary olfactory structures [31], however the authors did not investigate areas beyond the primary olfactory cortex. Building on this previous work, we sought to determine whether electrical stimulation of the olfactory nerve, compared to stimulation of the intranasal trigeminal nerve, enhances neural connectivity within the salience network and diminishes the salience-to-DMN connectivity. This research marks an initial step toward exploring olfactory nerve stimulation as a potential therapeutic approach for treatment-resistant depression. As the first study of its kind, we investigated these effects in healthy individuals.
Methods
Participants
We recruited 45 healthy individuals with a mean age of 29.6 years (SD = 12.8, range = 19–64 years of age) through advertisement at the University Clinic Carl Gustav Carus in Dresden, Germany (see Supplementary Material for power calculation). Following the skewed gender proportion in depression, we investigated 15 male and 30 female participants. Exclusion criteria were mental disorders as screened with the Patient Health Questionnaire (PHQ-D) [32] and disorders related to loss of olfactory function (e.g. Parkinson’s disease, renal insufficiency, inflammatory diseases in the nose) or neurodevelopmental disorders. To maximize the potential effect of olfactory nerve fiber stimulation, we furthermore excluded dysosmic individuals, as screened by the Sniffin’ Sticks identification test (Burghart Messtechnik GmbH, Germany) with the cut off of 12/16 [33].
The study was approved by the ethics committee of the Technical University Dresden (application number EK417092019) and performed under the ethical guidelines of the Helsinki Declaration [34]. All participants provided written informed consent. They received moderate financial compensation.
Research design
Procedure
Following an initial session to assess normosmia and calibration of electrical stimulation based in individual thresholds, participants attended two randomized experimental sessions. In each session, they received either olfactory or trigeminal nerve stimulation, followed by MRI scans (see Fig. 1). To minimize the delay between end of stimulation and start of MRI assessment, electrical stimulations were conducted in a room adjacent to the MRI scanner, replicating the successful protocol established by Weiss and colleagues [31].
A shows the sagittal cross-section of the nasal cavity. The positioning of the electrode head in the olfactory stimulation condition is highlighted in purple. The positioning of the electrode head for the trigeminal stimulation condition is highlighted in turquois. B shows the schematic representation of the timeline of the study. Participants attended two randomized experimental sessions. In each session, they received either olfactory or trigeminal nerve stimulation, followed by an MRI scan. Created with Biorender.com.
To monitor potential associated phenomena of the stimulation, participants rated the intensity of sensations “tingling”, “stabbing”, “burning”, and “cooling” (based on Weiss et al. [31]) on a 5-point Likert scale (ranging from no sensation to very strong) after the MRI session. Based on our previous experience with such stimulation, we also asked participants to assess further potential associated phenomena, namely “toothache”, “headache”, “numbness” and “paresthesia” on a 5-point Likert scale (ranging from none to very strong). Furthermore, we encouraged them to report any further sensations.
To monitor potential changes in implicit affect resulting from electrical stimulation, participants completed the Implicit Positive and Negative Affect Test (IPANAT) [35] before and after both the olfactory and trigeminal stimulation.
Electrical stimulation
The major difference between the olfactory and trigeminal conditions was in the intranasal electrode placement (see Fig. 1). For the olfactory condition, electrodes were positioned on the upper part of the middle nasal turbinate close to its insertion at the lateral wall, following the methodology of Weiss et al. [31], whereas for the trigeminal condition, electrodes were placed on the lower nasal turbinate, where trigeminal sensitivity to electrical stimulation is typically higher [36]. To ensure stimulation specificity, the lower nasal turbinate was selected for the trigeminal condition because it lies outside the known distribution of olfactory sensory neurons. While the olfactory epithelium can reach from the olfactory cleft up to the middle turbinate, especially in younger individuals [37, 38], there is no anatomical or histological evidence supporting the presence of olfactory receptors in the lower turbinate.
