Abstract
Major depressive disorder (MDD) is a debilitating mental health disorder that has a wide impact on many patients and has imposed a heavy burden on society in recent years. However, the specific pathogenesis of depression remains to be elucidated. Numerous studies have shown that metabolic disorders and molecules play important roles in MDD. Here, we demonstrate a preliminary mechanism through which TGR5 functions in the hippocampus during bile acid synthesis dysfunction in mice subjected to chronic social defeat stress (CSDS). According to the enzyme-linked immunosorbent assay (ELISA), susceptible mice subjected to CSDS presented reduced expression of key bile acid enzymes in the serum and total bile acids (TBAs) in the hippocampus. The expression of the bile acid-related receptor TGR5 in the hippocampus was lower in CSDS-exposed susceptible mice than in control mice. By analyzing the potential downstream signaling pathways of TGR5, we found that specific TGR5/cAMP/PKA regulation effectively increased the plasticity of Schaffer collateral (SC)–CA1 synapses in the hippocampus and further alleviated anxiety- and depression-like behavior in susceptible mice. These findings suggest that CSDS susceptibility is accompanied by dysfunction of TGR5 in the hippocampus and the downstream cAMP/PKA signaling pathway. Activating cAMP/PKA signaling can ameliorate behavioral deficits in susceptible mice. This study may support the development of potential effective pharmacotherapies for the treatment of MDD.
Similar content being viewed by others
Introduction
Recently, depression has become increasingly recognized worldwide as a devastating psychiatric disease that limits psychosocial function and reduces quality of life. In addition to emotional fluctuations in daily life, patients with depression have difficulty recovering from negative emotions, which, in the worst cases, can lead to suicide; therefore, this disease represents a serious health problem worldwide [1, 2].
There is currently no consensus about the cause of depression, but many hypotheses exist regarding the mechanism of its pathogenesis, including hypothalamic‒pituitary‒adrenal axis hyperactivity [3, 4], neuroinflammation [5], microbiome perturbation [6] and genetic abnormalities [7]. The evidence for the roles of these mechanisms in depression pathogenesis comes from research on different animal models and patients. Diverse perspectives from research on different species will help us the mechanisms related depression from a multidimensional perspective. Our team has focused on depression research and has investigated the occurrence and development of this disease by constructing advanced research models, including rodent [8], primate [9, 10], and clinical [11, 12] models.
Humans experience a variety of social stressors in their daily lives, and these factors can significantly affect their emotional state. Therefore, our research focuses on social defeat stress, a stressor that is highly relevant to depression. Many studies have shown that interpersonal stress, social rejection and social defeat are among the strongest proximal risk factors for depression [13]. The chronic social defeat stress (CSDS) model has been widely used to simulate affective disorders caused by social stress in rodents and has been used extensively in previous studies by our laboratory. Multiple brain regions, including the medial prefrontal cortex (mPFC) [14, 15], basolateral amygdala (BLA) [16] and nucleus accumbens (NAc) [17], are involved in the response to social defeat. In addition to these regions, the hippocampus is an essential part of the emotional regulation network. Studies have confirmed that the hippocampus shows marked changes in gene expression consistent to some degree with those in the cortex and amygdala after CSDS exposure [18]. From a more global perspective, neural circuits between multiple brain regions and the hippocampus, including the CA1 [19, 20] and CA3 [21] regions and DG [22], which are interconnected with other brain regions, are also involved in the effects of CSDS. Multiple subregions contribute to the activity and regulation of neural networks, together forming the hippocampal circuit. These findings suggest that the hippocampus is an extremely important bridge in the neural network linking CSDS and depression. Clinical investigations have revealed that the hippocampus is involved in depression, as indicated by reductions in the volume of principal hippocampal substructures and neuroinflammation-related dysfunction [23]. This brain region has also been linked to metabolic disorders. Some metabolites in the hippocampus have been confirmed to be associated with depression in clinical studies. For example, a previous study revealed that the concentration of taurine in the hippocampus of young women with depression decreased significantly [24], and another study revealed that the bioactive lipid metabolites EPA and DHA derived from LOX and CYP450 are targets for maintaining hippocampal neurogenesis and protecting against depression in humans [25]. Similarly, studies on rodents have revealed associations among metabolites, the hippocampus and depression. For example, adiponectin/AdipoR1 pathway activation can alleviate hippocampal neuroinflammation in mice exposed to chronic unpredictable stress [26].
