Abstract
Prenatal maternal stress (PNMS), anxiety, and depression are associated with altered trajectories of infant socio-emotional and brain development, including the amygdala and prefrontal cortex (PFC). During the COVID-19 pandemic, prenatal anxiety and depression was significantly elevated, yet the impact on infant neurodevelopment remains uncertain. The objective of this study was to determine whether PNMS and mental health during the pandemic was associated with infant amygdala and PFC volumes as well as temperament. Participants were enrolled in the Canadian ‘Pregnancy during the COVID-19 Pandemic’ cohort study. Pregnant individuals had their perceived stress, pandemic-related objective hardship, and mental health measured via questionnaires. Infant magnetic resonance imaging (MRI) scans (n = 100) were conducted at 3 months of age, and parents reported on infant temperament at 6 months of age. General linear models were used to examine the associations among PNMS, mental health, brain volumes, and developmental outcomes. Prenatal maternal anxiety negatively predicted 3-month left infant amygdala volumes (B = −5.919; p = 0.016; 95% CI, −10.748 to −1.089). Smaller left amygdala volumes were associated with greater infant 6-month negative affectivity (B = −0.003; p = 0.002; 95% CI, −0.006–−0.001). This study provides evidence for infant brain alterations related to prenatal maternal anxiety, indicating that the impact of anxiety on infant development during the COVID-19 pandemic may have long-lasting implications for children’s health. Our findings suggest that prenatal anxiety may be a key area for screening and intervention during pregnancy to best support healthy infant development.
Similar content being viewed by others
Introduction
At the beginning of March 2020, a multitude of public health measures were implemented in Canada to combat the spread of the severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), including changes to prenatal care [1,2,3]. Pregnant individuals were faced with uncertainties and challenges, leading to higher perinatal anxiety and depression symptoms during the pandemic compared to pre-pandemic levels [4,5,6,7]. This is concerning given that prenatal maternal stress (PNMS) and prenatal mental health is associated with negative health outcomes for both pregnant individuals and their future children [8, 9]. PNMS is linked to poorer neurodevelopment, cognitive development, temperament, and behavioral problems in childhood as well as negative birth outcomes [10, 11].
PNMS due to an adverse event can be broken down into two components: objective hardship (i.e., level of exposure to an external stressor) and a more subjective psychological component of perceived stress (e.g., worries regarding infection and its impact on the fetus). In addition to stress indicators, distress indicators also exist. Pregnant individuals are at risk for psychological distress, including prenatal anxiety and depression, especially in conjunction with stressful life experiences [12]. While prenatal anxiety and depression can often be comorbid with each other, they are also both distinct constructs [12, 13]. Depression measures the presence of sad, empty, or irritable mood, loss of interest in pleasurable activities, feelings of worthlessness, and thoughts of death. On the other hand, anxiety assesses an individual’s level of worries, nervousness, and trouble relaxing [13]. Anxiety and depression symptoms vary depending on the individual, leading to different trajectories: anxiety dominant, depression dominant, or anxiety and depression comorbidity [14]. Prenatal maternal stress, anxiety, and depression can all impact social-emotional development in infants and children. Prenatal anxiety and depression have been associated with both higher negative affectivity (i.e., sadness, fearfulness, distress) and difficult temperament (i.e., excessive crying, irritability, slow adaptability) in infants and toddlers [11, 15, 16]. However, both constructs may impact child development differently, with comorbid anxiety and depression potentially having the greatest effects [11, 14, 17, 18]. In the 1998 Quebec Ice storm study, higher prenatal subjective stress and maternal illness/infection (e.g., flu, fever, pre-eclampsia) were associated with negative aspects of temperament (e.g., fussy/difficult, dullness, needs attention) in 6-month infants [19]. Similarly, higher prenatal maternal objective hardship due to the 2011 Queensland floods was associated with lower problem solving skills, more difficult temperament (moderated by infant sex and gestational age at the time of the flood), and marginally associated with lower personal-social skills in infants at 6-months of age [20, 21]. Additionally, greater levels of both forms of PNMS have been associated with higher levels of internalizing, externalizing, and other psychiatric problems in childhood, mostly independent of postnatal mood [11, 22].
Stressful maternal experiences during pregnancy influence the uterine environment and alter the development of the fetal central nervous system, which can then “set probabilistic parameters for future brain and behavior development” in infancy and childhood [8]. Particularly, PNMS has been associated with differences in amygdala as well as prefrontal cortex (PFC) volumes in children [9]. Both the amygdala and PFC, as well as the white matter fibers connecting the two brain regions, play a key role in emotional regulation [23,24,25]. The amygdala is a bilateral structure of the limbic system. Psychological distress in mid-pregnancy was negatively related to left amygdala volumes in newborn males [26]. At the same time, higher levels of depression and maternal cortisol (a biomarker of physiological stress) in the second trimester have been associated larger right amygdala volumes in girls during childhood, where amygdala volumes partially mediated the effect of high cortisol levels on affective problems in girls [27, 28]. Furthermore, Jones et al., [29] found that larger amygdala volumes mediated the association between greater objective hardship due to the 1998 Quebec ice storm and externalizing symptoms in 11-year-old girls [29]. One study, however, found no association between prenatal depression and amygdala volumes in neonates [30]. Smaller amygdala volumes have been associated with greater levels of internalizing problems in children [31, 32], although other studies reported the opposite association [33, 34].