Small spherical electrodes (6 mm; COP06S1-80, SEI EMG s.r.l., Cittadella IT) were applied to the designated locations under endoscopic guidance. Electrical impulses were generated and controlled using a high-voltage direct current stimulator (DSA7, Digitimer, UK) and monitored with an oscilloscope (GoldStar Oscilloscope OS-9020G; Rigol, Suzhou, China). To conceal the stimulation condition from participants, both electrodes, olfactory and trigeminal, were attached in each condition and the position was stabilized during stimulation using a glasses-like frame.
For the electrical stimulation, participants were instructed to lie quietly with their eyes closed on a stretcher. Stimulation was administered for 12 min at a voltage of 250V, with an on-time of 60 s, and an off-time of 60 s. During on-time stimulation blocks occurred in 6 on-time cycles, 200 µs pulse width, 10 Hz square wave.
These parameters were adapted from Weiss et al. [31] and refined based on a pre-study involving 16 participants (see Supplementary Material). To ensure perceptual consistency across participants and conditions, individual calibration was performed in the initial session. Therefore, we determined the intranasal perception thresholds for both olfactory and trigeminal stimulation by gradually increasing the current of single impulses by 0.05 milliamperes (mA) starting from 0.5 mA, with a pulse duration of 500 μs until participants reported to perceive the stimulus. During the experimental sessions, stimulation was delivered at twice the perception threshold (average over all participants; olfactory, threshold: M = 1.50 mA, SD = 0.401 mA; stimulation: M = 3.00 mA, SD = 0.801 mA, range = 0.75–2.65 mA; trigeminal, threshold: M = 0.917 mA, SD = 0.278 mA; stimulation: M = 1.83 mA, SD = 0.556 mA, range = 0.50–1.65 mA).
Image acquisition
Imaging data were acquired using a 3T MAGNETOM Prisma scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 64 channel head coil. In two cases, a 20-channel head coil was used due to the large head size and the need for MRI compatible glasses which did not fit the 64 channel head coil.
First, a T1-weighted anatomical image was obtained with a magnetization prepared – rapid gradient echo (MPRAGE) sequence (volumes: 1, axial slices: 160, slice thickness: 1.0 mm, FoV: 256 mm, TR: 2300 ms, TE: 3.43 ms, TA: 5 min 12 s, flip angle: 9°, voxel size: 1.0 × 1.0 × 1.0 mm3). This was followed by a resting state scan (volumes: 250, axial slices: 35, slice thickness: 2.2 mm, FoV: 220 mm, TR: 2100 ms, TE: 30.0 ms, TA: 8 min 53 s, flip angle: 90°, voxel size: 2.2 × 2.2 × 2.2 mm3), during which participants were instructed to close their eyes. Lastly, a task-based sequence was acquired where the participants performed a salience evoking Oddball-paradigm [13]. This task is not within the scope of the current study and is presented in full in the Supplementary Information for reasons of transparency [13].
Statistical analysis
Analysis of associated phenomena and sensations
Potential associated phenomena and sensations were descriptively analyzed. Furthermore, an aggregated total score was computed by summing the ratings of phenomena per participant. This was done separately for both the olfactory and trigeminal stimulation. Wilcoxon singed-rank tests (two-tailed) were performed in R (https://www.r-project.org) to analyze the statistical difference from zero and between the two conditions, both for the individual phenomena and for the total score. Identically, an aggregated intensity score for all perceived sensations during the stimulation was calculated and Wilcoxon singed-rank tests (two-tailed) were performed.
Resting-state functional MRI data
Preprocessing was conducted using the CONN toolbox preprocessing pipeline including slice time correction and smoothing with a 7 mm FWHM Gaussian kernel [39]. Subsequent denoising steps involved the regression of five principal components from white matter, five from cerebrospinal fluid, 12 from realignment, and 38 from scrubbing, along with linear detrending. To further minimize noise related to cardiac and respiratory activity, a temporal band-pass filter of 0.01–0.1 Hz was applied to the time series as suggested by previous research [40, 41].