Takeda G protein-coupled receptor 5 (TGR5), also known as G-protein-coupled bile acid receptor (Gpbar1), belongs to the G protein-coupled receptor (GPCR) superfamily and is mainly regarded as a receptor for bile acids and a metabolic regulator [27]. However, the function of this receptor in the brain is still unclear. Several studies have shown that in the hypothalamus, TGR5 is a key mediator of the neural mechanism that combats diet-induced obesity [28]. TGR5 can also regulate food intake, modulate cognitive impairment [29, 30], alleviate neurodegeneration [31], mediate analgesia [32], and suppress neuroinflammation [33]. Previous studies reported an association between TGR5 and affective disorders and described the role of TGR5 in the hippocampus and its related neural circuits in mediating the antidepressant response [34, 35], which revealed that the expression of TGR5 is decreased in the hippocampus of CSDS-susceptible mice and that genetic manipulation of its expression level can significantly ameliorate depression-like behavior caused by CSDS. The electrophysiological changes in the hippocampus and the downstream pathways of TGR5 remain unknown. In addition, the specific relationship between TGR5 and bile acid has not been explored. This prompted us to explore the mechanism by which TGR5 alleviates depression from other perspectives and investigate its connection with bile acid metabolism.
In the present study, we explored the role of TGR5 in the hippocampus in CSDS-induced depressive-like behaviors and the underlying molecular mechanism. Using a combination of enzyme-linked immunosorbent assay (ELISA), western blotting (WB), behavioral tests, and patch-clamp recording, we revealed the antidepressant effect of TGR5 in the hippocampus of CSDS-exposed mice. We established a CSDS model and discovered that susceptibility to stress resulted in bile acid-related metabolic dysfunction. Additionally, CSDS susceptibility led to reduced TGR5 expression in the hippocampus. Pharmacological treatment could enhance synaptic plasticity and alleviate the depressive-like behaviors induced by CSDS. In summary, our findings provide evidence that TGR5 may be critically related to susceptibility to CSDS in model mice and that stress susceptibility can be alleviated by regulating the downstream cAMP/PKA signaling pathway. These results indicate the potential of TGR5 as a therapeutic target for depression.
Materials and methods
Animals
The animals used in this study were all purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., and included male C57BL/6 J mice (8–10 weeks old) and male CD-1 mice (18–20 weeks old). The two different types of mice were raised separately and left untreated until the experiments began. The mice were given free access to standard food and water and were maintained on a 12-hour light/dark cycle (8:00 AM–8:00 PM daylight time) in a relatively stable environment (constant temperature of 23 ± 1 °C, relative humidity of 50 ± 5%). The mice were allowed to adapt to the environment for 1 week before the start of the formal experiments. All animal experimental procedures were approved by the Animal Care and Use Committee of Chongqing Medical University and were conducted in strict accordance with the guidelines for animal care published by the National Institutes of Health.
CSDS paradigm
A mouse model of CSDS was constructed on the basis of a standard protocol [36] and our previous studies. After 7 days of adaptation, aggressive CD-1 mice were identified, and modeling was performed according to a standard protocol for 10 days. Mice were exposed to defeat stress for 5 min every day. After defeat stress exposure, the C57BL/6J and CD-1 mice were placed in the same cage and separated on a transparent plastic plate. C57BL/6J mice in the control group were maintained in the same type of hamster cage as those in the experimental group, but the left and right sides of the cage both contained C57BL/6J mice; the control mice were exposed to different control group mice each day. During modeling, the mice were treated ethically, and adequate care and attention were provided.