The PFC is a region of the cerebral cortex that is heavily involved in social-emotional and cognitive processes, which emerge in early infancy [35, 36]. Psychological distress has also been associated with reductions in gray matter volume of the frontal lobes in children [9]. For example, Buss et al. [37] found that higher pregnancy anxiety at 19 weeks of gestation was associated with gray matter volume reductions in the prefrontal cortex of 6–9 year old children, independent of postnatal stress [37]. Maternal depression at 25 gestational weeks was associated with cortical thinning of the entire cortex, and particularly the frontal lobes in children. Cortical thinning of the PFC mediated the association between prenatal depression and child externalizing behaviors [38]. However, by studying volume, we can gain a better understand of the impact of PNMS on the PFC more broadly. Volume is a more comprehensive measure of structural integrity than thickness, as it also takes into account surface area, thickness, gyrification, and overall size [39, 40]. At birth, cortical thickness is more developed than surface area. While cortical thickness reaches adult levels at around 1 year of age, surface area continues to grow and accounts for most of the cortical volume growth after 1 years old [41]. Furthermore, research has shown that PFC volume strongly correlates with differences in child and young adult outcomes, including executive functioning and mental health (e.g., depression, schizophrenia, suicidality) [42,43,44,45,46].
A variety of mechanisms have been proposed to explain the associations of PNMS with brain development and behavior, including maternal inflammation, altered placental functioning, and greater fetal cortisol exposure [8, 47]. Abnormal or inappropriate levels of maternal cortisol due to hypothalamic-pituitary-adrenal (HPA) axis activation caused by high stress can have a neurotoxic effect on the fetus’ developing brain [8, 9]. The amygdala in the fetus is rich with cortisol receptors and may be vulnerable to maternal activation of the HPA axis [48]. PNMS mid-pregnancy may have the strongest impact on infant neuroanatomy [26]. However, a positive postnatal environment, such as a sensitive mother-infant attachment, may alter neurodevelopmental changes associated with PNMS [9].
Recent studies have examined infant neurodevelopment during the COVID-19 pandemic. More severe pandemic-related prenatal maternal stress has been linked to greater negative affect and changes to amygdala-prefrontal functional connectivity in infancy [49, 50]. Greater prenatal maternal distress during the pandemic was associated with delayed social-emotional development in infants at 2-months [51]. Further, infants born during the pandemic have been shown to have higher levels of negative affectivity and a higher risk of communication and personal-social impairment compared to pre-pandemic [52,53,54].
It is of utmost importance to study the impact of PNMS on infant development. However, there is a dearth of studies examining the effect of pandemic-related PNMS and psychological distress on infant brain development. To fill this gap, we investigated the association between PNMS, psychological distress, infant temperament, and brain development, with a particular focus on the amygdala and PFC. Our study had two main objectives; first, to investigate the association among PNMS (prenatal objective hardship and perceived stress), mental health (prenatal depression and anxiety), and infant amygdala/PFC volumes. The second aim was to determine whether amygdala and PFC volumes are associated with temperament in infants.
We hypothesized that greater prenatal stress (objective hardship and perceived stress) and higher levels of maternal mental health symptomatology (depression and anxiety) would predict larger amygdala volumes and smaller PFC volumes. Secondly, we hypothesized that larger amygdala volumes and smaller PFC volumes will be associated with more challenging temperaments in infants.
Methods and materials
Procedure & participants
Data were collected as part of the pan-Canadian Pregnancy During the COVID-19 Pandemic (PdP) Study. The PdP study was a prospective longitudinal cohort study that included multiple follow-up surveys completed during the prenatal and postpartum period. Recruitment was conducted between April 5th 2020 and April 30th 2021. The initial online survey was advertised on social media platforms. To be eligible for the study, individuals must have been pregnant, ≥17 years of age, living in Canada, able to answer questions in English or French, and ≤35 weeks of gestation at recruitment [55].
The initial survey was completed by participants during pregnancy using REDCap (Research Electronic Data Capture; [56]). It collected their demographic, socioeconomic, and obstetric characteristics (e.g., age, ethnicity, household income, health before and during pregnancy), in addition to their mental health, pandemic-related hardships, and other measures. Participants were asked to complete a follow-up survey once a month for the first three months, then every other month during their prenatal period. In total, participants could have completed a maximum of five prenatal follow-up surveys, which assessed their experiences since the last survey. Once a participant gave birth, they reported on their pregnancy outcomes, such as their mode of delivery as well as their infant’s birthweight, length, and gestational age. During the postpartum period, participants were sent follow-up surveys when their child reached 3, 6, and 12 months of age.
The PdP study also included neuroimaging in the Calgary area. Infants were scanned at Alberta Children’s Hospital using magnetic resonance imaging (MRI) at 3 months of age. Only infants born at full-term (>36 weeks) were eligible for neuroimaging. Infants were excluded if they had a major birth complication or were diagnosed with any severe genetic or neurologic conditions. 506 families/infants were invited for imaging at 3 months. 140 scans were scheduled. 119 infants attempted imaging; however, 18 scans were unsuccessful as the infants woke up, were crying, and/or were not sleeping.
All pregnant individuals who participated in the study provided informed consent. All participants signed an electronic informed consent form before starting the first questionnaire; at the time of the MRI, parents provided consent for infants. The study procedures were conducted in alignment with the Declaration of Helsinki.
Participants who completed the prenatal surveys were entered in a monthly draw for a gift card. Individuals who also participated in the follow-up surveys and imaging part of study received a small stipend. The data were manually checked for bots and invalid responses. This project received ethical approval from the University of Calgary Conjoint Health Research Ethics Board (REB20-0500).
Measures
Objective hardship
The PdP study team developed the Pandemic Objective Hardship Index (POHI) to measure participants’ level of objective hardship due to the pandemic over the duration of pregnancy [57]. This measure has four subscales: Scope, Loss, Threat, and Change, each with a maximum score of 50 points. Scope refers to the duration and intensity of the hardship. Loss (i.e., financial, social or physical loss), Threat (i.e., physical and health-related consequences of the pandemic), and Change (i.e., changes to daily routines, prenatal care, work, and social interactions) were also measured. The sum of all four subscales creates the total score, with a maximum of 200 points.
Perceived stress
At final follow-up, the Perceived Stress Scale (PSS-10) was used to measure participants’ subjective level of psychological stress during the previous month. Participants are asked 10 questions regarding how often they appraised their life as “unpredictable, uncontrollable, and overloaded” during the first prenatal follow-up questionnaire [58]. Each item is scored from 0 (never) to 4 (often), with total scores ranging from 0–40. Higher scores indicate a greater level of perceived stress. The PSS-10 has high internal consistency (coefficient alpha reliability = 0.84–0.86) and validity. This scale is strongly correlated with other mental health measures, such as Beck Depression (r = 0.67) and Anxiety Inventory (r = 0.58) [58, 59].
Depression
At recruitment, the Edinburgh Postpartum Depression Scale (EPDS) measured prenatal depression symptoms over the past week [60]. The EPDS contains 10 items, each scored on a scale from 0–3. Total scores range from 0–30, where a higher score indicates a greater level of depressive symptoms. Individuals who score above the clinical cut-off score of ≥13 are at risk for depressive disorder. The EPDS has good reliability, with a split-half reliability of 0.88 and a standardized alpha coefficient of 0.87 [61, 62].
Anxiety
The Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety–Adult Short Form at recruitment measured participants’ general anxiety symptoms within the past week through a 7-item questionnaire [63]. Raw scores were converted to T-scores using the US general population norms. Possible T-score values ranged from 36.3–82.7 with a mean of 50 (SD = 10). T-scores between 60–69.9 indicate moderate anxiety levels, while scores ≥70 indicate severely elevated anxiety levels [64].
Infant temperament
Infant temperament was measured using the Infant Behavior Questionnaire–Revised Very Short Form (IBQ-R) at 6-months of age [65, 66]. There are 37 items total, each asking parents to report on specific infant behaviors over the past week. The IBQ-R assesses three dimensions of temperament: negative affectivity, positive affectivity/surgency, and regulatory capacity/orienting. Parents score each item on a 7-point Likert scale ranging from 0 (never) to 7 (always), where higher scores indicate higher levels of each dimension of temperament. This scale has strong psychometric properties, with good overall test-retest reliability (r = 0.54–0.93), interparent agreement averaging r = 0.41, and high internal consistency (Cronbach alpha > 0.70), with specific scale internal consistencies of 0.81 (negative emotionality), 0.80 (positive affectivity), and 0.74 (Orienting/Regulatory Capacity) [66].
Image acquisition & analysis
100 infants were scanned at 3 months of age using a GE 3T MR750w MRI with a 32-channel head coil at the Alberta Children’s Hospital to acquire brain imaging data. All infants were scanned while asleep atop an inflatable MedVac infant scanning bed. T1-weighted images were obtained (repetition time = 5200 ms, echo time = 2200 ms, inversion time = 540 ms, field of view = 1900 mm, matrix = 512 × 512, bandwidth = 41.67, voxel 1 × 1 × 1 mm3, flip angle = 12°, 136 slices, total time = 3:32). The brain images were rotated according to the standard atlas and subsequently segmented using infant Freesurfer (https://surfer.nmr.mgh.harvard.edu/). This program uses the intensity of each voxel to estimate the probability of whether it belongs to a particular brain structure [67]. 97 brain scans were successfully processed by FreeSurfer. One participant’s MRI data was removed from the study due to extremely high levels of motion in the image, which was determined through visual inspection. No quantitative motion metrics were used. All amygdala segmentations were visually inspected for segmentation quality by a trained expert. Amygdala volumes that were identified as outliers, where their masks were smaller or larger than they should have been, were subsequently edited for segmentation errors. If FreeSurfer over/underestimated the boundaries of the amygdala, the edges of the masks were outlined or erased using manual segmentation tools. The masks were reviewed from axial, sagittal, and coronal views to ensure that they did not overlap with other structures. The PFC volumes were manually segmented using ITK-SNAP. The cross-hairs were aligned by the corresponding section that, in a brain aligned in Talairach space, would be y = 26, as described by Rajkowska & Goldman-Rakic [68]. Only certain brain regions were selected to be visible (e.g., frontal pole, caudal middle frontal, lateral/medial orbitofrontal, pars opercularis/triangularis/orbitalis). The area anterior to the cross-hairs were selected as the PFC volume. Two PFC segmentations were removed from the study due to extremely large holes (missing sections) in their segmentations.
Covariates
Covariates included maternal education (i.e., highest education obtained), as well as infant sex, gestational age at birth, and total cerebral volume [TCV]. Age at scan and ethnicity were added as covariates to models where these variables were correlated with the dependent variable. Ethnicity is an important covariate to include in temperament models. Research has shown that temperament varies between cultures and countries. For example, negative affectivity is reportedly higher in Asian and South American countries, who often value collectivism, compared to European countries [69].
Data analysis
Statistical analyses were conducted using IBM SPSS (v26, Statistics for the Social Sciences) and the PROCESS Macro [70].
To address our first aim, generalized linear models (GLM), using identity link functions, were used to determine the association between PNMS (objective hardship and perceived stress) and mental health (anxiety and depression) during pregnancy and infant brain volumes (left/right amygdala and total PFC volumes) at 3 months of age. In a basic model, infant brain volumes were the dependent variables and objective hardship as well as perceived stress were the independent variables, adjusting for biological sex, parent education, gestational age at birth, and TCV. In a subsequent extended model, maternal anxiety and depression and their interaction were added to the models to determine their moderating effects of maternal mental health on infant brain volume and PNMS. Models were run for the left and right amygdala, and PFC separately. As two brain regions were assessed, the models were Bonferroni corrected for multiple comparisons (p = 0.05/2 or 0.025). Missing data were handled through listwise deletion.