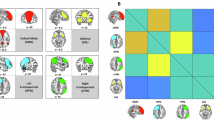
We focused analysis on three key networks: the salience network (including the left and right anterior insula and the ACC), the DMN (including the posterior cingulate cortex (PCC) and the MPFC), both based on own prior investigations [11, 14], and the olfactory network (including the left and right amygdala and the left and right piriform cortex). ROI definitions were based on anatomical locations provided in the CONN toolbox, except for the piriform cortex which was manually defined following the recommendations of Thaploo and colleagues [42]. The ROIs are visualized in Fig. 2.
First-level ROI-to-ROI analysis was performed on the preprocessed data. Second level ROI-to-ROI analysis was calculated separately for the olfactory and the trigeminal condition, as well as for between-condition contrasts (olfactory > trigeminal; olfactory < trigeminal). Contrast results and effect sizes were visualized and extracted using CONN’s results explorer tool. Two key measures were used to describe networks of strongly connected ROIs: Network Size (the number of strong connections) and Network Mass (the strength of those connections, calculated as the sum of statistical values [T-squared] across all connections in a given network). To control for multiple comparisons, we applied the false discovery rate (FDR) correction at the network level, using a significance threshold of p < 0.05. For the initial thresholding of the ROI-to-ROI matrix however, an uncorrected p-value of <0.05 was applied to reduce the risk of type II errors in this initial study.
To further assess how different stimulations influenced connectivity within and between the key brain networks, individual estimates were extracted from CONN for each participant, connection, and condition and entered in repeated measurement ANOVAs using JASP (version 0.18.3.0). The within subject effects of condition (2) and connection were modeled as main and interaction effects, with Greenhouse Geiser correction applied to account for sphericity violations. The analyses were conducted both within each network (salience [6 connections], default-mode [2 connections], olfactory [10 connections]), as well as for salience- default mode [8 connections] and salience-olfaction [18 connections] network connectivity.
Analysis of implicit mood
Wilcoxon singed-rank tests (two-tailed) were performed to examine whether total scores for positive and negative implicit affect differed between pre- and post-stimulation within each condition. Analyses were performed in R (https://www.r-project.org), separately for positive and negative affect, and for olfactory and trigeminal stimulation.
Results
Evaluation of associated phenomena and sensations for the olfactory and trigeminal stimulation
Participants rated associated phenomena and sensations significantly above zero (all p < 0.005), but with very low intensity. The intensity was higher during trigeminal than during olfactory stimulation (associated phenomena (total score): p = 0.0036; sensations (total score): p = 0.0386; see Fig. 3).
During the electrical stimulation and after the MRI session, participants were asked to rate both the olfactory and the trigeminal stimulation for the intensities of the associated phenomena (toothache, headache, numbness and paresthesia) and the intensities of the sensation (tingling, stabbing, burning, cooling). They rated the intensities of each phenomenon and sensation on a 5-point Likert scale ranging from 0 (no sensation) to 4 (very strong). An aggregated total score for phenomenon intensity was computed by summing the ratings of each phenomenon for each participant, for both the olfactory and trigeminal stimulation respectively. The same procedure was repeated for the total score for sensation intensity. The x-axis represents the intensity of associated phenomena and sensations. The y-axis represents the count. Purple olfactory stimulation, Turquoise trigeminal stimulation, Blue overlap between olfactory and trigeminal stimulation.
