Abstract
Background
Depression is are often insufficiently managed in cancer patients globally. To address this, we conducted a comprehensive systematic review and network meta-analysis to evaluate and compare the effectiveness of pharmacological and non-pharmacological interventions in alleviating depressive symptoms in adult cancer patients.
Methods
We searched PubMed, EMBASE, the Cochrane Library, and ClinicalTrials.gov from inception until 31 July 2024, with an updated search conducted on 10 January 2025. Eligible studies were randomised controlled trials evaluating pharmacological or non-pharmacological interventions for depressive symptoms in adult patients with cancer (aged ≥18 years). Studies involving paediatric populations, lacking complete outcome data, or not reporting intervention outcomes were excluded. A Bayesian network meta-analysis was undertaken to compare the effectiveness of included interventions. The review protocol was prospectively registered in PROSPERO (CRD42023465056).
Findings
A total of 95 RCTs involving 17,260 participants were included. Several non-pharmacological interventions indicated potential benefit compared with usual care, notably massage and touch therapy (standardised mean difference [SMD]: −0.76, 95% CI: −1.37 to −0.16; low certainty), relaxation therapy (SMD: −0.59, 95% CI: −1.06 to −0.11; low certainty), psychotherapy (SMD: −0.43, 95% CI: −0.56 to −0.30; low certainty), and education and support of person with cancer (SMD: −0.30, 95% CI: −0.45 to −0.14; low certainty). Among pharmacological approaches, preliminary findings suggest the combination of mirtazapine and methylphenidate may offer benefits compared with placebo (SMD: −2.46, 95% CI: −4.24 to −0.70; low certainty). However, the overall evidence quality was low, reflecting substantial variability and limited data.
Interpretation
Non-pharmacological interventions such as massage, relaxation therapies, and psychotherapy show promise in alleviating depressive symptoms in cancer patients. Limited preliminary evidence also suggests possible benefits of combined pharmacological treatment (mirtazapine plus methylphenidate). More rigorous research is required to strengthen these findings and better inform clinical practice.
Similar content being viewed by others
Introduction
Cancer is a common and life-threatening chronic disease, with its incidence steadily rising worldwide, despite overall improvements in survival rates [1]. However, within this growing population of cancer survivors, patients often face a substantial disease burden, frequently accompanied by complex comorbidities. Among these comorbidities, depression is a common and serious mental health issue. Statistics indicate that the average prevalence of depression among all cancer patients is 21.2% [2], rising to 24.6% [3] in patients with advanced cancer. Notably, studies have shown that cancer patients with concurrent mental health disorders face a higher risk of mortality and have a shorter life expectancy compared to the general population [4, 5].
Effective management of stress and negative emotion may positively impact tumor initiation, growth, metastasis, and the cancer immunotherapy process through neuroendocrine regulation [6]. Despite the common occurrence of psychological symptoms among cancer patients, these symptoms are often inadequately treated [7, 8]. The prevalence of severe adverse outcomes—including shortened survival, increased suicide risk, more frequent euthanasia requests, and markedly diminished treatment adherence—starkly evidences this therapeutic gap, demanding urgent attention in clinical practice [9].
Addressing the mental health of cancer patients is crucial for improving overall treatment outcomes. Interventions for depression in cancer patients are divided into non-pharmacological treatments, such as group psychosocial interventions (e.g., stress reduction, positive coping, enhanced social support from friends/family, managing physical symptoms and changes, and promoting healthy behavior changes), and individual psychosocial interventions (e.g., cognitive restructuring, behavioral activation, biobehavioral strategies, education, and/or relaxation techniques), as well as psychopharmacological treatments.
Antidepressants, including selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs), are the most commonly prescribed medications for patients with depression. However, randomized controlled trials evaluating the effectiveness of antidepressants in cancer patients are relatively scarce, and their results are often contradictory, leaving the effectiveness of these medications in this population uncertain. The latest meta-analyses [10] suggest that antidepressants may offer potential benefits to cancer patients with depression. Although these analyses generally supports the use of antidepressants in alleviating cancer-related depression, they do not thoroughly investigate the differences in effectiveness among various antidepressants.
In contrast, non-pharmacological treatments are gaining increasing attention as effective interventions for depression in cancer patients. The ASCO guidelines indicate that the evidence supporting the effectiveness of psychosocial interventions is stronger than that for pharmacological treatments [11]. Recent high-quality clinical trials have further validated the effectiveness of these non-pharmacological approaches [12, 13].
Although various interventions have been proposed for managing depression in cancer patients, few trials provide direct head-to-head comparisons, particularly among non-pharmacological treatments. Evidence for pharmacological interventions is also limited and heterogeneous, complicating the synthesis of findings across studies.
To address this, we conducted a Bayesian network meta-analysis to compare the relative effectiveness of multiple treatment options. This method combines direct and indirect evidence within a single analytical framework, enabling comparisons even when head-to-head trials are lacking. Unlike traditional pairwise meta-analyses, the Bayesian approach allows for more flexible modelling and provides treatment rankings based on probability estimates. This analysis aims to inform clinical decision-making by offering a clearer understanding of which interventions may be most effective for managing depression in people with cancer.
Materials and methods
Our systematic review and network meta-analysis adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement (Supplementary Table 1). This study has been registered with PROSPERO (registration number CRD42023465056).
Search strategy
We conducted a systematic review and network meta-analysis without language restrictions. Databases including PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov were searched from their inception until 31 July 2024, with an updated search conducted on 10 January 2025. Detailed search strategies are outlined in Supplementary Table 2. Reference lists from included studies and relevant systematic reviews were also screened to identify additional eligible studies.
Inclusion criteria
Included studies were randomized controlled trials evaluating pharmacological interventions approved by relevant regulatory authorities (e.g., Food and Drug Administration) and non-pharmacological interventions (e.g., psychotherapy, music therapy, exercise therapy) for depressive symptoms in adults with cancer. Editorials, commentaries, case reports, duplicate publications, and studies with incomplete outcome data were excluded.
Data extraction and bias risk assessment
Three reviewers independently reviewed the identified literature and finalised the studies for inclusion. From these studies, we extracted pertinent data encompassing study features (e.g., authors, year of publication), patient demographics (e.g., average or median age, percentage of female patients, education, marital status, initial depression scores), specifics of the disorders described (e.g., type of cancer, cancer stage, period since diagnosis), details of the interventions, and the employed depression rating scales. Two psychiatrists delineated all interventions following the descriptions provided by the original authors. For depression scores, we collected mean changes and their standard deviations. If authors provided pre- and post-intervention measurements, we calculated the mean change and its standard deviation using methods outlined in the Cochrane Handbook. Additionally, we liaised with study authors to source supplementary or hitherto unpublished data absent from the original manuscripts.
The risk of bias was assessed independently by three reviewers using the Cochrane risk-of-bias tool 2.0 (RoB2) [14], with disagreements resolved through discussion and consensus.
Data analysis
The standardized mean difference (SMD) with a 95% confidence interval (CI) was used as the effect size for changes in depression scores.
STATA MP (version 17.0) was used to produce network graphs to assess the connectivity of available evidence. By R software (version 4.2.1) using the gemtc package (versions 1.0-1) and JAGS software (version 4.3.2), the Markov Chain Monte Carlo simulation technique was used in Bayesian network meta-analysis of random-effects models. We used uninformative and normal prior distributions and built four independent Markov chains. For each analysis, we ran 50,000 burn-ins and 100,000 sample iterations to obtain the posterior distribution. Trajectory plots (Supplementary Fig. 1) and Brooks-Gelman-Rubin diagnostic plots (Supplementary Fig. 2) were used to assess the degree of model convergence visually. To rank each intervention, we used the surface under the cumulative ranking curve (SUCRA) to assess the probability of each intervention being the best intervention. An area of 1 is the best, and an area of 0 is the worst. The node-splitting method assessed local inconsistencies within the network, while Egger’s and Begg’s tests were utilized to evaluate publication bias through funnel plot symmetry. In addition, we prespecified summary estimates of year of study publication, proportion of women, sample size, used meta-regression to determine the impact of these potential effect modifiers. Subgroup analyses explored outcomes based on different psychotherapies (symptom management education and coping skills for negative emotions, meaning centered psychotherapy, supportive psychotherapy, cognitive-behavioral therapy, mindfulness, dignity therapy), age (<55 and ≥55 years), cancer type (breast and non-breast cancers), intervention duration (<12 and ≥12 weeks), and baseline depression severity (no or mild and moderate or severe). Sensitivity analyses assessed the stability of results by excluding studies with small sample sizes (<40 participants) or unclear intervention durations.
Furthermore, We conducted a pairwise meta-analysis of head-to-head comparisons using the frequentist approach and compared the results with the corresponding pooled estimates from the Bayesian framework. Statistical heterogeneity were assessed using the inconsistency index (I²). I² values were interpreted as indicating low heterogeneity (<25%), moderate heterogeneity (25–50%), and high heterogeneity (>50%).
Assessment of certainty of evidence
We assessed the certainty of effect estimates for the primary outcomes using the CINeMA (Confidence in Network Meta-Analysis) framework and web-based application [15, 16]. This evaluation covered six key domains: within-study bias (risk of bias), across-study bias (e.g., small-study effects or publication bias), indirectness, imprecision, heterogeneity, and incoherence (i.e., inconsistency between direct and indirect evidence). For each domain, we assigned one of three ratings—no concerns, some concerns, or major concerns—and then integrated these ratings to determine an overall confidence level for each comparison (high, moderate, low, or very low).
Results
Systematic evaluation and characteristics
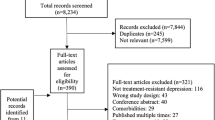
From 37,159 identified citations, we excluded 34,815 after screening titles and abstracts, leaving 2344 full-text articles for detailed review. Of these, 95 randomized controlled trials (RCTs) met our inclusion criteria, comprising 85 non-pharmacological and 10 pharmacological interventions (Fig. 1). These trials enrolled a total of 17,260 cancer patients, evaluating 10 distinct non-pharmacological and 10 pharmacological intervention strategies. Classifications of non-pharmacological interventions are presented in Supplementary Table 3. Participants in most trials had a mean age under 65 years, with a majority being female. Intervention durations predominantly ranged up to 12 weeks (Table 1). No significant differences in baseline characteristics among studies were observed, supporting our assumption of transitivity. Supplementary Tables 4 and 5 outline individual study characteristics, and patient baseline data is detailed in Supplementary Table 6. Risk of bias assessments are summarized in Supplementary Fig. 3.
Heterogeneity, inconsistency, and reporting bias
Pairwise meta-analyses under the frequentist framework and network meta-analyses using the Bayesian framework are detailed in Supplementary Table 7. Significant heterogeneity was detected among non-pharmacological interventions, notably psychotherapy versus usual care (I² = 88.10%), education and support versus usual care (I² = 69.90%), and education and support versus psychotherapy (I² = 74.50%). For pharmacological interventions, substantial heterogeneity appeared in the comparison of paroxetine versus placebo (I² = 53.90%). The node-splitting method revealed inconsistencies between direct and indirect evidence for psychotherapy versus usual care (P = 0.02) and education and support versus usual care (P = 0.04) (Supplementary Table 8). Although potential publication bias existed among non-pharmacological interventions, robustness analyses using the Metatrim method confirmed persistent statistically significant intervention effects after bias correction (Supplementary Fig. 4).
Comparisons of depression change
We conducted a primary network meta-analysis comparing the effectiveness of non-pharmacological and pharmacological interventions in reducing depressive symptoms among cancer patients. 85 studies involving 16,128 participants evaluated non-pharmacological interventions, while 10 studies with 1132 participants assessed pharmacological approaches (network diagram: Fig. 2). Our findings indicate that non-pharmacological interventions, including massage and touch therapy (SMD: −0.76; 95% CI: −1.37 to −0.16; low certainty), relaxation therapy (SMD: −0.59; 95% CI: −1.06 to −0.11; low certainty), psychotherapy (SMD: −0.43; 95% CI: −0.56 to −0.30; low certainty), and education and support of person with cancer (SMD: −0.30; 95% CI: −0.45 to −0.14; low certainty), significantly reduced depressive symptoms compared to usual care. Among pharmacological interventions, only mirtazapine combined with methylphenidate showed a statistically significant improvement relative to placebo (SMD: −2.46; 95% CI: −4.24 to −0.70; low certainty) (Fig. 3).
A: Network diagram of effectiveness of non-pharmacological interventions designed to reducing depressive symptoms in cancer patients. B: Network diagram of effectiveness of pharmacological interventions designed to reducing depressive symptoms in cancer patients. Each intervention is represented as a node, and direct comparisons of the two interventions in the RCT are shown as links between nodes. The size of the nodes reflects the number of patients treated, and the width of each edge is proportionally weighted according to the number of comparisons.
Summary of pooled standardized mean differences (SMD) (95% credible intervals) for non-pharmacological interventions (upper triangle) and pharmacological interventions (lower triangle). Data in each cell are SMD (95% credible intervals) for the comparison of column-defining treatment versus row-defining treatment (upper triangle) and SMD (95% credible intervals) for the comparison of row-defining treatment versus column-defining treatment (lower triangle). Ranking first means this treatment provides the best effectiveness.
Rankings
Rankings based on probabilities and SUCRA values are detailed in Supplementary Tables 9 and 10, supporting the pooled results. Non-pharmacological interventions with higher rankings included massage and touch therapy (SUCRA: 0.86), relaxation therapy (SUCRA: 0.75), and psychotherapy (SUCRA: 0.65). Among pharmacological interventions, mirtazapine combined with methylphenidate (SUCRA: 0.97) and mianserin (SUCRA: 0.77) had higher rankings.
Meta-regression and sensitivity analysis
Meta-regression analyses revealed no significant influence of publication year, proportion of females, or sample size on outcomes (Supplementary Table 11). Sensitivity analyses excluding potential confounders did not alter primary conclusions, confirming robustness of results. Supplementary Figs. 5 and 6 illustrate network diagrams and detailed analyses from these assessments.
Evidence confidence assessment
The evidence assessment using the CINeMA approach is detailed in Supplementary Tables 12 and 13, including the number of primary studies contributing to each treatment comparison. Most comparisons were informed by only one to three trials, except for psychotherapy versus usual care (n = 37) and education and support versus usual care (n = 22).
Subgroup analysis
We performed subgroup analyses based on psychotherapy type, patient age, cancer type, intervention duration, and baseline depression severity:
Psychotherapy type
Reminiscence therapy, cognitive behavioural therapy, symptom management education and coping skills for negative emotions, meaning-centered psychotherapy, and mindfulness interventions were significantly superior to usual care (Supplementary Figs. 7, 8).
Age
In patients under 55 years, massage and touch, relaxation therapy, and psychotherapy significantly improved depressive symptoms compared to usual care. No pharmacological treatments demonstrated superiority. For patients aged 55 years and older, psychotherapy and education and support of person with cancer outperformed usual care; again, no pharmacological interventions showed advantage (Supplementary Figs. 9, 10).
Cancer type
Among non-breast or mixed cancer populations, psychotherapy and education and support of person with cancer were effective compared to usual care, while pharmacological interventions were not significantly better than placebo. In breast cancer patients, relaxation therapy, psychotherapy, and education and support of person with cancer were superior to usual care, with no significant pharmacological benefit (Supplementary Figs. 11, 12).
Intervention duration
For interventions lasting less than 12 weeks, massage and touch, relaxation therapy, and psychotherapy demonstrated effectiveness. For interventions of 12 weeks or longer, psychotherapy, education and support of person with cancer, and exercise significantly reduced depressive symptoms compared to usual care. For pharmacological interventions lasting less than 12 weeks, none showed significant superiority over placebo. For interventions of 12 weeks or longer, only two studies were available, limiting detailed analysis (Supplementary Figs. 13, 14).
Depression severity
In patients with mild or no depression, relaxation, massage, and psychotherapy significantly outperformed usual care, whereas pharmacological treatments showed no advantage. For moderate to severe depression, psychotherapy was significantly effective (Supplementary Figs. 15, 16).
Discussion
In this systematic review and network meta-analysis, we explored a variety of interventions aimed at reducing depressive symptoms in adults with cancer. The analysis included data from 95 clinical trials: 85 trials evaluating non-pharmacological interventions and 10 assessing pharmacological treatments. These studies collectively involved 17,260 patients, comprising 16,128 participants assigned to non-pharmacological interventions and 1132 to pharmacological interventions. To our knowledge, this is among the first network meta-analyses comparing the effectiveness of both non-pharmacological and pharmacological interventions for managing depression in cancer patients, providing an indicative ranking based on their relative effectiveness. Nevertheless, it is essential to acknowledge the limitations in available evidence for certain interventions due to the small number of studies.
Our analysis suggested that several non-pharmacological strategies, such as massage and touch therapy, relaxation therapy, psychotherapy, and educational and support programs for cancer patients, were associated with improvements in depressive symptoms compared with usual care. Among pharmacological treatments, the combination of mirtazapine and methylphenidate appeared to provide notable benefits over placebo, though the certainty of this evidence was limited. Subgroup analyses revealed notable variability in outcomes, suggesting that certain non-pharmacological interventions may be especially effective in specific contexts. Psychotherapy consistently demonstrated high efficacy across all subgroups, supporting its role as a foundational element of psychosocial care. Evidence-based modalities—such as cognitive behavioural therapy, symptom management and emotional coping skills training, meaning-centred psychotherapy, and mindfulness—should be prioritised in routine clinical practice. Beyond psychotherapy’s broad utility, our analysis identified opportunities for further targeted intervention: massage and touch therapy was most effective for patients younger than 55 years and for shorter-term interventions, while relaxation therapy showed optimal benefits for breast cancer patients and those with mild or no depression. In these specific instances, clinicians might prioritize these indicated therapeutic modalities as frontline approaches, with psychotherapy serving as a robust complementary option, always tailored to individual patient needs and preferences. Over the past few decades, numerous studies have evaluated non-pharmacological treatments in cancer patients, finding that interventions such as psychotherapy [17,18,19,20,21,22], relaxation therapy [23], massage [24], and touch therapy can significantly alleviate depressive symptoms. These findings are consistent with our results, further emphasizing the importance of these interventions as part of a comprehensive approach to cancer treatment. Given the variety of psychotherapeutic approaches, we differentiated between different therapeutic modalities and found that reminiscence therapy, cognitive-behavioral therapy, symptom management, psychoeducation for coping with negative emotions, meaning-centered psychotherapy, and mindfulness therapy all significantly outperformed usual care, further highlighting the effectiveness of psychotherapies. Notably, our findings also indicate that educational and support programs for cancer patients are effective in alleviating depressive symptoms, which aligns with the growing body of evidence in the digital health era [25], where e-health interventions have shown promise in supporting the mental health of cancer patients. However, despite previous meta-analyses demonstrating that music and art therapy [26, 27], as well as yoga [28, 29], can significantly reduce depressive symptoms, these interventions did not show the same benefits in our study. Lastly, the existing literature provides inconsistent evidence regarding the effectiveness of exercise interventions. Although researchers such as Abdul Salam [30], Lynette L. Craft [31], and Cho Yin Joyce Law [32] have highlighted the significant benefits of exercise therapy in alleviating depressive symptoms in cancer patients, this was not confirmed by Joke Bradt’s [33] study. Consistent with this, our study also did not observe any significant improvements in depressive symptoms related to exercise interventions. However, in further subgroup analysis, we found that exercise interventions lasting more than 12 weeks could alleviate depressive disorders in cancer patients, suggesting that further exploration of the potential benefits of exercise is warranted.
For pharmacological interventions, previous meta-analyses have provided preliminary evidence on the effectiveness of antidepressants in treating depressive symptoms among cancer patients. Riblet et al.’s [34] systematic review compared the effects of antidepressants and placebo across different cancer types and stages, suggesting that specific drugs, such as paroxetine, fluoxetine, and mianserin, may have advantages in alleviating cancer-related depression. In a recent 2023 meta-analysis, Ostuzzi and colleagues [10] supported the potential benefits of antidepressants for patients with cancer-related depression. In our own study, we analyzed the use of antidepressants and found that, in preliminary research, only a specific combination of two drugs showed a trend toward greater effectiveness compared to either drug used alone, although the certainty of evidence was low. This finding is particularly noteworthy as it suggests that certain drug combinations may be more effective than monotherapy, offering a potential new avenue for clinical practice.
Previous meta-analyses typically focused on specific pharmacological or non-pharmacological interventions for a single cancer type, limiting the generalizability of their findings. To address this limitation, our network meta-analysis included a broad range of interventions across various cancer types and stages, providing a relative ranking of their effectiveness. However, many treatment comparisons were supported by only one or two studies, increasing the risk of small-study effects and potentially leading to overestimation of effect sizes. Health professionals should therefore interpret such findings with caution—particularly for interventions based on limited evidence—and prioritise those supported by more consistent and robust data when making treatment decisions for patients with cancer-related depression.
These results have important implications for clinical decision-making. The effectiveness of non-pharmacological interventions suggests they should be considered as key components of depression management in cancer care, particularly because of their generally favorable safety profiles and patient acceptability. In cases where depressive symptoms are more severe, pharmacological treatments like mirtazapine and methylphenidate may be appropriate, though careful consideration of the evidence and individual patient circumstances is essential.Our study also emphasizes the importance of early psychological screening and timely intervention in cancer care. Tailoring depression management strategies to the specific needs of cancer patients, considering factors such as age, cancer type, and depression severity, could lead to better outcomes and improved quality of life.Additionally, the long-term sustainability of these interventions warrant further investigation to guide more informed clinical decisions.
Our findings contribute to the ongoing effort to integrate effective mental health interventions into cancer care. By prioritizing evidence-based non-pharmacological strategies and carefully evaluating pharmacological options, healthcare providers can better support the psychological well-being of cancer patients and potentially enhance their overall outcomes.
Limitations of this review
This study has several limitations. Firstly, maintaining blinding for participants and providers in non-pharmacological intervention studies presents significant challenges [35]. The inability to achieve full blinding inevitably introduces observer bias, potentially compromising the objectivity of intervention effects and the reliability of study results. This potential bias must be carefully considered when interpreting the findings. Secondly, although disease severity is a critical factor influencing depressive symptoms, most included studies lack consistent and comparable tumor staging information, impeding stratified analyses based on disease severity. This limitation restricts our understanding of patient responses across different stages of the disease. Thirdly, the lack of standardized classification for non-pharmacological interventions adds to this complexity. Although the ten intervention categories were determined after careful deliberation by several psychiatrists, the classification remains somewhat subjective. Fourth, some studies did not report extractable outcome data, preventing their inclusion in the analysis. Although we contacted the authors for additional information, not all responded or provided usable data. This may have reduced the completeness of the evidence and introduced selection bias. Furthermore, the unusually large effect sizes observed for some interventions, such as the combination of mirtazapine with methylphenidate, raise concerns about the reliability and generalizability of these results. These effect sizes are notably higher than those typically reported for antidepressant treatments. Several factors could contribute to these discrepancies, including potential confounding effects of antidepressants on pain management in cancer patients, as well as the unique context of cancer-related depression, where symptomatology and treatment responses may differ from the general population.
Conclusion
Our network meta-analysis offers a comprehensive evaluation of the effectiveness of both pharmacological and non-pharmacological interventions for alleviating depressive symptoms in adult cancer patients. The findings of this study not only have the potential to influence current clinical practice but also to shape future treatment strategies significantly. We identified that certain interventions, both pharmacological and non-pharmacological, may provide substantial benefits to cancer patients. Consequently, we recommend that interdisciplinary teams—including professionals from oncology, psychiatry, nursing, sociology, and related fields—collaborate closely to develop integrated and coordinated treatment plans for these patients. Overall, our study offers preliminary evidence to guide healthcare providers in selecting appropriate treatment strategies to improve depressive symptoms in cancer patients. Additionally, our findings highlight key research priorities, emphasizing the need for direct comparative randomized controlled trials between non-pharmacological and pharmacological interventions, as well as combination therapies. Moreover, high-quality, large-scale trials are particularly crucial for evaluating the effectiveness of new therapeutic approaches in this area.
Modification of the protocol
If we need to amend this protocol, we will give the date of each amendment, describe the change and give the rationale in this section. Changes will not be incorporated into the protocol.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Riedl D, Schuessler G. Prevalence of depression and cancer - a systematic review. Z Psychosom Med Psychother. 2022;68:74–86.
Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–74.
Wang YH, Li JQ, Shi JF, Que JY, Liu JJ, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. 2020;25:1487–99.
Zeng Y, Hu CH, Li YZ, Zhou JS, Wang SX, Liu MD, et al. Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer. Nat Med. 2024;30:1680–88.
Chang A, Sloan EK, Antoni MH, Knight JM, Telles R, Lutgendorf SK. Biobehavioral pathways and cancer progression: insights for improving well-being and cancer outcomes. Integr Cancer Ther. 2022;21:15347354221096081.
Carlson LE, Waller A, Groff SL, Giese-Davis J, Bultz BD. What goes up does not always come down: patterns of distress, physical and psychosocial morbidity in people with cancer over a one year period. Psychooncology. 2013;22:168–76.
Limb M. Three in four cancer patients with depression are not getting adequate treatment, studies find. BMJ. 2014;349:g5358.
Kissane DW. Unrecognised and untreated depression in cancer care. Lancet Psychiatry. 2014;1:320–1.
Vita G, Compri B, Matcham F, Barbui C, Ostuzzi G. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2023;3:CD011006.
Carlson LE, Ismaila N, Addington EL, Asher GN, Atreya C, Balneaves LG, et al. Integrative oncology care of symptoms of anxiety and depression in adults with cancer: society for integrative oncology-ASCO guideline. J Clin Oncol. 2023;41:4562–91.
Lepore SJ, Buzaglo JS, Lieberman MA, Golant M, Greener JR, Davey A. Comparing standard versus prosocial internet support groups for patients with breast cancer: a randomized controlled trial of the helper therapy principle. J Clin Oncol. 2014;32:4081–6.
Dionne-Odom JN, Azuero A, Lyons KD, Hull JG, Tosteson T, Li Z, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III Randomized Controlled Trial. J Clin Oncol. 2015;33:1446–52.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16:e1080.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082.
Faller H, Schuler M, Richard M, Heckl U, Weis J, Kuffner R. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013;31:782–93.
Oberoi S, Yang J, Woodgate RL, Niraula S, Banerji S, Israels SJ, et al. Association of mindfulness-based interventions with anxiety severity in adults with cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2012598.
Piet J, Wurtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta-analysis. J Consult Clin Psychol. 2012;80:1007–20.
McCloy K, Hughes C, Dunwoody L, Marley J, Gracey J. Effects of mindfulness-based interventions on fatigue and psychological wellbeing in women with cancer: a systematic review and meta-analysis of randomised control trials. Psychooncology. 2022;31:1821–34.
Liu T, Xu J, Cheng H, Zhang Y, Wang S, Lin L, et al. Effects of internet-based cognitive behavioral therapy on anxiety and depression symptoms in cancer patients: a meta-analysis. Gen Hosp Psychiatry. 2022;79:135–45.
Li J, Li C, Puts M, Wu YC, Lyu MM, Yuan B, et al. Effectiveness of mindfulness-based interventions on anxiety, depression, and fatigue in people with lung cancer: A systematic review and meta-analysis. Int J Nurs Stud. 2023;140:104447.
Han J, Cheng HL, Bi LN, Molasiotis A. Mind-body therapies for sleep disturbance among patients with cancer: a systematic review and meta-analysis. Complement Ther Med. 2023;75:102954.
Tian EJ, Veziari Y, Leach MJ, Kumar S. The effectiveness of reflexology on mental health in cancer patients: a systematic review and meta-analysis of randomised controlled trials. Complement Ther Clin Pract. 2023;50:101708.
Singleton AC, Raeside R, Hyun KK, Partridge SR, Di Tanna GL, Hafiz N, et al. Electronic health interventions for patients with breast cancer: systematic review and meta-analyses. J Clin Oncol. 2022;40:2257–70.
Bradt J, Dileo C, Magill L, Teague A Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev 2016;CD006911. https://doi.org/10.1002/14651858.CD006911.pub4
Li Y, Xing X, Shi X, Yan P, Chen Y, Li M, et al. The effectiveness of music therapy for patients with cancer: a systematic review and meta-analysis. J Adv Nurs. 2020;76:1111–23.
Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802.
Gonzalez M, Pascoe MC, Yang G, de Manincor M, Grant S, Lacey J, et al. Yoga for depression and anxiety symptoms in people with cancer: a systematic review and meta-analysis. Psychooncology. 2021;30:1196–208.
Salam A, Woodman A, Chu A, Al-Jamea LH, Islam M, Sagher M, et al. Effect of post-diagnosis exercise on depression symptoms, physical functioning and mortality in breast cancer survivors: a systematic review and meta-analysis of randomized control trials. Cancer Epidemiol. 2022;77:102111.
Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:3–19.
Law CYJ, Yu THJ, Chen T. Effectiveness of aerobic and resistance exercise in cancer survivors with depression: a systematic review and meta-analysis of randomized controlled trials. J Psychosom Res. 2023;173:111470.
Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2015;1:CD007103.
Riblet N, Larson R, Watts BV, Holtzheimer P. Reevaluating the role of antidepressants in cancer-related depression: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2014;36:466–73.
Boutron I, Guittet L, Estellat C, Moher D, Hrobjartsson A, Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 2007;4:e61.
Funding
the National Key Research & Development Programme (2024ZD0520004, 2024ZD0520000, 2022YFC2505105); China National Science Foundation (Grant No. 82373121); the Science and Technology Planning Project of Guangzhou (grant number 2024A03J1222); Supported by Major Project of Guangzhou National Laboratory (Grant No. GZNL2023A02007, SRPG22-017).
Author information
Authors and Affiliations
Contributions
WHL and KGL obtained funding. WHF, JXH, KGL and WHL designed the study. YXL, and YJ collected the data. HJC, RBS and PLC analyzed the data. XZ and CCL drafted the manuscript. WHF, YXL, YJ and HJC contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors have read and approved the final manuscript. KGL and WHL are the study guarantors.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare: no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, W., Liu, Y., Jiang, Y. et al. Treatments of depressive symptoms in cancer patients: A systematic review and network meta-analysis. Transl Psychiatry 15, 327 (2025). https://doi.org/10.1038/s41398-025-03507-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03507-z