To address our second aim, GLMs were used to determine the association between infant brain volumes (i.e., amygdala and PFC) at 3 months and each of the three subscales (negative affectivity, positive affectivity/surgency, and regulatory capacity/orienting) of temperament at 6 months of age assessed with the IBQ-R, adjusting for biological sex, maternal education, gestational age at birth, maternal ethnicity, and TCV. As we assessed three subscales on the IBQ-R the models were corrected for multiple comparisons using the Bonferroni method (p = 0.05/3 or 0.0125).
Results
Participants
A total of 100 mother-infant dyads (mean maternal age = 33.3 years old, SD = 4.33) were recruited from the Calgary area during the COVID-19 pandemic. Participants completed the recruitment questionnaires at an average of 18.3 weeks (SD = 16.38) after the initial Canadian country-wide shut down period on March 11, 2020. Approximately one third (28.6% [n = 28]) of individuals were enrolled in the study in the first trimester of pregnancy, 45.9% (n = 45) were in the second trimester, and 25.5% (n = 25) were in the third trimester. The majority of our participants were white (Caucasian), married or living with their partner, had a university education, and had an annual household income >$70,000 CAD. Approximately half of participants (53.5% [n = 53]) did not have any other children. At intake, 18.8% (n = 19) of individuals exhibited clinically elevated prenatal depression symptoms above the EPDS cut-off score of 13, while 30.1% (n = 28) exhibited clinically elevated prenatal anxiety levels compared to a general US population reference sample. Descriptive maternal characteristics are reported in Table 1. Participants gave birth at an average of 39.6 weeks gestation (SD = 1.28). Infants were 57% (n = 57) male and 43% (n = 43) female. Infant sample characteristics are presented in Table 2.
Maternal stress, mental health, and infant brain volumes
Our first aim was to examine the relationship between prenatal objective hardship, perceived stress, and infant brain volumes at 3 months of age. None of the regions of interest (i.e., amygdala, PFC) were associated with either maternal stress variables (all, p > 0.05). However, when examining the moderating effects between maternal mental health (i.e., prenatal depression and anxiety) and infant brain volumes, greater prenatal anxiety was significantly associated with smaller left amygdala volumes (B = −5.919, p = 0.016, Fig. 1, Table 3), independent of prenatal stress.
PFC & amygdala volumes and emotional regulation
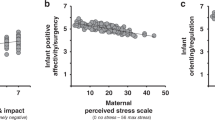
Our second aim was to examine the relationship between infant brain volumes at 3 months and temperament at 6 months of age. Left amygdala volumes negatively predicted infant negative affectivity (B = −0.003, p = 0.002, Fig. 2, Table 4); right amygdala volumes were not associated with temperament. A mediation analysis was run post hoc to determine whether infant left amygdala volumes mediated any relationship between prenatal anxiety and infant negative affectivity. The mediation was not significant. Despite prenatal anxiety predicting left amygdala volumes, and left amygdala volumes predicting negative affectivity, there was no direct effect between prenatal anxiety and negative affectivity.
Discussion
As part of a larger longitudinal pan-Canadian study of pregnant individuals during the pandemic, we examined the association of prenatal maternal stress, anxiety, and depression with 3-month infant amygdala and PFC volumes. We showed that greater prenatal maternal anxiety was associated with smaller left amygdala volumes in infants at 3 months. In addition, we also determined that smaller left amygdala volumes were associated with greater negative affectivity at 6 months.
The negative relationship between maternal anxiety and infant amygdala volumes adds to the growing body of literature describing the vulnerability of the amygdala to early stress. Our finding is supported by previous research that found altered amygdala volumes and functional changes in association with maternal stress during the intrauterine period [9]. Higher prenatal depression [28] and pregnancy-related anxiety [71] symptoms have been associated with larger amygdala volumes in young girls. Conversely, Lehtola et al. [26] found that prenatal psychological distress, a combination of anxiety and depression symptoms, predicted smaller amygdala volumes in newborn males [26]. Importantly, the direction of the effect of PNMS on amygdala volumes may vary based on the age of the children studied. The growth of the amygdala varies with age, with “rapid increases in volumes at early ages [that] decline as youth enter adolescence” [72]. PNMS could potentially affect the developmental trajectory of amygdala growth throughout time. In animal models, prenatal stress has resulted in reduced amygdala volumes (including decreases in amygdala neurons and glial cells) in offspring early postnatally [73]. However, in later developmental stages, prenatal stress was related to greater amygdala volumes compared to controls [73]. In children, greater maternal depression during pregnancy was associated with more curvilinear left amygdala volume trajectories [74]. Infant sex, PNMS severity, timepoint in gestation, HPA-axis activation, and genetic influences could all affect the resulting offspring amygdala volumes [8, 75].
PFC volumes were not associated with prenatal maternal stress or mental health in this study. The lack of a significant relationship could be due to a number of factors. First, the PFC was treated as a broad region of interest made up of smaller subregions (e.g., frontal pole, orbitofrontal cortex, ventrolateral prefrontal cortex). Subregional variability could have masked effects. Second, PFC volumes are a function of both surface area and cortical thickness. Variability in both factors could have also masked effects. Future research can use surface-based analyses (e.g., vertex-wise cortical thickness via infant FreeSurfer) to tease apart the effect of PNMS on both factors. However, this type of analysis would be difficult in the current study due to various constraints (sample size, scanner time), which would limit that level of spatial granularity.
Overall, in our study, higher prenatal anxiety predicted smaller left amygdala volumes, which in turn predicted greater negative affectivity. Previous research has shown that PNMS is related to greater difficult/negative temperament, with more irritability and crying [11]. Pandemic-related stress during pregnancy has also been linked to greater negative affect in infants [49]. However, in this study, there was no association between prenatal anxiety and negative affectivity, and thus no significant mediation by left amygdala volumes. However, the association between amygdala volumes and temperament is mixed. Previous research found that smaller amygdala volumes were related to higher levels of infant negative emotionality, excessive crying and irritability [76, 77]. Filippi et al. [78] showed that infants with more negative temperament had slower growth in left amygdala volumes in childhood [78]. In children, smaller amygdalae were related to pediatric depression and anxiety [31, 79, 80]. The reduction of amygdalae volumes could be due to an excitotoxic process caused by PNMS (i.e., exposure to cortisol, inflammation) [8]. At the same time, some studies reported larger amygdalae volumes predict worse internalizing problems in children [27, 34] and greater infant fear [81]. The current study also provides some evidence towards lateralization of amygdala structure and function, where the left amygdala is more involved in negative emotions and local processing [82]. The left hemisphere also grows more quickly in early gestation [83], potentially making it more vulnerable to PNMS, similar to our results.
Genetics could have also played a moderating role in this study. Research has shown that prenatal maternal mental health (anxiety, depression) interacts with genotype to predict infant outcomes [84, 85]. First, the GUSTO study found that infants’ brain-derived neurotropic factor (BDNF) Val66Met gene (which effects synaptic plasticity) moderates the association between prenatal anxiety and DNA methylation of infants as well as the association between DNA methylation and amygdala volumes. Prenatal maternal anxiety had a greater effect on DNA methylation in the Met/Met polymorphism group compared to the Met/valine (Val) and Val/Val genotype groups [84]. The group also found that the impact of prenatal depression on right amygdala volume varied as a function of the infants’ genomic profile risk for major depressive disorder [86]. Furthermore, they determined that Genotype X Environmental models best predicted amygdala volumes compared to models which included genotype or environment (prenatal anxiety, depression, socio-economic status) variables alone [87]. Thus, the relationship between prenatal anxiety and amygdala volumes in this study could be similarly moderated by genetics. The group also studied the effect of prenatal anxiety on neonatal PFC cortical thickness. They found no main effect of prenatal anxiety on PFC cortical thickness. However, the association was moderated by single-nucleotide polymorphisms (SNPs) of the catechol-O-methyltransferase (COMT) gene (which regulates PFC catecholamine signaling). Cortical thickness in the right ventrolateral PFC decreased as prenatal anxiety increased for met homozygous infants. For val homozygous infants, prenatal anxiety was associated with an increase in cortical thickness [85]. Thus, the negative findings in our study could be due to a hidden moderating effect of infant genotype on PFC volumes, leading to cortical thinning in some and cortical thickening in others. Other studies have also shown that genotype also moderates the relationship between prenatal mental health and infant/child negative emotionality [11]. Together, these findings show that the associations between PNMS, infant brain volumes, and temperament in this study could have potentially been influenced by the infant’s genotype.
Possible limitations should be considered when interpreting our findings. Infant movements during scanning affected the quality of some scans. The scans were performed at 3 months of age, thus postnatal factors, such as infant feeding, sleep, and infection, could have also influenced infant brain development. Almost all the maternal data were collected using self-report questionnaires, potentially introducing reporting biases. Participants were recruited primarily from social media advertising, which could have contributed to selection biases. No genetic information was collected from parent or infant, limiting our understanding of the influences of genetics on the brain-behavior associations in this study. The moderating effects of genetics in relation to the impact of PNMS during the pandemic on infant outcomes should be studied in future research. Furthermore, in this study, we examined the interaction between prenatal anxiety and depression and its effect on infant brain volumes. The interaction was non-significant. However, given the study’s sample size, our analyses are likely underpowered to detect an interaction. Finally, participants were mostly white, financially stable, highly educated, and living in a country with a relatively contained outbreak and universal healthcare, both limiting the generalizability of our results and potentially underestimating the true effect of prenatal psychological distress on infant brain development in populations with greater vulnerabilities.
This study also had several major strengths. Its prospective longitudinal design allowed us to examine the associations between maternal stress variables, infant brain volumes at 3 months, and infant temperament at 6 months of age. We had a relatively large sample size of 100 mother-infant dyads, resulting in a unique and well-characterized cohort. Participant recruitment and data collection started at the early phases of the pandemic. Thus, our study was able to capture maternal stress and mental health levels related to the initial government mandated restrictions and rise of COVID-19 cases. The PdP study team developed a novel measure of Objective Hardship that captured participants level of loss, threat, and change due to the pandemic, as well as the duration and intensity of their hardship. Thus, we were able to disentangle the relative effects of objective hardship, perceived stress, and maternal mental health on infant development while they were exposed to a relatively independent stressor (the pandemic). In addition, the study team collected information on many potential confounding variables, and used measures that had strong psychometric properties, such as the EPDS and PROMIS.
Our findings have implications for perinatal policy and health care. The results suggest that screening for prenatal anxiety could help identify at-risk individuals during pregnancy. Anxiety reduction interventions could also mitigate its impact on infant brain development [88]. The brain remains quite plastic throughout infancy and childhood. Thus, offspring of at-risk individuals may also benefit from early evidence-based interventions, such as those targeting emotional regulation. Future research should use longitudinal scanning to help us determine the effect of prenatal anxiety on the trajectory of brain development throughout childhood. Long-term follow-ups with these children will allow us to examine the link between pandemic-related prenatal stress, brain volumes, and many measures of child development, such as motor, behavioral, and social-emotional development. Future studies should also investigate potential personal and environmental factors that could protect infant brain development from the negative effects of pandemic-related stress.
Going beyond the current study, examining the impact of PNMS during the pandemic on functional and structural connectivity can further offer additional insight that should be pursued in future work. In general, previous research shows that prenatal maternal stress and mental health is associated with infant brain connectivity. For example, greater PNMS has been associated with less positive resting state functional connectivity but greater structural connectivity between the medial PFC and the amygdala in 5-week-old infants [89]. Next, in terms of maternal mental health, greater prenatal depression symptoms have been linked to an increase in infant negative amygdala-dorsal PFC functional connectivity, a decrease in structural connectivity between the right ventral PFC and the right amygdala, as well as higher functional connectivity between the infants’ amygdala and other brain structures, including the left temporal cortex, insula, and anterior cingulate cortex [90, 91]. Prenatal maternal anxiety may also play a role. Research has shown that higher prenatal anxiety is associated with functional connectivity between infants’ amygdala and brain regions important for fear learning (e.g., lower amygdala-fusiform gyrus functional connectivity, higher amygdala-thalamus connectivity) as well as between young children’s amygdala and brain regions important for sensorimotor functioning (e.g., increased negative connectivity between the amygdala and bilateral parietal regions) [92, 93]. According to another PdP study, social support could moderate the association between prenatal distress during the pandemic and infant amygdala-prefrontal functional connectivity. Infants of participants with low social support had a significant relationship between higher prenatal distress and weaker amygdala-PFC functional connectivity, while participants with high social support showed no effect [50]. Finally, the impact of prenatal stress and mental health on brain connectivity could have subsequent impacts on children’s behavior. One study found that weaker amygdala-frontal structural connectivity, which was associated with greater prenatal depression, in turn predicted externalizing behavior in young boys [94]. All in all, structural and functional connectivity is another area of research that should be understood in the context of PNMS and the pandemic.
In conclusion, the present study described the relationship between prenatal stress, including symptoms anxiety and depression, during the COVID-19 pandemic, infant brain volumes, and temperament. We observed a negative association between prenatal anxiety and infant left amygdala volumes, and lower left amygdala volumes predicted higher negative affectivity. Infant temperament may be partially programmed in utero and influenced by the development of the amygdala. The current study supports the hypothesis that exposure to stress in utero influences infant brain and behavioral development.
Data availability
Data may be made available on reasonable request and subject to institutional data sharing agreements and ethical approval.
References
Groulx T, Bagshawe M, Giesbrecht G, Tomfohr-Madsen L, Hetherington E, Lebel CA. Prenatal care disruptions and associations with maternal mental health during the COVID-19 Pandemic. Front Glob Womens Health. 2021;2:648428 https://doi.org/10.3389/fgwh.2021.648428.
Public Health Agency of Canada. Pregnancy, childbirth and caring for newborns: advice for mothers during COVID-19 2020. https://www.canada.ca/en/public-health/services/publications/diseasesconditions/pregnancy-advise-mothers.html Accessed 26 May 2023.
Urrutia D, Manetti E, Williamson M, Lequy E. Overview of Canada’s answer to the COVID-19 pandemic’s first wave (January–April 2020). Int J Env Res Public Health. 2021;18:7131.
Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–72. https://doi.org/10.1016/S2214-109X(21)00079-6.
Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5–13. https://doi.org/10.1016/j.jad.2020.07.126.
Mateus V, Cruz S, Costa R, Mesquita A, Christoforou A, Wilson CA, et al. Rates of depressive and anxiety symptoms in the perinatal period during the COVID-19 pandemic: comparisons between countries and with pre-pandemic data. J Affect Disord. 2022;316:245–53.
Papadopoulos A, Nichols ES, Mohsenzadeh Y, Giroux I, Mottola MF, Van Lieshout RJ, et al. Depression in pregnant women with and without COVID-19. BJPsych Open. 2021;7:e173.
Gunnar MR, Doyle C The effects of prenatal stress on offspring development. Wiley Encycl Health Psychol. 2020:275–86. https://doi.org/10.1002/9781119057840.ch32.
Lautarescu A, Craig MC, Glover V. Prenatal stress: effects on fetal and child brain development. Int Rev Neurobiol. 2020;150:17–40. https://doi.org/10.1016/bs.irn.2019.11.002.
Papadopoulos A, Nichols ES, Mohsenzadeh Y, Giroux I, Mottola MF, Van Lieshout RJ, et al. Prenatal and postpartum maternal mental health and neonatal motor outcomes during the COVID-19 pandemic. J Affect Disord Rep. 2022;10:100387.
Van den Bergh BR, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2020;117:26–64.
Falah-Hassani K, Shiri R, Dennis C-L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47:2041–53.
American Psychiatric Association issuing body. Diagnostic and statistical manual of mental disorders: DSM-5-TR. 5th edition, text revision. Washington, DC: American Psychiatric Association Publishing;2022.
Yang Z, Wang X, Wang M, Yan S, Wu F, Zhang F. Trajectory of prenatal anxiety and depression and its association with fetal growth development. Early Hum Dev. 2023;187:105875.
Austin M-P, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Hum Dev. 2005;81:183–90.
Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46:1454–63.
Field T. Prenatal anxiety effects: a review. Infant Behav Dev. 2017;49:120–8.
Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34:1–14.
Laplante DP, Brunet A, King S. The effects of maternal stress and illness during pregnancy on infant temperament: Project Ice Storm. Pediatr Res. 2016;79:107–13.
Simcock G, Elgbeili G, Laplante DP, Kildea S, Cobham V, Stapleton H, et al. The effects of prenatal maternal stress on early temperament: the 2011 Queensland Flood Study. J Dev Behav Pediatr. 2017;38:310–21.
Simcock G, Laplante DP, Elgbeili G, Kildea S, Cobham V, Stapleton H, et al. Infant neurodevelopment is affected by prenatal maternal stress: the QF 2011 Queensland Flood Study. Infancy. 2017;22:282–302.
King S, Dancause K, Turcotte‐Tremblay A, Veru F, Laplante DP. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res Part C Embryo Today Rev. 2012;96:273–88.
Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–85.
Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–71.
Tottenham N, Gabard-Durnam LJ. The developing amygdala: a student of the world and a teacher of the cortex. Curr Opin Psychol. 2017;17:55–60.
Lehtola SJ, Tuulari JJ, Scheinin NM, Karlsson L, Parkkola R, Merisaari H, et al. Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. NeuroImage Clin. 2020;28:102380.
Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci. 2012;109:E1312–9.
Wen D, Poh J, Ni S, Chong Y, Chen H, Kwek K, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7:e1103.
Jones SL, Dufoix R, Laplante DP, Elgbeili G, Patel R, Chakravarty MM, et al. Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: sex specific effects in project ice storm. Front Hum Neurosci. 2019;13:144.
Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT, et al. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry. 2013;74:837–44.
Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–6.
Warnell KR, Pecukonis M, Redcay E. Developmental relations between amygdala volume and anxiety traits: Effects of informant, sex, and age. Dev Psychopathol. 2018;30:1503–15.
Albaugh MD, Nguyen T-V, Ducharme S, Collins DL, Botteron KN, D’Alberto N, et al. Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biol Psychol. 2017;124:133–40.
De Bellis MD, Casey B, Dahl RE, Birmaher B, Williamson DE, Thomas KM, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–7.
Grossmann T. Mapping prefrontal cortex functions in human infancy. Infancy. 2013;18:303–24.
Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res. 2012;195:191–218. https://doi.org/10.1016/B978-0-444-53860-4.00009-X.
Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35:141–53.
Sandman CA, Buss C, Head K, Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry. 2015;77:324–34. https://doi.org/10.1016/j.biopsych.2014.06.025.
Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 2013;23:2521–30.
Kolk SM, Rakic P. Development of prefrontal cortex. Neuropsychopharmacology. 2022;47:41–57.
Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 2015;25:2204–12.
Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. 2019;85:443–53.
Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42:180–92.
Gosnell SN, Velasquez KM, Molfese DL, Molfese PJ, Madan A, Fowler JC, et al. Prefrontal cortex, temporal cortex, and hippocampus volume are affected in suicidal psychiatric patients. Psychiatry Res Neuroimaging. 2016;256:50–6.
Ohtani T, Levitt JJ, Nestor PG, Kawashima T, Asami T, Shenton ME, et al. Prefrontal cortex volume deficit in schizophrenia: a new look using 3 T MRI with manual parcellation. Schizophr Res. 2014;152:184–90.
Lagopoulos J, Hermens DF, Naismith SL, Scott EM, Hickie IB. Frontal lobe changes occur early in the course of affective disorders in young people. BMC Psychiatry. 2012;12:1–7.
Glover V, Bergman K, Sarkar P, O’Connor TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34:430–5.
Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68.
Buthmann JL, Miller JG, Gotlib IH. Maternal–prenatal stress and depression predict infant temperament during the COVID-19 pandemic. Dev Psychopathol. 2022;36:161–9.
Manning KY, Long X, Watts D, Tomfohr-Madsen L, Giesbrecht GF, Lebel C. Prenatal maternal distress during the COVID-19 pandemic and associations with infant brain connectivity. Biol Psychiatry. 2022;92:701–8.
Duguay G, Garon-Bissonnette J, Lemieux R, Dubois-Comtois K, Mayrand K, Berthelot N. Socioemotional development in infants of pregnant women during the COVID-19 pandemic: the role of prenatal and postnatal maternal distress. Child Adolesc Psychiatry Ment Health. 2022;16:28.
Giesbrecht GF, Lebel C, Dennis C-L, Silang K, Xie EB, Tough S, et al. Risk for developmental delay among infants born during the COVID-19 pandemic. J Dev Behav Pediatr. 2023;44:e412–20.
Hessami K, Norooznezhad AH, Monteiro S, Barrozo ER, Abdolmaleki AS, Arian SE, et al. COVID-19 pandemic and infant neurodevelopmental impairment: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2238941–e2238941.
Morris AR, Saxbe DE. Differences in infant negative affectivity during the COVID‐19 pandemic. Infant Ment Health J. 2023;44:466–79.
Giesbrecht GF, Bagshawe M, van Sloten M, MacKinnon AL, Dhillon A, van de Wouw M, et al. Protocol for the pregnancy during the COVID-19 pandemic (PdP) study: a longitudinal cohort study of mental health among pregnant Canadians during the COVID-19 pandemic and developmental outcomes in their children. JMIR Res Protoc. 2021;10:e25407.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Giesbrecht G, van de Wouw M, Rioux C, Lai B, King S, Tomfohr-Madsen L, et al. Cumulative and compounding effects of pre-pandemic vulnerabilities and pandemic-related hardship on psychological distress among pregnant individuals. Gen Hosp Psychiatry. 2023;83:93–100.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Lee E-H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res. 2012;6:121–7. https://doi.org/10.1016/j.anr.2012.08.004.
Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8:99–107.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–6.
Cox JL, Chapman G, Murray D, Jones P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J Affect Disord. 1996;39:185–9.
Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18:263–83.
Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94.
Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav Dev. 2003;26:64–86.
Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of short and very short forms of the Infant Behavior Questionnaire–revised. J Pers Assess. 2014;96:445–58.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55.
Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the talairach coordinate system. Cereb Cortex. 1995;5:323–37.
Putnam SP, Sehic E, French BF, Gartstein MA, Lira Luttges B. The global temperament project: parent-reported temperament in infants, toddlers, and children from 59 nations. Dev Psychol. 2024.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications; New York, NY, 2017.
Acosta H, Tuulari JJ, Scheinin NM, Hashempour N, Rajasilta O, Lavonius TI, et al. Maternal pregnancy-related anxiety is associated with sexually dimorphic alterations in amygdala volume in 4-year-old children. Front Behav Neurosci. 2019;13:175.
Russell JD, Marsee MA, Weems CF. Developmental variation in amygdala volumes: modeling differences across time, age, and puberty. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:117–25.
Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79.
Donnici C, Long X, Reynolds J, Giesbrecht GF, Dewey D, Letourneau N, et al. Prenatal depressive symptoms and childhood development of brain limbic and default mode network structure. Hum Brain Mapp. 2023;44:2380–94.
Manning KY, Jaffer A, Lebel C. Windows of opportunity: how age and sex shape the influence of prenatal depression on the child brain. Biol Psychiatry. 2024;97:227–47.
Demers CH, Hankin BL, Hennessey E-MP, Haase MH, Bagonis MM, Kim SH, et al. Maternal adverse childhood experiences and infant subcortical brain volume. Neurobiol Stress. 2022;21:100487.
Sammallahti S, Serdarevic F, Tiemeier H. Excessive crying, behavior problems, and amygdala volume: a study from infancy to adolescence. J Am Acad Child Adolesc Psychiatry. 2023;62:675–83.
Filippi CA, Sachs JF, Phillips D, Winkler A, Gold AL, Leibenluft E, et al. Infant behavioral reactivity predicts change in amygdala volume 12 years later. Dev Cogn Neurosci. 2020;42:100776.
Burrows CA, Lasch C, Gross J, Girault JB, Rutsohn J, Wolff JJ, et al. Associations between early trajectories of amygdala development and later school-age anxiety in two longitudinal samples. Dev Cogn Neurosci. 2024;65:101333.
Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–6.
Bezanson S, Nichols ES, Duerden EG. Postnatal maternal distress, infant subcortical brain macrostructure and emotional regulation. Psychiatry Res Neuroimaging. 2023;328:111577.
Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev. 2004;45:96–103.
Andescavage NN, Du Plessis A, McCarter R, Serag A, Evangelou I, Vezina G, et al. Complex trajectories of brain development in the healthy human fetus. Cereb Cortex. 2017;27:5274–83.
Chen L, Pan H, Tuan TA, Teh AL, MacIsaac JL, Mah SM, et al. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism influences the association of the methylome with maternal anxiety and neonatal brain volumes. Dev Psychopathol. 2015;27:137–50.
Qiu A, Tuan TA, Ong ML, Li Y, Chen H, Rifkin-Graboi A, et al. COMT haplotypes modulate associations of antenatal maternal anxiety and neonatal cortical morphology. Am J Psychiatry. 2015;172:163–72.
Qiu A, Shen M, Buss C, Chong Y-S, Kwek K, Saw S-M, et al. Effects of antenatal maternal depressive symptoms and socio-economic status on neonatal brain development are modulated by genetic risk. Cereb Cortex. 2017;27:3080–92.
Ong M, Tuan TA, Poh J, Teh AL, Chen L, Pan H, et al. Neonatal amygdalae and hippocampi are influenced by genotype and prenatal environment, and reflected in the neonatal DNA methylome. Genes Brain Behav. 2019;18:e12576.
Graham AM, Doyle O, Tilden EL, Sullivan EL, Gustafsson HC, Marr M, et al. Effects of maternal psychological stress during pregnancy on offspring brain development: considering the role of inflammation and potential for preventive intervention. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:461–70.
Humphreys KL, Camacho M, Roth MC, Estes EC. Prenatal stress exposure and multimodal assessment of amygdala–medial prefrontal cortex connectivity in infants. Dev Cogn Neurosci. 2020;46:100877.
Posner J, Cha J, Roy A, Peterson B, Bansal R, Gustafsson H, et al. Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry. 2016;6:e935–e935.
Qiu A, Anh T, Li Y, Chen H, Rifkin-Graboi A, Broekman MM, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry. 2015;5:e508.
Hill TL, Na X, Bellando J, Glasier CM, Ou X. Functional connectivity to the amygdala in the neonate is impacted by the maternal anxiety level during pregnancy. J Neuroimaging. 2025;35:e70004.
Donnici C, Long X, Dewey D, Letourneau N, Landman B, Huo Y, et al. Prenatal and postnatal maternal anxiety and amygdala structure and function in young children. Sci Rep. 2021;11:4019.
Hay RE, Reynolds JE, Grohs MN, Paniukov D, Giesbrecht GF, Letourneau N, et al. Amygdala-Prefrontal Structural Connectivity Mediates the Relationship between Prenatal Depression and Behavior in Preschool Boys. J Neurosci. 2020;40:6969–6977.
Acknowledgements
This work was supported by the Alberta Children’s Hospital Research Institute, the Owerko Centre for Neurodevelopment and Mental Health, and the Canadian Institutes of Health Research (UIP 178826). CL, EGD, LT-M receive funding from the Canada Research Chairs Program. We would like to thank Sarah Al-Saoud for her assistance in processing the brain scans and segmenting the regions of interest for this study. Additionally, this project would have not been completed without the participation from all the individuals in the PdP study.
Author information
Authors and Affiliations
Contributions
Catherine Lebel, Lianne Tomfohr-Madsen, and Gerald Giesbrecht all conceived, designed, and developed the protocol for the larger PdP study. Emily Nichols processed the brain scans using infant Freesurfer. Kathryn Manning collected infant imaging data. Amber Di Paolo wrote the manuscript, segmented the prefrontal cortex, and conducted the statistical analyses/data interpretation, under the supervision of Emma Duerden. Emma Duerden significantly contributed to the development of the manuscript and data processing. All co-authors reviewed and edited the final manuscript. There are no financial interests or potential conflicts of interest to report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Di Paolo, AL., Nichols, E.S., Tomfohr-Madsen, L. et al. The association between prenatal maternal anxiety, infant brain volumes, and temperament during the COVID-19 pandemic. Transl Psychiatry 15, 283 (2025). https://doi.org/10.1038/s41398-025-03527-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-025-03527-9