Looking at individual items, toothache was the only associated phenomena that significantly deviated from zero. This was true both in the olfactory (p = 0.0263) and the trigeminal condition (p < 0.0004), but stronger in the trigeminal one (p = 0.0016). Additionally, one participant reported an associated phenomenon of “a pulling feeling in the nasal cavity” (intensity = 1), one a “throbbing in the hand” (intensity = 1), and one a “pressure” (intensity = 1) during the olfactory condition. During the trigeminal condition, one participant reported throbbing in the tooth (intensity = 2), and another reported pressure in the head (intensity = 2). For sensations, intensities for stabbing, tingling, and burning deviated significantly from zero for the olfactory and trigeminal condition (all p < 0.005) and cooling was significantly reported in the trigeminal condition only (p = 0.0369). The sensation of stabbing was significantly stronger for the trigeminal than for the olfactory condition (p = 0.0333), for all other sensations, there was no significant group difference (see Fig. 3). Additionally, six participants reported sensations described as throbbing (intensities = 1, 1, 2, 3, 3, 3), one as pulsing (intensity = 2), one as pressure (intensity = 1), one tapping in wrist (intensity = 1) during the olfactory condition, while four participants reported throbbing (intensities = 1, 1, 2, 2), one tapping (intensity = 1), one pulsing (intensity = 3), and one pressure (intensity = 1) during the trigeminal condition. One participant reported the smell of leaves after the olfactory stimulation. No further experiences of smell were reported.
Connectivity within and between networks
After both, olfactory and trigeminal stimulation, we observed pronounced functional connectivity within regions of the salience network, DMN, and the olfactory network (olfactory: mass value = 5595.42, pFDR-corrected < 0.001; trigeminal: mass value = 4241.33, pFDR-corrected < 0.001; see Fig. 4A). After both stimulations, the most pronounced connectivity was observed within the salience network, particularly between the left and right anterior insula (olfactory: T[44] = 21.98, pFDR-corrected < 0.001; trigeminal: T[44] = 18.77, pFDR-corrected < 0.001). Moreover, both stimulations showed significantly negative functional connectivity between the salience network and DMN. This negative functional connectivity was most pronounced between the PCC of the DMN and the left anterior insula of the salience network (olfactory: T[44] = −12.80, pFDR-corrected < 0.001; trigeminal: T[44] = −7.21, pFDR-corrected < 0.001).
A Functional connectivity during olfactory and trigeminal stimulation separately. B Functional connectivity compared between conditions (olfactory vs. trigeminal stimulation). Red lines indicate increased connectivity within networks, while blue lines indicate decreased connectivity between networks. r right hemisphere, l left hemisphere, ACC anterior cingulate cortex, MPFC medial prefrontal cortex, PCC posterior cingulate cortex.
Comparing olfactory and trigeminal stimulation conditions revealed significant differences in functional connectivity patterns (overall connectivity: mass value = 82.51, pFDR-corrected = 0.026, see Fig. 4B). Specifically, olfactory compared to trigeminal stimulation increased functional connectivity within the salience network and between the salience network and the piriform cortex of the olfactory network. Furthermore, olfactory stimulation decreased functional connectivity between the PCC of the DMN and all regions of the salience network (see Table 1).
Analysis of extracted data allowed for combined analysis of all network areas and unsurprisingly confirmed the increased connectivity after the olfactory vs trigeminal stimulation within the hubs of the salience network (F[1,43] = 5.5, p = 0.024, η2 = 0.113). The analysis also showed a significantly increased connectivity within the bilateral core hubs of the olfactory network (F[1,43] = 4.2, p = 0.046, η2 = 0.089) during olfactory stimulation as opposed to the trigeminal stimulation. In tendency, the same pattern was observed in the default mode network, but missed statistical significane (F[1,43] = 4.0, p = 0.051, η2 = 0.086). Between-network connectivity did not differ between olfactory and trigeminal stimulation, neither for the salience-olfactory network (F[1,43] = 0.1, p = 0.808, η2 = 0.001), nor for the salience-default mode network (F[1,43] = 3.1, p = 0.087, η2 = 0.066). However, the latter between-network connectivity was in tendency reduced during olfactory stimulation (see Fig. 5).
The plot combines a boxplot (center) representing the median and interquartile range, a violin plot (cloud, right) illustrating the density distribution of the data, and individual data points (rain, left) overlaid for clarity. This visualization highlights both the central tendency and variability of the data, as well as the individual observations within each condition.