Behavioral tests
Before the behavioral test, mice were transferred to a dedicated behavioral testing room for adaptation. The behavioral tests in this study included social interaction test (SIT), elevated plus maze test (EPM), open field test (OFT), sucrose preference test (SPT). All behavioral tests were performed in strict accordance with published protocol standards. The entire behavioral tests were recorded with high-definition camera and analyzed using EthoVisionXT13 software. The social interaction (SI) ratio was calculated after the SIT as follows: SI ratio = time spent in the interaction zone in phase 2/ time spent in the interaction zone in phase 1. An SI ratio < 1 indicated susceptibility, and mice that were deemed susceptible were the central focus of this study.
Drug treatment
Hyodeoxycholic acid (HDCA) was purchased from MedChemExpress (MCE). HDCA (HY-N0169) was dissolved in 10 mM dimethyl sulfoxide (DMSO) to prepare a stock solution and then diluted to 50 µM in artificial cerebrospinal fluid (aCSF) for field excitatory postsynaptic potential (fEPSP) recording. H-89 (MCE, HY-15979) was dissolved in 5 mM DMSO to prepare a stock solution and then diluted to 10 µM in aCSF for fEPSP recording. Forskolin (MCE, HY-15371) was dissolved in DMSO to prepare a stock solution of 10 mM. The solution was subsequently diluted to 50 µM in aCSF for fEPSP recording and to 0.1 mg/kg for intraperitoneal injection.
ELISA
The serum concentrations of CYP7A1 (Kanglang Biotech, KL-CYP7A1-Mu), CYP8B1 (Kanglang Biotech, KL-CYP8b1-Mu), and CYP27A1 (Kanglang Biotech, KL-CYP27A1-Mu) were measured by using ELISA kits. TBA concentrations in the hippocampus and mPFC (Kanglang Biotech, KL-TBA-Mu) were measured by using ELISA kits. The specific experimental steps were as follows: standards and samples were added to the plate, the plate was incubated, washing solution was added, enzyme-labeled reagent was added, chromogen A and chromogen B were added, and stop solution was added to each well. The samples were processed and the results were analyzed in accordance with the manufacturer’s instructions.
WB
Protein expression levels were quantified via WB. The samples were homogenized, and the protein concentrations were standardized; then, the samples were boiled at 100 °C for 5 min. Equal amounts of the samples were separated by 15% sodium dodecyl sulfate‒polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore). A 5% nonfat milk solution was used for blocking. Primary antibodies against the following proteins were used: TGR5 (1:1000, Abcam, ab72608), PXR (1:1000, Abcam, ab192579), S1PR2 (1:1000, Proteintech, 21180-1-AP), FXR (1:1500, Proteintech, 25055-1-AP) and β-actin (1:8000, Proteintech, 66009-1-Ig). Next, the membranes were washed with TBST and incubated with the appropriate secondary antibodies, including HRP-conjugated goat anti-rabbit IgG (H+G) (1:8000, Bio-Rad, 1706515) and HRP-conjugated goat anti-mouse IgG (H+G) (1:8000, Bio-Rad, 1706516). The membranes were developed using an enhanced chemiluminescence (ECL) reagent.
fEPSP recording
The brains of the mice were dissected in ice-cold artificial cerebrospinal fluid (124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.5 mM MgSO4, 2.5 mM CaCl2, 26 mM NaHCO3, and 10 mM glucose) bubbled with 95% O2/5% CO2. Brain slices (300 µm) were cut with a vibratome and kept in an incubation chamber at 33 °C at the interface of bubbled ACSF for more than 1 h. The slices were then moved to a submersion-type recording chamber and perfused with ACSF at 27 ± 1 °C (flow rate = 1–2 mL/min). For the fEPSP recording, a bipolar stimulation electrode was placed near the Schaffer collaterals (SCs) close to the CA3 area, and the electrodes were filled with ACSF (2–3 MΩ). A 700b amplifier (Axon, USA) was used to stimulate the dendritic and pyramidal cell layers of the CA1 region of the hippocampus. For electrical stimulation, 0.1-ms constant current paired pulses were delivered to SCs–commissural fibers at 30-s intervals. Evoked responses with an intensity that was 50–60% of the maximum excitatory postsynaptic potential were adjusted and used as baseline intensities (set to 100%).
Statistical analysis
All behavioral analyses were performed in a double-blind manner. Other experiments were studied in a single-blind manner. The sample size was determined based on the assumption of normal distribution and similar variability between experimental groups. All the data obtained in our study were statistically analyzed with SPSS 21.0 software, and GraphPad Prism 9.0 and Adobe Illustrator CS6 were used to visualize the results. Normality and equality of variances were tested for each group of data. For normally distributed data with equal variances, t tests and analysis of variance (ANOVA) followed by post hoc tests were performed for comparisons between groups. If the data did not conform to a normal distribution, nonparametric tests were performed. All the results are reported as the mean ± SEM, and significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ns- not significant.
Results
CSDS induces significant anxiety and depression-like behaviors
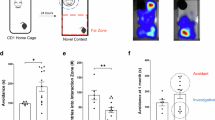
As illustrated in the experimental timeline (Fig. 1A), we primarily used a CSDS mouse model, which is a validated model for simulating social avoidance, anxiety and depression-like behaviors in rodents. After 10 days of CSDS exposure, the SIT was used to compare the stress susceptibility (indicated by an SI ratio < 1) of the CSDS-exposed mice to that of the control mice. After the SIT, we performed the following behavioral tests: the SPT, OPT, and EPM test. Compared with control mice, susceptible mice presented significantly shorter social interaction times and higher susceptibility in the SIT test (Fig. 1B–D), lower sucrose preference ratios in the SPT (Fig. 1J), and longer times spent in the closed arms in the EPM test (Fig. 1E–G), but there was no different in locomotor activity in the OFT (Fig. 1H). The time spent in the central area was not significantly different between the susceptible group and the control group (Fig. 1I). The behavioral results suggested that the CSDS model was successfully established.
A Schematic timeline of the experimental procedure. B, C Results of the SIT (Kruskal‒Wallis one-way ANOVA, F (2,44) = 10.119, F (2,44) = 9.227). D Percentage of time spent in the interaction zone by the resilient group and susceptible group (control, n = 26; susceptible, n = 16; resilient, n = 5). E–G EPM test results (Mann‒Whitney test and t test). H, I OFT results (t test and Mann‒Whitney test). J SPT results (Mann‒Whitney test; control, n = 26; susceptible, n = 16). (A) was created in BioRender. Chen, X. (2025) https://BioRender.com/p92m786. The data are expressed as the means ± SEMs. *p < 0.05, **p < 0.01, ***p < 0.001; ns not significant.
Changes in the classical bile acid synthesis pathway and hippocampal bile acid levels induced by CSDS in mice
To further study the role of bile acids in the CSDS model, the expression of key enzymes of various pathways upstream of bile acid synthesis was measured in susceptible mice [37]. The expression of key enzymes of classic pathways related to bile acid synthesis, including CYP7A1 and CYP8B1, was significantly lower in the susceptible group than in the control group (Fig. 2A, B). However, there was no significant difference in the expression of CYP27A1 (Fig. 2C), the key enzyme of the alternative bile acid synthesis pathway, indicating that downregulation of the expression of key enzymes in the upstream classic bile acid synthesis pathway may cause bile acid-related dysfunction in susceptible mice. Next, we tested the TBA concentration in the mPFC and hippocampus, two brain regions closely related to depression. The results revealed that the TBA concentration in the hippocampus of susceptible mice was significantly lower than that in the hippocampus of control mice (Fig. 2D). There was no significant difference in the TBA level in the mPFC between the two groups (Fig. 2E).
A–C Serum concentrations of (A) CYP7A1 (Mann‒Whitney test; control, n = 15; susceptible, n = 17), B CYP8B1 (t test; control, n = 15; susceptible, n = 17), and C CYP27A1 (t test; control, n = 11; susceptible, n = 13). D Concentration of TBA in the hippocampus (t test; control, n = 10; susceptible, n = 10). E Concentration of TBA in the mPFC (t test; control, n = 10; susceptible, n = 10). F–I Expression levels of (F) FXR, G TGR5, H PXR, and I S1PR2 (t test; control, n = 6; susceptible, n = 6). The data are expressed as the means ± SEMs. *p < 0.05, ***p < 0.001; ns not significant.
The expression of the bile acid-related receptor TGR5 is decreased in the hippocampus of susceptible mice
To investigate the role of bile acid receptors in stress susceptibility, we measured the protein expression levels of TGR5 [27], FXR [38], S1PR2 [39] and PXR [40] in the hippocampus via WB. Among these receptors, FXR and TGR5 are specific receptors for bile acids, whereas PXR and S1PR2 are nonspecific receptors. By measuring the expression levels of these receptors, we hope to identify receptors whose expression is altered in response to abnormal bile acid levels in the hippocampus of susceptible mice. Only TGR5 protein expression was decreased in CSDS-exposed mice (susceptible mice) compared with control mice (Fig. 2G), and there was no significant difference in the expression of FXR (Fig. 2F), PXR (Fig. 2H), or S1PR2 (Fig. 2I). We further assessed TGR5 levels in multiple brain regions, including the caudate putamen (CPu) (Supplementary Fig. 1A), amygdala (Supplementary Fig. 1B), and thalamus (Supplementary Fig. 1C), and found that TGR5 levels in these regions were not significantly different between susceptible mice and control mice.
TGR5-mediated potentiation of SC‒CA1 synaptic transmission in the hippocampus requires the cAMP/PKA signaling pathway
To identify the function of TGR5 in the hippocampus, we performed electrophysiological recording of SC–CA1 neural projections in the hippocampus (Fig. 3A). TGR5-related potentiation of fEPSPs in hippocampal slices from control mice was induced by the TGR5 agonist HDCA according to patch-clamp recording [41]. This effect was abolished by H-89 [42] (Fig. 3B). These results showed that the TGR5-mediated increase in synaptic transmission is PKA dependent. As previous studies reported that cAMP is the major upstream signal of PKA, we speculated that cAMP might also be involve in the TGR5 signaling pathway. To further confirm the role of cAMP in the TGR5 signaling pathway, we conducted an blockade experiment. Before the administration of HDCA, slices were perfused with the cAMP agonist forskolin. Previous studies reported that synaptic potentiation is induced by forskolin [43]. No further synaptic potentiation was induced by HDCA after forskolin pretreatment (Fig. 3C). These results confirmed the ability of HDCA to activate TGR5 through the cAMP/PKA pathway.
A Schematic diagram of the method used to record fEPSPs in mouse brain slices. B Effects of HDCA on fEPSPs at SC‒CA1 synapses in brain slices from the CON+aCSF and CON+H-89 groups and representative traces of fEPSPs before (black) and after (red) HDCA perfusion. C Effects of HDCA on fEPSPs at SC‒CA1 synapses in brain slices from the CON+aCSF and CON+forskolin pretreatment groups and representative traces of fEPSPs before (black) and after (red) HDCA perfusion. D Effects of HDCA and forskolin on fEPSPs at SC‒CA1 synapses in the brain slices of susceptible mice and representative traces of fEPSPs before (black) and after (red) drug perfusion (n = 3 for each group).
However, HDCA did not exert a similar effect on brain slices from susceptible mice, possibly because of the downregulation of TGR5 in the hippocampus (Fig. 2G). Interestingly, we directly perfused brain slices with forskolin and observed that forskolin could significantly induce fEPSP potentiation in susceptible mice. These patch-clamp experiments revealed the potential for TGR5 to function through the cAMP/PKA signaling pathway and revealed the potential role of target molecules in its downstream signaling pathway.
The cAMP/PKA pathway downstream of TGR5 can reverse CSDS-induced anxiety and depression-like behaviors
As illustrated in the experimental timeline (Fig. 4A), we designed a subsequent study to explore whether the TGR5/cAMP/PKA signaling pathway can alleviate anxiety and depression-like behaviors and whether forskolin treatment is effective in ameliorating these behaviors. The mice in the CSDS group were administered saline or 0.1 mg/kg forskolin (intraperitoneally (i.p.)) for 10 days. Compared with saline treatment, forskolin treatment did not effectively alleviate the social avoidance behavior of CSDS-exposed mice (Fig. 4B) but effectively reduced the proportion of mice susceptible to CSDS (Fig. 4C). According to the results of the EPM test (Fig. 4D, E) and SPT (Fig. 4H), stress susceptibility-related behaviors, including anxiety and depression-like behaviors, were effectively alleviated in the forskolin treatment group compared with the control + saline group and the CSDS+saline group. We found no deficits in the locomotor abilities of the mice in the OFT (Fig. 4F), and there was no significant difference in the time spent in the central area (Fig. 4G). Similarly, we conducted a study on susceptible mice. After CSDS exposure (Supplementary Fig. 2B–G), we selected susceptible mice and divided them into a susceptible + saline group and a susceptible + forskolin group. The results were similar to those shown in Fig. 4; that is, forskolin treatment did not effectively ameliorate the social avoidance behavior of susceptible mice (Supplementary Fig. 2H, I), but anxiety and depression-like behaviors were effectively alleviated (Supplementary Fig. 2J–N).
A Schematic timeline of the experimental procedure. B SIT results (Tukey’s HSD test, F(2,31) = 3.987). C Percentage of time spent in the interaction zone by the resilient group and susceptible group. D, E EPM test results. D Number of entries into the open arms (Kruskal‒Wallis test, one-way ANOVA, F(2,31) = 3.897). E Time spent in the closed arms (Dunnett’s T3 test, F(2,31) = 6.447). F, G OFT results. F Total distance traveled in the OFT (Kruskal‒Wallis test, one-way ANOVA, F(2,31) = 0.130). F Time spent in the central area (Tukey’s HSD test, one-way ANOVA, F(2,31) = 1.278). H SPT results (Kruskal‒Wallis test, one-way ANOVA, F(2,31) = 8.347). (A) was created in BioRender. Chen, X. (2025). https://BioRender.com/m26q803. The data are expressed as the means ± SEMs. *p < 0.05, **p < 0.01; ns not significant.
Discussion
The CSDS model was used in this study to simulate the psychopathological changes observed in humans with depression and to study social avoidance and affective disorder-like behavioral deficits after exposure to uncontrollable social defeat stress. Previous research has shown that the classic 10-day CSDS paradigm does not lead to stable behavioral deficits in the tail suspension test (TST) or forced swim test (FST) [44]. However, it can induce stable behavioral changes in the SPT, which reflects anhedonia, which is associated with depressive-like behavior. On the basis of these findings and numerous previous experiments by our laboratory, the SIT, OFT, EPM test, and SPT, were used in this study. Using the classic CSDS paradigm, we successfully induced depressive behaviors in the susceptible group but not in the control group.
In recent years, a close relationship between depression and metabolic disorders, in which lipids play an important role, was established. Studies have shown the potential value of assessing lipid metabolism in patients with depression to help address this important clinical challenge [45,46,47]. Furthermore, a study on CSDS model rodents revealed that social defeat stress and lipid metabolism disorders interact and facilitate the development of a depression-like phenotype [48]. Although the mechanism underlying this phenomenon has not been well explained at the molecular level, these findings provide a framework to better understand the relationship between social defeat stress and lipid metabolism disorders. Past research revealed a significant 4% correlation between bile acid levels and depression [49], and other clinical data revealed a strong correlation between blood bile acid levels and the severity of depressive symptoms [50]. Notably, anhedonia is a core symptom of clinical depression, so the above studies reflect the basic connection between bile acid metabolism and depression in clinical settings. In addition, studies on animal models have shown that the development of depressive-like behaviors, including a decrease in sucrose preference, reflecting anhedonia, is often accompanied by bile acid metabolism disorders [35, 51, 52]. There are many types of stimuli used to induce depression in animals, including CSDS stimuli, which are related to social behavior. Some studies have confirmed that bile acids can alleviate depressive-like behaviors in animals [53]. Considering the previous findings related to the effects of antidepressants on bile acid receptors, we hypothesize that the manipulation of bile acids and their associated pathways has potential for antidepressant treatment. In this study, the ELISA results revealed that the levels of CYP7A1 and CYP8B1, the key enzymes in the classical bile acid synthesis pathway, were significantly lower in susceptible mice than in control mice, suggesting that bile acid synthesis was impaired in CSDS-susceptible mice. Newly published research shows that there is a decrease in the serum bile acid level in CSDS-susceptible mice, which also supports our findings. In addition, the levels of various bile acids in the hypothalamus were measured, all of which tended to be decreased to varying degrees [35]. Does a similar phenomenon occur in the mPFC and hippocampus, which are closely related to affective disorders? Next, we measured TBA levels in these two brain regions, and the results revealed that the TBA level in the hippocampus but not the mPFC was significantly decreased in the susceptible group, suggesting that the hippocampus is the core region involved in the downstream effects of these changes.
Previous studies have shown that bile acids can protect the central nervous system (CNS) from harmful stimuli and inflammation [54, 55], in part through SC–CA1 synapses. However, the mechanisms through which bile acids, their specific receptors and social defeat interact remain unclear. A series of studies have demonstrated the vital role of TGR5, a GPCR, in the nervous system and highlighted its potential value as a research target. To explore the biological mechanism underlying the effect of social defeat stress, we analyzed the expression of TGR5 in the hippocampus and preliminarily confirmed its association with the effects of CSDS, and the results were consistent with the findings of a recent study [34]. In general, TGR5 is involved in various biological processes in the brain. For example, it participates in the anti-inflammatory function of microglia and plays a neuroprotective role. TGR5 has been reported to be associated with a variety of neurological and neuropsychiatric diseases, such as subarachnoid hemorrhage, middle cerebral artery occlusion, anorexia, and epilepsy. Many signaling pathways are involved in TGR5 function. For example, the cAMP pathway is involved in TGR5-mediated anti-inflammatory effects and has been shown to attenuate oxidative stress and neuronal apoptosis in subarachnoid hemorrhage [56]. In addition, the BRCA1/Sirt1 [57] and Sirt3 [57] pathways are involved in the physiological effects of TGR5 in the brain. Previous studies by others have examined the expression level of TGR5 in the mPFC, hypothalamus, NAc, ventral tegmental area (VTA) and dorsolateral striatum (DLS). The results revealed that the TGR5 expression level was decreased in the hippocampus, mPFC and hypothalamus. Considering that the mechanism underlying the antidepressant effect of TGR5 was previously shown to involve the neural circuit from the hypothalamus to the hippocampus to the septal nucleus, we speculate that there may be a neural network in the brain that has the potential to mediate depressive-like behavior in which TGR5 is the main target molecule. With respect to the molecular mechanisms underlying affective disorders, especially depression, recent research has shown that TGR5 can mediate antidepressant effects through hippocampal pyramid neurons. CSDS significantly reduces TGR5 expression levels in hippocampal CA3 pyramidal neurons, which is similar to our findings regarding TGR5 levels throughout the hippocampus. The manipulation of TGR5 expression by AAV injection can significantly mediate depressive-like phenotypes, whereas the infusion of specific agonists can prevent CSDS-induced behavioral deficits. However, in TGR5 knockout mice, the overexpression of TGR5, but not the infusion of a specific agonist, significantly ameliorates depressive-like behaviors caused by exposure to CSDS. Our results confirm this result from another perspective; that is, HDCA could not promote hippocampal synaptic plasticity in CSDS mice-exposed with reduced TGR5 expression levels. However, by targeting its downstream signaling pathways, we show that hippocampal synaptic plasticity can be increased without manipulating TGR5 expression, which presents another possible treatment option. Further research on neural circuits has revealed that TGR5 in LHAGABAergic neurons exerts an antidepressant-like effect by relieving the inhibition of dCA3CaMKIIa neurons that project to the DLS. This finding suggests that the hippocampus plays an important role as an intermediate brain region in the neural network underlying the antidepressant effect of TGR5, suggesting that it is highly important to study the overall changes in the hippocampus and the its subregions. Although multiple findings have shown that the hippocampus is highly involved in the mechanism underlying the antidepressant effect of TGR5, the downstream pathway through which this effect is mediated remains to be further clarified. Overall, we speculate that there may be a signaling pathway downstream of TGR5 in the hippocampus that functions as a signal amplifier and mediates the biological effects of TGR5.
The SC‒CA1 circuit, which is commonly used to study synaptic transmission, is a key component of the hippocampal circuitry, and multiple antidepressants have been found to affect SC‒CA1 synapses. As a part of the complex neural network in the hippocampus, the CA3 region plays a key role in information integration and transmission. It receives information from the DG via mossy fibers and transmits this information to the CA1 region through SCs, whereas the CA1 region is responsible for transmitting processed information to other brain regions. These regions are interconnected and jointly support the function of the hippocampus. Studies on antidepressants have shown that ketamine can mediate the rapid and persistent enhancement of fEPSPs at SC–CA1 synapses through AMPA receptors [58]. Additionally, chronic fluoxetine administration is associated with enhanced synaptic dynamics atypical of SC–CA1 synapses, elevated hippocampal plasticity, and improved hippocampus-dependent behavior [59]. As mentioned in the introduction, neural circuits linking the CA1 and CA3 regions and other regions of the hippocampus mediate the depression-like phenotype and social behavior changes caused by CSDS; therefore, exploring the functions of connections among hippocampal subregions is highly important. As potential targets of antidepressants, SC–CA1 synapses show great promise, and whether these synapses mediate the effects of social defeat and the function of TGR5 is worthy of further study.
To confirm our hypothesis, we used the natural TGR5-specific agonist HDCA and recorded fEPSPs via the patch-clamp technique, and we detected significant enhancement of SC–CA1 synaptic plasticity in the hippocampus. Further experiments revealed that TGR5 enhanced neural plasticity at SC–CA1 synapses in the hippocampus and that synaptic transmission could be blocked by the protein kinase A (PKA) antagonist H-89. H-89 is a potent and selective cAMP-dependent PKA inhibitor that inhibits PKA in a competitive manner with ATP. Furthermore, we perfused forskolin and HDCA successively in blockade experiments to determine the specific relationship between TGR5 and the cAMP/PKA pathway. These experiments proved that the ability of HDCA-mediated TGR5 activation to enhance synaptic plasticity in the hippocampus of control mice specifically involves the cAMP/PKA pathway, indicating that this pathway has another important function. Combined with the WB results, these results suggest that the decrease in TGR5 expression in the hippocampus of susceptible mice may result in a decrease in the number of HDCA receptors. A study involving the administration of the cAMP agonist forskolin to susceptible mice revealed that it can continuously enhance hippocampal synaptic plasticity in these mice, which further reinforces our hypothesis. Forskolin treatment can circumvent the impact of insufficient TGR5 expression, revealing that the upstream signaling molecule cAMP can act as a signal amplifier to improve synaptic function.
Increasing neuroplasticity is considered the basis of alleviating depression. Our results showed that TGR5 and its downstream signaling molecules can be used as targets to increase neuroplasticity, but further behavioral data are needed for verification. Given the results observed in brain slices, we focused on whether drug administration through commonly used methods, such as intraperitoneal injection, can mediate behavioral changes in animals. Forskolin (coleonol) is an effective activator of adenylate cyclase and an inducer of intracellular cAMP formation. Previous clinical studies have shown reduced adenylyl cyclase activity and disturbances in the postreceptor cAMP signaling cascade in the postmortem temporal cortex of depressed suicide victims [60]. Correspondingly, forskolin-stimulated changes in adenyl cyclase activity appear to reflect qualitative differences in adenyl cyclase activity in patients with major depressive disorder (MDD). In rats, forskolin has been shown to exert strong antidepressive effects in the FST [61]. At a concentration of 0.01–0.1 mg/kg, forskolin dose-dependently reduces the immobility time of rats in the FST. