Abstract
Suicide is a critical global public health issue, where traditional risk assessment tools have limited predictive value, warranting the identification of novel risk assessment factors. Metabolic syndrome (MetS) has been linked to poor cognition and brain volumes, which may lead to abnormal behaviors. The relationship between MetS and suicide risk has been less studied. This study aims to explore the association of MetS on suicide attempt leveraging data from the UK Biobank and genomic analyses. We first explored the cross-sectional and longitudinal relationships between MetS and suicide attempt, while also exploring the mediating role of cognitive performance. Second, using summary data from the largest genome-wide association studies, the genetic associations between MetS, suicide attempt, and cognitive performance were examined. Of 380,557 participants tracked over 13 years, we identified 1847 new cases of suicide attempt. The presence of MetS was found to significantly increase the risk of suicide attempt (HR = 1.250, 95% CI = 1.134–1.379). In participants lacking traditional suicide risk factors, such as being female, younger, and having higher educational attainment, MetS still presented a greater risk in predicting future suicide attempts. Additionally, MetS and suicide attempt exhibited significant genetic correlation (rg = 0.080 ± 0.026), and Mendelian randomization analysis suggested MetS had a significant negative effect on suicide attempt (β = 0.156, 95% CI = 0.077–0.235). These findings highlight a significant association between MetS and increased suicide risk. Addressing MetS may offer an avenue for improved suicide management.
Similar content being viewed by others
Introduction
Suicide poses a significant public health challenge worldwide [1], culminating in more than 700,000 deaths annually [1, 2]. The cause of suicide is linked to complex factors, including genetic, socio-environmental, and psychopathological factors, rendering existing suicide risk assessment tools inadequate [1], with positive predictive values below 20% [3, 4]. Recent data indicate that in the UK, up to 90% of patients with mental disorders who died by suicide were assessed as having no risk or low risk of suicide by their clinical teams [5]. These facts underscore the urgent need for objective indicators to predict suicide risk at an early stage, especially in patients who lack traditional suicide risk factors. In response, the World Health Organization has initiated the Mental Health Action Plan, focusing on the development of prevention strategies that include identifying novel risk factors and early monitoring indicators [6].
Emerging evidence indicates a complex relationship between MetS and suicide risk. MetS and its components have been associated with structural and functional brain alterations, particularly in the reduction of volumes in regions crucial for emotion processing and cognitive function, such as the cingulate cortex and hippocampus [7, 8]. Notably, similar morphological changes have been observed in individuals with suicide behaviors [9]. In addition, MetS has been linked to systemic inflammation and oxidative stress [10, 11], which are increasingly recognized as important biological mechanisms in suicidal behaviors 12,27. Furthermore, MetS has been associated with cognitive impairment, particularly in the domains of executive function, processing speed, and memory [12,13,14]. These cognitive deficits correspond to those observed in individuals with suicidal behaviors, where impaired decision-making and cognitive flexibility have been identified as potential risk factors for suicide attempts [1, 12, 13, 15].
Recent studies examining the relationship between MetS and suicide risk have shown promising results, though findings vary across populations. A community-based study reported an up to an 88% higher risk of suicide attempts in individuals with MetS [16]. However, the relationship appears more complex when the presence of psychiatric disorders was considered [17, 18]. These mixed results may be partially explained by divergent methodological approaches and the potential mediating role of cognitive function, which has been independently associated with both MetS and suicide risk. Therefore, further exploration is needed to investigate the potential of MetS as an early indicator for monitoring suicide risk in the general population, with consideration of cognitive function as a mediating factor.
Based on these findings, this study aimed to investigate the relationship between MetS and suicide risk in the general population, specifically focusing on cognitive function as a mediator. We hypothesized that MetS would be associated with increased suicide risk and that cognitive function would mediate this relationship. To test these hypotheses, we utilized longitudinal data from the UK Biobank. Our objectives were to evaluate the potential of MetS as an objective indicator for monitoring suicide risk and to explore the underlying mechanisms linking metabolic health to suicidal behaviors.
Methods
Ethics approval and study population
Ethical approval was obtained from the NHS National Research Ethics Service (11/NW/0382). Informed consent was obtained from each participant, before data collection. Our research utilized the prospective UK Biobank cohort initiated between 2006 and 2010. Initial evaluations collected individual-level information through self-administered questionnaires and a nurse-led verbal interview. Medical conditions were linked from the national death registration, hospital inpatient records, primary care data, and self-report health conditions during the assessment. Cognitive performance tests were assessed at the time of initial evaluation. All methods were performed in accordance with the relevant guidelines and regulations.
Figure 1 and Supplementary Fig. 1 present an overview of the study design. The initial cohort comprised 502,204 participants, all of whom underwent MetS screening during baseline evaluations. Participants were excluded if they lacked complete data for all five MetS diagnostic criteria (n = 121,627), resulting in 380,577 individuals eligible for suicide risk assessment. Online questionnaires identified 41,976 participants reporting suicide ideation or non-hospitalized suicide attempts. Through linkage with hospitalization records and mortality registries, 3493 participants were confirmed to have at least one suicide attempt resulting in hospitalization or death. We evaluated the MetS-suicide risk association using both cross-sectional and longitudinal analytic approaches, and assessed the mediating role of cognitive function. Data sources are detailed in Supplementary Table S1.
This study aimed to identify the association between metabolic syndrome and suicide risk, as well as to investigate the mediating effect of cognitive function. The research consisted of two main parts. First, a population-based cohort study was conducted using UK Biobank data to examine the prospective association between metabolic syndrome and the risk of suicide attempt. Second, summary-level data from large-scale genome-wide association studies (GWAS) were used to explore the genetic correlation and potential causal relationship between metabolic syndrome and suicide risk.
Definition of metabolic syndrome
MetS was defined in accordance with the criteria established by the International Diabetes Federation (IDF) [19]. Specifically, participants were diagnosed with MetS if they exhibited more than two of the following four components alongside evidence of central obesity: hypertension, hyperglycaemia, hypertriglyceridemia, and dyslipidemia for high-density lipoprotein (HDL) cholesterol.
Central obesity is defined as an unhealthy waist circumference or body mass index (BMI) ≥ 30 kg/m2. The cut-off points for unhealthy waist circumference are ≥88 cm for females, ≥ 94 cm for males in Europeans, and ≥90 cm for males of other ancestries. Hypertension was defined as a systolic blood pressure (SBP) ≥ 130 mmHg or a diastolic blood pressure (DBP) ≥ 85 mmHg, or a history of treatment with antihypertensive drugs. Due to the limited number of participants with fasting glucose test results, hyperglycaemia was determined based on glycated haemoglobin (HbA1c) levels, with a cut-off point >6% (42 mmol/mol), aligning with the current study’s strategy for evaluating MetS in the UK Biobank cohort [20]. Participants who received treatment with any antihyperglycemic drug were also classified as having hyperglycaemia. Hypertriglyceridemia was defined as ≥1.7 mmol/L in triglyceride (TG) or a history of treatment with triglyceride-lowering drugs. Dyslipidemia was defined as HDL cholesterol levels <1.03 mmol/L in males and <1.29 mmol/L in females, or a history of treatment with cholesterol-lowering drugs. Codes for identifying the above drugs are listed in Supplementary Table S2.
When considering MetS as the exposure, it was treated as a binary variable (presence or absence of MetS). Additionally, we calculated the summed number for MetS traits and treated it either as an ordinal categorical variable (scores from 0 to 5, with 0 as the reference) to explore the risk of suicide attempt at each MetS score level, or as a linear variable to calculate the P value for trend.
Identification of suicide events
We used two source of data to identify suicide events: (1) Online mental-health questionnaires [21] and (2) Hospitalization and death registrations [22]. (1) The online questionnaires included “ever thought or contemplated suicide” (suicide ideation) and “ever attempted self-harm or suicide” (suicide attempt) without a specific time of events, therefore only allowing a cross-sectional analysis. The data fields for corresponding questionnaires are listed in Supplementary Table S1. (2) The hospitalization and death registrations recorded suicide attempts using the three-character International Classification of Disease (ICD) codes with corresponding times of event. Specifically, death by suicide was defined using the ICD-10 codes X60-84 and Y10-34 through the death registration. Suicide attempt causing hospitalization was defined using the ICD-10 codes X60-84 and ICD-9 codes E950-958 through hospital inpatient records. The time between the first suicide attempt either causing hospitalization or death and recruitment to the UK Biobank cohort was calculated in years. Suicide attempt before the recruitment was considered as the suicide history. The primary outcome in this study was suicide attempt causing hospitalization or death.
Cognition data
To assess the mediating effect of cognitive performance on suicide attempt, we calculated a weighted cognitive z-score using the following five domains according to previous studies [23, 24]: fluid intelligence, numeric memory, reaction time, pairs matching, and prospective memory.
The fluid intelligence score was calculated as the unweighted sum of the number of correct responses to 13 fluid intelligence questions. Participants who failed to complete all items within the two-minute time limit received zero points for unanswered questions. Numeric memory tests involved sequential recall trials raging from 2-digit to 12-digit sequences, with the longest correctly recalled sequence length recorded as the test outcome. In the reaction time assessment, paired cards were presented on the screen and the response latency of participant was measured when judging if the two cards were the same. Pairs matching test evaluated spatial working memory by requiring memorization of card positions, with performance quantified as the average number of correctly identified matching pairs. Prospective memory included an instructional phase where participants were directed: “At the end of the games we will show you four colored shapes and ask you to touch the Blue Square. However, to test your memory, we want you to touch the Orange Circle instead.” The prospective memory result was coded as 0 for incorrect recall or skipped tests, 1 for correct recall on the second attempt, and 2 for correct recall on the first attempt.
Initially, we computed the z-score for each cognitive test. For reaction time, where higher values indicate worse cognitive performance, we inverted the values to align the directional interpretation with that of other cognitive tests. Subsequently, we applied a weighted averaging system for z-scores to ascertain the composite score of cognitive performance. The allocation of weights was determined by the number of tests each participant completed. Finally, we scaled the new scores to obtain the overall cognitive performance [23, 24].
Covariates
We incorporated four types of covariates when analyzing the impacts of MetS and the risk factors for suicide [14, 21]: (1) Basic demographic factors, including age at recruitment, sex, age2, and educational level (categorized as high school equivalent or lower). (2) Household income as the socioeconomic factor. (3) Smoking status (categorized as never, former, or current smoker) as a lifestyle factor. (4) Significant suicide risk factors, including the number of previous suicide attempts and medical histories of schizophrenia, bipolar disorder, and major depressive disorder. When assessing the associations of cognition with MetS and its components, we adopted well-documented covariates including age, sex, age2, and educational level. The correlations between these covariates and suicide attempt/MetS are shown in Supplementary Tables S3 and S4.
Among the 380,577 participants, 3963 lacked educational level, 1441 had missing smoking status records, and 54,739 were missing household income information. Missing covariates were imputed using the most frequent value (Supplementary Table S5). The coding strategies for the covariates are shown in Supplementary Table S6.
Sensitivity analysis
To investigate the predictive utility of MetS in individuals without traditional suicide risk factors, we conducted subgroup analyses stratified by suicide history (presence/absence), age groups (<55 years or ≥55 years), sex, educational attainment, smoking status, household income (median-dichotomized), and psychiatric histories (schizophrenia, bipolar disorder, or major depressive disorder).
Given documented elevated suicide attempt rates during the first two years post-recruitment in the UK Biobank and inherent data latency issues in registry linkages [22, 25], we excluded suicide cases recorded within two years after recruitment and re-performed the analyses. This sensitivity analysis aimed to disentagle potential measurement biases from the true biological associations.
Genetic correlation and Mendelian randomization
To assess the genetic associations between MetS, cognition, and suicide attempt, we obtained public summary data of Genome-wide association studies (GWAS) (Supplementary Table S7). The summary data of MetS was downloaded from the Complex Trait Genetics lab, with an estimated sample size of 461,920 [26]. The summary data of cognitive performance was downloaded from the Social Science Genetic Association Consortium, with a total sample size of 257,818 [27]. The summary data of suicide attempt (including cases causing death) was obtained through the International Suicide Genetics Consortium, with a sample size of 815,178 [28]. All data were based on European ancestry with hg19 as the reference genome.
We used linkage disequilibrium score regression (LDSC) to access the genetic correlations between MetS, cognitive performance, and suicide attempt. Since all three summary data included participants from the UK Biobank, we used generalized summary-data based Mendelian randomization (GSMR) to interpret the potential genetic associations between these traits, which is unbiased by sample overlap between the exposure and the outcome [29]. GSMR was performed using GCTA software (Version 1.91.7), utilizing the Haplotype Reference Consortium (HRC) panel as the reference sample, and adopting a threshold P value of 5E-08 to select SNPs and an LD r2 threshold of 0.01 for clumping analysis. The mediating effects of trait were estimated using the Delta method according to previous studies [30].
Statistical analysis
Statistical analyses were conducted using R software (Version 4.2.2). Continuous variables were summarized for description using mean values with standard deviations (SD) or median values with minimum to maximum values. Categorical variables were summarized using absolute counts and percentages. Comparisons between participants with or without suicide attempt were performed using Student’s t-tests or chi-square tests.
Logistics regressions were performed to explore the cross-sectional relationships between MetS, suicide events, and cognitive performance. Cox proportional hazard models were performed to evaluate the impact of MetS on future suicide risk. Since participants may have multiple suicide attempts during the follow-up, we took the first suicide attempt as the endpoint. For those in the UK Biobank cohort without suicide attempt records, the study endpoint was defined by loss to follow-up or the data censoring date of November 2022. Linear regressions were used to assess the relationships between MetS and cognitive performance. To evaluate whether individual MetS components might be a better predictor for suicide risk than the integrated MetS diagnosis or a MetS composite score, we defined all 31 possible combinations of the five MetS components as exposure variables and accessed their strength of association with suicide risk and cognitive performance. The mediating effect of cognitive performance was explored using the lavaan package in R (Version 0.6-16). P values less than 0.05 were considered statistically significant.
Results
Demographic characteristics
A total of 380,557 participants were included, the demographic characteristics are presented in Table 1. The mean age of the participants was 56.6 ± 8.09 years, and 53.8% (204,773) were female. The mean follow-up duration of this study was 13.3 ± 2.08 years. MetS was diagnosed in 32.7% (124,363) of the participants. 3493 (0.92%) participants had suicide attempts causing hospitalization, and 1847 (0.49%) occurred after recruitment. The presence of MetS was significantly higher in participants with subsequent suicide attempt (37.2%) than those without (32.7%, P < 0.001). In addition, participants with suicide attempt after recruitment were younger (53.6 ± 8.5 vs 56.6 ± 8.1, P < 0.001) and had higher BMI (27.9 ± 5.4 vs 27.4 ± 4.8, P < 0.001). The proportion of female was smaller (49.9 vs 53.8% P < 0.001). For the social factors, participants with suicide attempt showed lower educational attainment (57.6 vs 66.2%, P < 0.001) and lower household income (P < 0.001). Suicide history was strongly associated with subsequent suicide attempt, with 12.6% of the participants with subsequent suicide attempt having a suicide history (P < 0.001).
Additionally, 32.5% (123,630 of the 380,577) of the participants completed the online mental-health questionnaires after recruitment, allowing us to identify life-time suicide ideation and attempt among this subgroup of participants. Among them, 33,649 (27.2%) participants reported a history of suicide ideation without actions, and 4834 (3.9%) participants reported suicide attempt that did not require hospitalization. Bold values indicate statisitcal significant.
MetS was associated with increased suicide risk
We first explored the cross-sectional associations between MetS and suicide risk (Fig. 2). The presence of MetS was associated with an increased trend in the risk of suicide ideation, suicide attempt without hospitalization, and suicide attempt causing hospitalization or death, with odds ratios (OR) and 95% confidence interval (CI) of 1.071 (1.040–1.105), 1.159 (1.079–1.246), and 1.596 (1.471–1.731), respectively (Fig. 2A, Supplementary Table S8). When considering all suicide events collectively, MetS remained significantly associated with an increased risk (OR = 1.108, 95% CI: 1.077–1.140).
A, B Define suicide events using online mental-health questionnaires, using logistics regressions; A Taking metabolic syndrome as a binary variable. B Using the number of components of metabolic syndrome as an independent variable. C Define suicide attempt using hospitalization records and death registration, using cox regressions. *P value < 0.05, **P value < 0.01, ***P value < 0.001.