Evaluation of implicit mood
Implicit positive affect did not significantly differ between pre- and post-stimulation in either condition (olfactory: p = 0.054; trigeminal: p = 0.083). In contrast, implicit negative affect showed a significant reduction following stimulation in both conditions (olfactory: p < 0.001; trigeminal: p < 0.001), suggesting a decrease in negative affect post-stimulation regardless of condition (see Fig. 6).
The plot combines a boxplot (center) representing the median and interquartile range, a violin plot (cloud, right) illustrating the density distribution of the data, and individual data points (rain, left) overlaid for clarity. This visualization highlights both the central tendency and variability of the data, as well as the individual observations pre- and post-stimulation within each condition.
Discussion
This study investigated the effects of electrical stimulation of the olfactory mucosa on salience network connectivity in healthy individuals. In line with the olfactory processing pathway, olfactory nerve stimulation significantly increased functional connectivity within the olfactory cortex, between the olfactory and the salience network, and within the salience network regions. Furthermore, olfactory nerve stimulation decreased functional connectivity between the salience network and the PCC of the DMN.
The increased olfactory cortex connectivity indicates that electrical stimulation of the olfactory area leads to synchronization of activation patterns in the piriform cortex and the amygdala – a result similarly observed by Weiss and colleguages [31]. The increased connectivity between the olfactory and the salience network furthermore suggests that electrical stimulation can influence brain activity of the salience network through olfactory processing pathways. Notably, functional connectivity between the piriform cortex and the salience network was also observed in the trigeminal stimulation condition. This may reflect a baseline level of connectivity between the piriform cortex and salience-related structures (as indicated by structural connectivity [43]). However, this functional connectivity was significantly stronger in the olfactory condition. Given the complexity of innervation in the nasal cavity [44, 45], we cannot completely rule out the possibility that trigeminal stimulation may have inadvertently engaged some olfactory nerve fibers. It is, however, highly unplausible that olfactory nerve fibers were activated in the trigeminal condition.
In the context of depression, it is particularly noteworthy that olfactory stimulation increased connectivity within the salience network and while decreasing connectivity between the salience and the DMN. The is striking because the opposite pattern, namely reduced functional connectivity within the salience network [8] alongside increased connectivity between the salience network and the DMN [10, 46, 47] is typical for depression. Additionally, connectivity within the salience network is inversely associated with the severity of depression symptoms [10], while increased DMN influence on the salience network has been linked to negative cognitive biases in processing positive stimuli [48]. Furthermore, reduced functional connectivity of the right insula within the salience network has been suggested as a marker for poor antidepressant response [49]. These findings raise the question of whether modulating resting-state functional connectivity through olfactory nerve stimulation could benefit individuals with depression. The functional changes observed in our study suggest that olfactory stimulation may counteract some psychopathological alterations, offering a potential minimal invasive neuromodulatory approach in the treatment of depression.
Our study was conducted with healthy individuals to test for effects, safety, and associated phenomena and sensations of olfactory nerve fiber stimulation. We did not observe significant differences in positive affect between pre- and post-stimulation in either condition. Negative affect significantly decreased following both stimulations, potentially reflecting reduced tension after ending the somewhat distressing experimental procedure. However, it should be noted that both positive and negative affect were generally low and within non-pathological ranges, as the study was performed in a health participant sample. Future studies should investigate the antidepressant potential of such stimulation in patients with depression, and analyze how its efficacy compares to established treatments, such as pharmacotherapy and auricular vagus nerve stimulation [50,51,52,53], both known to alter resting state network connectivity [53]. We do not yet know if olfactory stimulation is another promising intervention for depression treatment. While it shows a favorable associated phenomena profile in healthy participants, we have no data on its efficacy and phenomena — particularly regarding long-term associated phenomena in patients with depression.
Few participants reported toothache following stimulation, with slightly more reports after trigeminal than olfactory stimulation. To mitigate such symptoms and preserve patient compliance in clinical applications, reducing the electrical amplitude could be a feasible strategy. However, this must be carefully balanced against the potential risk of diminishing the desired neural engagement. Replicating our findings in clinical samples will provide critical insights into whether olfactory stimulation can serve as an effective and safer alternative to more invasive treatments, offering new avenues for individualized depression therapy with potentially fewer side effects.
Limitations
Some limitations must be noted. First, the generalizability of our findings is restricted due to the sample, that predominantly consists of younger, well-educated individuals. Individuals with treatment resistant depression are on average a little older, and less educated than our participants were [54]. Given that functional connectivity within and between brain networks change with age [55], these age-related changes could potentially alter the effects of olfactory stimulation in older individuals, which should be considered when interpreting the results. Second, the study sample presents an intended sex imbalance, with twice as many females (n = 30) as males (n = 15), reflecting the higher prevalence of depression in females. However, the smaller number of male participants hindered us to perform sex-specific analyses due to the increased risk of false positive or false negative results. Future studies with larger sample sizes and more balanced sex representation would help elucidate whether sex differences exist in salience network modulation due to olfactory stimulation and, in turn, provide insights into the sex-specific neural underpinnings of depression. Third, the stimulation effects were measured using resting-state fMRI taken approximately eight minutes after the electrical stimulation, which only allows for the assessment of short-term effects. The duration and persistence of the observed effects remain unclear. Additionally, the stimulation paradigm was novel, with parameters selected in a pilot-study to minimize side effects while ensuring effective stimulation. It is uncertain how variations in stimulus intensity might influence the results, and the potential impact of different stimulation thresholds on the observed changes in functional connectivity should be carefully considered in future research.
Conclusion
This study highlights the potential of olfactory nerve stimulation to modulate functional connectivity within the brain’s salience network, with observed increased connectivity within the salience network and decreased connectivity between the salience network and the DMN. The findings suggest that olfactory stimulation could influence brain activity in ways that contrast those observed in depression, potentially offering a new avenue for therapeutic intervention. Given that these results were observed in a healthy cohort, further research is needed to determine their applicability in clinical settings.
Data availability
Data is available from the corresponding author on request.
References
Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77.
Yang Y, Zhong N, Imamura K, Lu S, Li M, Zhou H, et al. Task and resting-state fMRI reveal altered salience responses to positive stimuli in patients with major depressive disorder. PLoS ONE. 2016;11:155092.
Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203.
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67.
Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61.
Bondi E, Maggioni E, Brambilla P, Delvecchio G. A systematic review on the potential use of machine learning to classify major depressive disorder from healthy controls using resting state fMRI measures. Neurosci Biobehav Rev. 2023;144:104972.
Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11.
Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2014;7:930 https://doi.org/10.3389/FNHUM.2013.00930.
Javaheripour N, Li M, Chand T, Krug A, Kircher T, Dannlowski U, et al. Altered resting-state functional connectome in major depressive disorder: a mega-analysis from the PsyMRI consortium. Transl Psychiatry. 2021;11:511 https://doi.org/10.1038/S41398-021-01619-W.
Zhang S, She S, Qiu Y, Li Z, Mao D, Zheng W, et al. Altered cortical myelin in the salience and default mode networks in major depressive disorder patients: a surface-based analysis. J Affect Disord. 2023;340:113–9.
Koeppel CJ, Herrmann T, Weidner K, Linn J, Croy I. Same salience, different consequences: Disturbed inter-network connectivity during a social oddball paradigm in major depressive disorder. Neuroimage Clin. 2021;31:102731 https://doi.org/10.1016/J.NICL.2021.102731.
Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4:33 https://doi.org/10.3389/FNSYS.2010.00033.
Takamiya A, Hirano J, Yamagata B, Takei S, Kishimoto T, Mimura M. Electroconvulsive therapy modulates resting-state EEG oscillatory pattern and phase synchronization in nodes of the default mode network in patients with depressive disorder. Front Hum Neurosci. 2019;13:1 https://doi.org/10.3389/FNHUM.2019.00001.
Wang J, Wei Q, Wang L, Zhang H, Bai T, Cheng L, et al. Functional reorganization of intra‐ and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Hum Brain Mapp. 2018;39:1403.
Northcutt RG. Understanding vertebrate brain evolution. Integr Comp Biol. 2002;42:743–56.
Lopes da Silva FH, Witter MP, Boeijinga PH, Lohman AHM. Anatomic organization and physiology of the limbic cortex. Physiol Rev. 1990;70:453–511.
MacLEAN PD. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949;11:338–53.
Maclean PD. The Limbic system (Visceral brain) and emotional behavior. AMA Arch Neurol Psychiatry. 1955;73:130–4.
Wilson DA, Xu W, Sadrian B, Courtiol E, Cohen Y, Barnes DC. Cortical odor processing in health and disease. Prog Brain Res. 2014;208:275.
Gottfried JA. Smell: central nervous processing. Adv Otorhinolaryngol. 2006;63:44–69.
Han P, Hummel T, Raue C, Croy I. Olfactory loss is associated with reduced hippocampal activation in response to emotional pictures. Neuroimage. 2019;188:84–91.
Croy I, Hummel T. Olfaction as a marker for depression. J Neurol. 2017;264:631–8.
Kohli P, Soler ZM, Nguyen SA, Muus JS, Schlosser RJ. The association between olfaction and depression: a systematic review. Chem Senses. 2016;41:479–86.
Rochet M, El-Hage W, Richa S, Kazour F, Atanasova B. Depression, olfaction, and quality of life: a mutual relationship. Brain Sci. 2018;8:80 https://doi.org/10.3390/BRAINSCI8050080.
Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, et al. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–21.
Rottstaedt F, Weidner K, Hummel T, Croy I. Pre-aging of the olfactory bulb in major depression with high comorbidity of mental disorders. Front Aging Neurosci. 2018;10:1–7.
Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–47.
Carlsen J, de Olmos J, Heimer L. Tracing of two-neuron pathways in the olfactory system by the aid of transneuronal degeneration: projections to the amygdaloid body and hippocampal formation. J Comp Neurol. 1982;208:196–208.
Weiss T, Shushan S, Ravia A, Hahamy A, Secundo L, Weissbrod A, et al. From nose to brain: un-sensed electrical currents applied in the nose alter activity in deep brain structures. Cereb Cortex. 2016;26:4180–91.
Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282:1737–44.
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Quirin M, Kazén M, Kuhl J. When nonsense sounds happy or helpless: the implicit positive and negative affect test (IPANAT). J Pers Soc Psychol. 2009;97:500–16.
Poletti SC, Hausold J, Herrmann A, Witt M, Hummel T. Topographical distribution of trigeminal receptor expression in the nasal cavity. Rhinology. 2019;57:147–52.
Lane AP, Gomez G, Dankulich T, Wang H, Bolger WE, Rawson NE. The superior turbinate as a source of functional human olfactory receptor neurons. Laryngoscope. 2002;112:1183–9.
Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417–21.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Wee CY, Yap PT, Denny K, Browndyke JN, Potter GG, Welsh-Bohmer KA, et al. Resting-state multi-spectrum functional connectivity networks for identification of MCI patients. PLoS ONE. 2012;7:e37828 https://doi.org/10.1371/JOURNAL.PONE.0037828.
Cordes D, Nandy RR, Schafer S, Wager TD. Characterization and reduction of cardiac- and respiratory-induced noise as a function of the sampling rate (TR) in fMRI. Neuroimage. 2014;89:314–30.
Thaploo D, Joshi A, Georgiopoulos C, Warr J, Hummel T. Tractography indicates lateralized differences between trigeminal and olfactory pathways. Neuroimage. 2022;261:119518.
Borghei A, Kelly R, Pearce JJ, Stoub TR, Sani S. Structural connectivity of the human piriform cortex: an exploratory study. Neurosurgery 2023. https://doi.org/10.1227/neu.0000000000002756.
Schaefer ML, Böttger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol. 2002;444:221–6.
Genovese F, Bauersachs HG, Gräßer I, Kupke J, Magin L, Daiber P, et al. Possible role of calcitonin gene-related peptide in trigeminal modulation of glomerular microcircuits of the rodent olfactory bulb. Eur J Neurosci. 2017;45:587–600.
Wei M, Qin J, Yan R, Bi K, Liu C, Yao Z, et al. Association of resting-state network dysfunction with their dynamics of inter-network interactions in depression. J Affect Disord. 2015;174:527–34.
Shao J, Meng C, Tahmasian M, Brandl F, Yang Q, Luo G, et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 2018;12:1708–19.
Guha A, Yee CM, Heller W, Miller GA. Alterations in the default mode-salience network circuit provide a potential mechanism supporting negativity bias in depression. Psychophysiology. 2021;58:e13918 https://doi.org/10.1111/PSYP.13918.
Geugies H, Opmeer EM, Marsman JBC, Figueroa CA, van Tol MJ, Schmaal L, et al. Decreased functional connectivity of the insula within the salience network as an indicator for prospective insufficient response to antidepressants. Neuroimage Clin. 2019;24:102064 https://doi.org/10.1016/J.NICL.2019.102064.
Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11:492–500.
Alexander L, Hawkins PCT, Evans JW, Mehta MA, Zarate CA. Preliminary evidence that ketamine alters anterior cingulate resting-state functional connectivity in depressed individuals. Translational Psychiatry. 2023;13:1–10.
Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm. 2013;120:821–7.
Saberi A, Ebneabbasi A, Rahimi S, Sarebannejad S, Sen ZD, Graf H, et al. Convergent functional effects of antidepressants in major depressive disorder: a neuroimaging meta-analysis. Mol Psychiatry. 2025;30:736–51. https://doi.org/10.1038/S41380-024-02780-6.
Liu X, Mukai Y, Furtek CI, Bortnichak EA, Liaw K-L, Zhong W. Epidemiology of treatment-resistant depression in the United States. J Clin Psychiatry. 2021;83:21m13964 https://doi.org/10.4088/JCP.21m13964.
Sala-Llonch R, Bartrés-Faz D, Junqué C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol. 2015;6:663 https://doi.org/10.3389/fpsyg.2015.00663.
Acknowledgements
This project was funded by the Deutsche Forschungsgemeinschaft (DFG HU441/28-1 to TH; DFG CR479/16-1 to IC). The funding source had no influence on the collection and interpretation of the data/results. We would like to thank Susanne Weise and Kristina Hernandez for their help with the stimulation of the olfactory mucosa.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CH was responsible for formal analysis, visualization, and writing – original draft. MG was responsible for formal analysis, data curation, and writing – review & editing. NM was responsible for investigation, data curation, formal analysis, and writing – review & editing. AT contributed to formal analysis, and writing – review & editing. MW contributed to writing – review & editing. TH contributed to conceptualization, methodology, supervision, funding acquisition, and writing – review & editing. IC was responsible for conceptualization, methodology, formal analysis, project administration, funding acquisition, supervision, and writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants signed an Informed Consent form, and the study was approved by the Ethics Committee of the Technical University in Dresden.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heller, C., Geisler, M., Mayer, N.L. et al. Modulating salience network connectivity through olfactory nerve stimulation. Transl Psychiatry 15, 303 (2025). https://doi.org/10.1038/s41398-025-03500-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03500-6
This article is cited by
-
Alterations of the amygdala in post-COVID olfactory dysfunction
Scientific Reports (2025)