A dose-response relationship was observed between the number of MetS traits and suicide events (Fig. 2B, Supplementary Table S9). Specifically, an increasing number of MetS traits was significantly associated with suicide attempt causing hospitalization or death (OR range: 1.227 to 3.577, P for trend <2 × 10−16). Participants fulfilled three or more traits of MetS showed a significantly increased risk of all suicide events (OR range: 1.088 to 1.352, P for trend = 2.37 × 10−12).
We then assessed the longitudinal associations between MetS and subsequent suicide risk, by considering suicide attempt before recruitment as a covariate and taking the first suicide attempt causing hospitalization or death as the endpoint (Fig. 2C, Supplementary Table S10). The presence of MetS at baseline significantly increased subsequent suicide risk by 25.0% (Hazard ratio [HR]: 1.250, 95% CI: 1.134–1.379). There was also a dose-response relationship between the number of MetS traits and subsequent suicide risk (HR range: 1.160 to 1.501, P for trend = 3.84 × 10−6).
To explore whether individual MetS components might be stronger predictors of suicide risk than the integrated MetS diagnosis or cumulative MetS traits, we evaluated all 31 potential combinations of metabolic components (Supplementary Fig. 2). Elevated suicide attempt risk was observed for the combination of central obesity, hypertension, and dyslipidemia, which exceeded the hazard associated with MetS alone. However, these combinations did not significantly outperform other three-component configurations.
Cognitive performance serves as a mediator from MetS to suicide risk
We further questioned whether the relationships between MetS and suicide risk was mediated by cognitive performance. Among the five cognitive domains assessed at baseline, enhanced performance was associated with lower suicide risk (Supplementary Table S11). We identified bidirectional relationships between MetS and cognition: MetS predicted poorer cognitive performance across all domains (Supplementary Table S12), while higher baseline cognition correlated with reduced incident MetS risk (Supplementary Table S13).
In addition, cumulative MetS components demonstrated a graded inverse relationship with global cognition (Supplementary Table S14). The combination of hyperglycemia, dyslipidemia, and central obesity exhibited stronger cognitive detriment that exceeding the predictive power of simple MetS component counts for pairs matching results (Supplementary Fig. 3).
We then calculated the weighted average of the five cognitive domains to represent the overall cognitive function, and subsequently analyzed its associations with MetS and suicide attempt (Fig. 3A, Supplementary Table S15). We found the presence of MetS was associated with impaired overall cognitive performance (β = −0.065, 95% CI: −0.072–−0.058). Conversely, enhanced overall cognitive performance demonstrated an inverse relationship with the likelihood of MetS (OR = 0.933, 95% CI: 0.927–0.940). In addition, both MetS (OR = 1.248, 95% CI: 1.160–1.342) and the overall cognitive performance (OR = 0.873, 95% CI: 0.851–0.895) exhibited significant associations with lifetime suicide attempt resulting in hospitalization or death.
A Associations between the presence of metabolic syndrome, overall cognitive performance, and lifetime suicide attempt causing hospitalization or death. B Results of mediation analyses. Grey lines illustrate path 1 (metabolic syndrome to cognitive performance, then to suicide attempt). Green lines illustrate path 2 (cognitive performance to metabolic syndrome, then to suicide attempt). c1 and c2 are the direct effects.
We then performed mediation analysis to dissect the relationships between MetS, overall cognitive performance, and suicide attempt (Fig. 3B). Cognitive performance may mediate the association between MetS and suicide attempt, while it could also reduce suicide risk by affecting MetS.
Sensitivity analyses
We performed sensitivity analysis by stratifying participants based on their suicide histories (Supplementary Table S16) and found that the presence of MetS significantly increased the risk of subsequent suicide attempt in participants without suicide histories (HR = 1.247, 95% CI: 1.122 − 1.385).
When grouping by age, sex, educational level, smoking status, and household income, the presence of MetS was identified as a significant risk factor for subsequent suicide attempt, except for current smokers, in whom MetS only showed a trend towards increasing suicide risk (P = 0.225). In addition, we found the impact of MetS on suicide attempt was higher than the general level in participants who were younger (< 55 years, HR = 1.378, 95% CI: 1.201–1.581), female (HR = 1.378, 95% CI: 1.191–1.595), had higher educational attainment (HR = 1.289, 95% CI: 1.131–1.470), and did not present medical history of major psychiatric disorders such as schizophrenia, bipolar disorder, and major depressive disorder (HR = 1.427, 95% CI: 1.075–1.894). These results suggest that MetS could be used as a supplementary indicator for the monitoring of suicide risk in those without traditional suicide risk factors.
Lastly, we excluded participants with their suicide attempt recorded within two years after recruitment, and found that the presence of MetS was still associated with an increase risk of suicide attempt (HR = 1.275, 95% CI: 1.143–1.423).
Genetic associations between MetS and suicide attempt
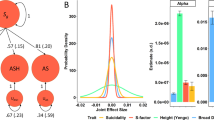
We utilized the largest GWAS summary data for MetS, cognitive performance, and suicide attempt (including cases causing death) to explore their genetic relationships (Fig. 4). We found suicide attempt was genetically correlated with both MetS (rg = 0.080 ± 0.026, P = 0.002) and cognitive performance (rg = −0.213 ± 0.026, P = 3.86 × 10−16). There was also a significant genetic correlation between MetS and cognitive performance (rg = −0.133 ± 0.019, P = 1.02 × 10−12) (Fig. 4A, Supplementary Table S17).
Mendelian randomization using GSMR suggested significant effects of MetS (β = 0.156, 95% CI: 0.077 − 0.235) and cognitive performance (β = −0.109, 95% CI: −0.183–−0.036) on suicide attempt. In addition, MetS and cognitive performance suggested bidirectional effects on each other (Fig. 4B, Supplementary Table S18, Supplementary Fig. 4). The mediation analysis indicated that cognitive performance was a mediator linking MetS and suicide attempt, with a mediated proportion of 8.96%. On the other hand, better cognitive performance decreased the risk of suicide attempt, which was mediated by MetS with a mediated proportion of 21.94% (Fig. 4C, D, Supplementary Table S18).
Discussion
This study represents a pioneering analysis of the correlation between MetS and suicide attempt by combining evidence from a population-based cohort and genetic data. We found that the presence of MetS at baseline is a significant risk factor for future suicide actions, and cognitiove performance and MetS exhibit bidirectional mediation in affecting future suicide risk. Our findings collectively suggest that MetS could be used as a modifiable factor in the monitoring and management of suicide risk.
The high prevalence of MetS brings major concerns to the public health. Besides affecting cardiovascular outcomes and all-cause mortality [31], recent evidence has linked MetS to worse brain health [14]. For instance, Qureshi et al. confirmed that MetS was associated with less brain volume and poor cognition in over 37,000 participants [14]. Since damage to brain health is one of the major causes of suicide, it is possible that MetS could be a novel risk factor for suicide.
Although several studies have reported the associations between MetS and suicide, most of them were conducted in specific populations (such as patients with psychiatric disorders) and yield inconsistent results [32]. Chang et al. reported that MetS was associated with a 88% increase in the risk of suicide death through a community-based cohort [16]. Mellor et al. reported that young individuals who attempted suicide were twice as likely to have comorbid MetS [18]. However, Stenzel et al. discovered that in patients with bipolar disorders, MetS was not linked to suicide [17]. It is possible that the limited sample size, heterogeneity of study population, and presence of confounding factors (such as socio-environmental factors and suicide history) lead to the controversial findings. Our study, leveraging the longitudinal cohort of the UK Biobank and focusing on over 380,000 participants, provides more robust results. Furthermore, our subgroup analyses revealed that MetS was associated with a higher suicide risk among females, those of younger age, had higher educational attainments, and without histories of suicide or psychiatric disorders, which have previously been considered protective factors against suicide [1]. While individuals lacking these traditional risk factors might typically be assessed as having low suicide risk, the presence of MetS could serve as a valuable supplementary indicator in suicide risk evaluation.
Our study found that MetS was associated with poor cognitive performance and that it exhibits bidirectional mediation in promoting suicide attempts. This phenomenon can be explained through several biological mechanisms. First, studies demonstrate that MetS may induce endothelial dysfunction, resulting in compromised cerebral blood flow regulation [33]. This vascular dysfunction is often accompanied by the disruption of the blood-brain barrier, allowing neurotoxic substances to enter the central nervous system, directly causing white matter damage that is strongly associated with cognitive decline [33, 34]. Second, MetS typically involves chronic systemic inflammation with elevated levels of inflammatory cytokines such as interleukin-6 (IL-6) [35, 36]. Our previous study has shown that elevated plasma IL-6 levels correlated with both brain volume loss and cognitive decline, as well as an increased risk of dementia [37]. Third, the five components of MetS appear to independently influence cognitive performance through distinct pathways; for instance, hyperglycemia has been shown to impair the cerebral energy metabolism and promote neuronal dysfunction [38].
Conversely, impaired cognitive function may also promote the development of MetS. First, impaired cognition may trigger reward-seeking behaviors, including unhealthy dietary choices and sedentary behaviors, directly exacerbating MetS [39]. Second, cognitive decline leads to chronic hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, including abnormally elevated cortisol levels [40]. This endocrine imbalance promotes central adiposity, insulin resistance, and dyslipidemia through overactivation of glucocorticoid receptor, all of which are key components of MetS [41]. Notably, chronic stress activation leads to an increase in inflammatory cytokines, which contribute to the development of both MetS and cognitive decline [41]. Collectively, these interconnected mechanisms create a deleterious feedback loop between cognitive decline and the progression of MetS.
Our subgroup analyses demonstrate that MetS elevates suicide risk across all demographic strata, including traditionally protected populations, mediated by distinct mechanisms operating across sex, age, and education. We propose several potential explanations for these findings. First, women with MetS experience greater societal pressure, such as social stigma [42], which may contribute to increased psychological distress and suicide risk [43]. Second, younger adults with MetS exhibit accelerated white matter hyperintensity progression and early-onset prefrontal metabolic dysregulation [44, 45], and disproportionately executive functions [46]. Regarding individuals with higher educational attainment, they may have stronger identity ties to their intellectual capabilities, making cognitive decline more perceptible and threatening to their self-concept and social-status maintenance, thereby intensifying their psychological burden [47,48,49].
Our genetic analyses revealed a significant genetic correlation between MetS and suicide attempt, and Mendelian randomization analysis indicated that MetS plays a negative effect on suicide attempt. These findings should be interpreted within the broader context of familial influences on suicide risk. Research has shown that suicide attempts have a substantial heritability, ranging from 41 to 55% [50], with individuals having a family history of suicide showing up to three times higher risk of suicide attempt [51]. However, genetic predisposition to suicide does not operate alone but rather interacts substantially with environmental factors [15].
The influence of MetS on suicide risk may function via two mechanisms: gene-environment interaction (GxE) and gene-environment correlation. Regarding GxE, individuals with increased genetic susceptibility to suicide may experience an elevated risk when they develop MetS [52, 53]. Additionally, environmental factors commonly shared among family members—such as dietary habits, lifestyle patterns, and socioeconomic status—contribute to both MetS and suicide risk. Thus, the observed association between MetS and elevated suicide risk might be attributable to socioeconomic factors that directly influence suicide risk. Furthermore, recent genome-wide association studies have identified substantial genetic overlap between suicide attempts and various psychiatric disorders, suggesting shared biological pathways [54]. The significantly higher prevalence of MetS in patients with psychiatric disorders may partially explain these findings. Moreover, individuals with a family history of suicide often face social stigma and bullying [55], which may not only lead to depression or suicidal ideation but also trigger emotional eating, potentially contributing to MetS [56]. Conversely, social stigma associated with individuals who have obesity or are overweight conditions may increase vulnerability to mental disorders and suicide [43]. However, due to the unavailability of family suicide history data in the UK Biobank, we could not definitively establish a relationship between family history of suicide and MetS. This aspect warrants investigation in future studies.
As a modifiable factor, interventions on MetS and its components have shown protecting effects on brain health [14]. Unfortunately, there is lack of evidence regarding the effects of managing MetS on suicide risk. A recent meta-analysis of randomized controlled trials indicated that exercise decreased the risk of suicide attempt by 77% [57], which is a promising result that supports the potential benefits of targeting MetS for managing suicide risk. Further intervention studies are needed to understand whether the reversal of MetS can reduce suicide risk.
There are several limitations of this study. First, the high educational level among UK Biobank participants, compared to the general population, may limit the generalizability of our findings, as education is a protective factor against suicide. Second, we did not perform a comprehensive mediator analysis between MetS and suicide attempt. Third, the lack of longitudinal MetS data restricts our ability to determine whether improvements in MetS correlate with decreased suicide risk. Furthermore, since the brain imaging data of the UK Biobank was acquired six years after participant recruitment, and most of the suicide occurred within the six years post-recruitment [22], we were unable to access the mediating role of brain structures in the pathway from MetS to suicide. In our mediation analysis examining cognition as a mediator, we calculated weighted averages for cognitive domains, applying equal weights to construct the overall cognitive score. However, given that certain domains demonstrated stronger associations with suicide than others, an alternative approach could have been to weight the cognitive results based on their respective associations with suicide risk. Most importantly, although family history of suicide is one of the strongest predictors of suicide attempt, we were unable to obtain this information in the UK Biobank dataset. Future studies should aim to address these gaps.
Conclusion
In conclusion, this study contributes to the understanding of MetS in increasing suicide risk. MetS could be a complementary indicator in the prediction and early intervention of suicide risk.
Data availability
Raw data can be accessed following approval from the UK Biobank team. Statistical data are available in the Supplementary Tables. Q.W. and Z.Z. are the guarantor of this work, and such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Knipe D, Padmanathan P, Newton-Howes G, Chan LF, Kapur N. Suicide and self-harm. The Lancet. 2022;399:1903–16.
Lange S, Cayetano C, Jiang H, Tausch A, Oliveira E Souza R. Contextual factors associated with country-level suicide mortality in the Americas, 2000–2019: a cross-sectional ecological study. Lancet Reg Health Am. 2023;20:100450.
Quinlivan L, Cooper J, Davies L, Hawton K, Gunnell D, Kapur N. Which are the most useful scales for predicting repeat self-harm? A systematic review evaluating risk scales using measures of diagnostic accuracy. BMJ Open. 2016;6:e009297.
Chan MKY, Bhatti H, Meader N, Stockton S, Evans J, O’Connor RC, et al. Predicting suicide following self-harm: systematic review of risk factors and risk scales. Br J Psychiatry. 2016;209:277–83.
Graney J, Hunt IM, Quinlivan L, Rodway C, Turnbull P, Gianatsi M, et al. Suicide risk assessment in UK mental health services: a national mixed-methods study. Lancet Psychiatry. 2020;7:1046–53.
Saxena S, Funk M, Chisholm D. WHO’s mental health action plan 2013–2020: what can psychiatrists do to facilitate its implementation? World Psychiatry. 2014;13:107–9.
Shen C, Liu C, Qiu A. Metabolism-related brain morphology accelerates aging and predicts neurodegenerative diseases and stroke: a UK Biobank study. Transl Psychiatry. 2023;13:233.
Shirzadi Z, Rabin J, Launer LJ, Bryan RN, Al-Ozairi A, Chhatwal J, et al. Metabolic and vascular risk factor variability over 25 years relates to midlife brain volume and cognition. J Alzheimers Dis. 2023;91:627–35.
Yun J-Y, Kim Y-K. Neural correlates of treatment response to ketamine for treatment-resistant depression: a systematic review of MRI-based studies. Psychiatry Res. 2024;340:116092.
Lind L. Endothelium-dependent vasodilation, insulin resistance and the metabolic syndrome in an elderly cohort: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis. 2008;196:795–802.
Esposito K, Ciotola M, Schisano B, Misso L, Giannetti G, Ceriello A, et al. Oxidative stress in the metabolic syndrome. J Endocrinol Invest. 2006;29:791–5.
Zhang H, Ye Y, Zhao Y, Li S, Jiao P, Yang Y, et al. Obesity is associated with lower levels of negative emotions in polycystic ovary syndrome in clinical and animal studies. Ann Med. 2024;56:2373199.
Gholami A, Doustmohammadian A, Shamshirgaran SM, Aminisani N, Azimi-Nezhad M, Abasi H, et al. Association between metabolic syndrome and health-related quality of life in older adults: findings from the Iranian Longitudinal study on ageing. Metab Syndr Relat Disord. 2024. https://doi.org/10.1089/met.2023.0049.
Qureshi D, Topiwala A, Al Abid SU, Allen NE, Kuźma E, Littlejohns TJ. Association of metabolic syndrome with neuroimaging and cognitive outcomes in the UK Biobank. Diabetes Care. 2024;47:1415–23.
Turecki G, Brent DA. Suicide and suicidal behaviour. The Lancet. 2016;387:1227–39.
Chang J-C, Yen AM-F, Lee C-S, Chen SL-S, Chiu SY-H, Fann JC-Y, et al. Metabolic syndrome and the risk of suicide: a community-based integrated screening samples cohort study. Psychosom Med. 2013;75:807–14.
Stenzel C, Dalkner N, Unterrainer H-F, Birner A, Bengesser SA, Fellendorf FT, et al. Effects of metabolic syndrome and obesity on suicidality in individuals with bipolar disorder. J Affect Disord. 2022;311:1–7.
Goldman-Mellor SJ, Caspi A, Harrington H, Hogan S, Nada-Raja S, Poulton R, et al. Suicide attempt in young people: a signal for long-term health care and social needs. JAMA Psychiatry. 2014;71:119–27.
International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrom. 2023. Sourced from The International Diabetes Federation, full text are available at: https://idf.org/media/uploads/2023/05/attachments-30.pdf.
Qureshi D, Collister J, Allen NE, Kuźma E, Littlejohns T. Association between metabolic syndrome and risk of incident dementia in UK Biobank. Alzheimers Dement. 2024;20:447–58.
Zhang B, You J, Rolls ET, Wang X, Kang J, Li Y, et al. Identifying behaviour-related and physiological risk factors for suicide attempts in the UK Biobank. Nat Hum Behav. 2024. https://doi.org/10.1038/s41562-024-01903-x.
Wang J, Qiu J, Zhu T, Zeng Y, Yang H, Shang Y, et al. Prediction of suicidal behaviors in the middle-aged population: machine learning analyses of UK Biobank. JMIR Public Health Surveill. 2023;9:e43419.
Wang X, Shi Z, Qiu Y, Sun D, Zhou H. Peripheral GFAP and NfL as early biomarkers for dementia: longitudinal insights from the UK Biobank. BMC Med. 2024;22:192.
Sabia S, Dugravot A, Dartigues J-F, Abell J, Elbaz A, Kivimäki M, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II Cohort study. BMJ. 2017;357:j2709.
Clifton L, Liu X, Collister JA, Littlejohns TJ, Allen N, Hunter DJ. Assessing the importance of primary care diagnoses in the UK Biobank. Eur J Epidemiol. 2024;39:219–29.
van Walree ES, Jansen IE, Bell NY, Savage JE, de Leeuw C, Nieuwdorp M, et al. Disentangling genetic risks for metabolic syndrome. Diabetes. 2022;71:2447–57.
Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21.
Docherty AR, Mullins N, Ashley-Koch AE, Qin X, Coleman JRI, Shabalin A, et al. GWAS Meta-analysis of suicide attempt: identification of 12 genome-wide significant loci and implication of genetic risks for specific health factors. Am J Psychiatry. 2023;180:723–38.
McGrath IM, International Endometriosis Genetics Consortium, Montgomery GW, Mortlock S. Genomic characterisation of the overlap of endometriosis with 76 comorbidities identifies pleiotropic and causal mechanisms underlying disease risk. Hum Genet. 2023;142:1345–60.
Yao S, Zhang M, Dong S-S, Wang J-H, Zhang K, Guo J, et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat Hum Behav. 2022;6:1569–76.
Abou Kassm S, Sánchez Rico M, Naja W, Alvarado JM, Halaby A, Limosin F, et al. Metabolic syndrome and risk of death in older adults with major psychiatric disorders: results from a 5-year prospective multicenter study. Int J Geriatr Psychiatry 2022;37. https://doi.org/10.1002/gps.5835.
Giménez-Palomo A, Gomes-da-Costa S, Dodd S, Pachiarotti I, Verdolini N, Vieta E, et al. Does metabolic syndrome or its component factors alter the course of bipolar disorder? A systematic review. Neurosci Biobehav Rev. 2022;132:142–53.
Devraj K, Kulkarni O, Liebner S. Regulation of the blood-brain barrier function by peripheral cues in health and disease. Metab Brain Dis. 2024;40:61.
Nyúl-Tóth Á, Patai R, Csiszar A, Ungvari A, Gulej R, Mukli P, et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience. 2024;46:6511–36.
Wang W, Li J, Cui S, Li J, Ye X, Wang Z, et al. Microglial Ffar4 deficiency promotes cognitive impairment in the context of metabolic syndrome. Sci Adv. 2024;10:eadj7813.
Valado A, Cunha M, Pereira L. Biomarkers and seaweed-based nutritional interventions in metabolic syndrome: a comprehensive review. Mar Drugs. 2024;22:550.
Zhao Z, Zhang J, Wu Y, Xie M, Tao S, Lv Q, et al. Plasma IL-6 levels and their association with brain health and dementia risk: a population-based cohort study. Brain Behav Immun. 2024;120:430–8.
Xu F, Shi J. Insulin signaling and oxidative stress: bridging the gap between type 2 diabetes mellitus and Alzheimer’s disease. J Alzheimers Dis. 2025;103:994–1004.
Zhang X, Han L, Lu C, McIntyre RS, Teopiz KM, Wang Y, et al. Brain structural and functional alterations in individuals with combined overweight/obesity and mood disorders: a systematic review of neuroimaging studies. J Affect Disord. 2023;334:166–79.
Zheng B, Tal R, Yang Z, Middleton L, Udeh-Momoh C. Cortisol hypersecretion and the risk of Alzheimer’s disease: a systematic review and meta-analysis. Ageing Research Reviews. 2020;64:101171.
Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15:525–34.
Limon VM, Lee M, Gonzalez B, Choh AC, Czerwinski SA. The impact of metabolic syndrome on mental health-related quality of life and depressive symptoms. Qual Life Res. 2020;29:2063–72.
Brochu PM. Weight stigma as a risk factor for suicidality. Int J Obes (Lond). 2020;44:1979–80.
Angoff R, Himali JJ, Maillard P, Aparicio HJ, Vasan RS, Seshadri S, et al. Relations of metabolic health and obesity to brain aging in young to middle-aged adults. J Am Heart Assoc. 2022;11:e022107.
Kobiec T, Mardaraz C, Toro-Urrego N, Kölliker-Frers R, Capani F, Otero-Losada M. Neuroprotection in metabolic syndrome by environmental enrichment. A lifespan perspective. Front Neurosci. 2023;17:1214468.
Haase Alasantro L, Hicks TH, Green-Krogmann E, Murphy C. Metabolic syndrome and cognitive performance across the adult lifespan. PLoS One. 2021;16:e0249348.
Muñoz IG, Santos-Lozada AR. Educational attainment and psychological distress among working-age adults in the United States. SSM - Mental Health. 2021;1:100003.
Jokela M. Why is cognitive ability associated with psychological distress and wellbeing? Exploring psychological, biological, and social mechanisms. Personality and Individual Differences. 2022;192:111592.
Sutin AR, Stephan Y, Terracciano A. Psychological distress, self-beliefs, and risk of cognitive impairment and dementia. J Alzheimers Dis. 2018;65:1041–50.
Edwards AC, Ohlsson H, Mościcki E, Crump C, Sundquist J, Lichtenstein P, et al. On the genetic and environmental relationship between suicide attempt and death by suicide. Am J Psychiatry. 2021;178:1060–9.
Tidemalm D, Runeson B, Waern M, Frisell T, Carlström E, Lichtenstein P, et al. Familial clustering of suicide risk: a total population study of 11.4 million individuals. Psychol Med. 2011;41:2527–34.
Fanelli G, Sokolowski M, Wasserman D, European College of Neuropsychopharmacology (ECNP) Network on Suicide Research and Prevention, Kasper S, Zohar J, et al. Polygenic risk scores for neuropsychiatric, inflammatory, and cardio-metabolic traits highlight possible genetic overlap with suicide attempt and treatment-emergent suicidal ideation. Am J Med Genet B Neuropsychiatr Genet. 2022;189:74–85.
Knowles EEM, Curran JE, Meikle PJ, Huynh K, Mathias SR, Göring HHH, et al. Disentangling the genetic overlap between cholesterol and suicide risk. Neuropsychopharmacology. 2018;43:2556–63.
Strawbridge RJ, Ward J, Ferguson A, Graham N, Shaw RJ, Cullen B, et al. Identification of novel genome-wide associations for suicidality in UK Biobank, genetic correlation with psychiatric disorders and polygenic association with completed suicide. EBioMedicine. 2019;41:517–25.
Burke CT, Calear AL, Cruwys T, Batterham PJ. Are parents the Key? How parental suicide stigma and suicide literacy affect help-seeking attitudes and intentions for their child. J Youth Adolesc. 2023;52:2417–29.
Mataracı Değirmenci D, Kalkan Uğurlu Y, Küçük Alemdar D. The relationship between coronavirus anxiety level and emotional eating in individuals with metabolic syndrome. Psychol Health Med. 2023;28:3156–62.
Fabiano N, Gupta A, Fiedorowicz JG, Firth J, Stubbs B, Vancampfort D, et al. The effect of exercise on suicidal ideation and behaviors: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2023;330:355–66.
Acknowledgements
We sincerely thank the participants of the UK Biobank, whose invaluable contributions were pivotal to this study. This research has been conducted using the UK Biobank Resource under Application Number 86920. This work uses data provided by patients and collected by the NHS as part of their care and support.
Funding
This work was funded by the Chinese National Programs for Brain Science and Brain-like Intelligence Technology (STI2030-Major Projects-2021ZD0200700), and the National Natural Science Foundation of China (82171499, 82301683, 82401763, and 82571712). Role of the funder/sponsor: the funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
QW contributed to the conception and design of the study. QW, and ZZ contributed to the data analysis and interpretation, and drafted the manuscript. MX, JC, ST, QL, and JZ critically reviewed and edited the manuscript. YL, YH, SL, and YW contributed to the interpretation of the data. QW, MX, JC, ST, and ZZ obtained the funding. All authors approved the final version of the manuscript. QW and ZZ are the guarantor of this work, and such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing of interest.
Ethics approval and consent to participate
The UK Biobank study received approval from the National Health Service North West Multicenter Research Ethics Committee. All participants were informed of consent via electronic signature prior to participation in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Z., Xie, M., Tao, S. et al. Metabolic syndrome increases the risk of suicide attempt: evidence from a population-based cohort and genomic analysis. Transl Psychiatry 15, 365 (2025). https://doi.org/10.1038/s41398-025-03575-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03575-1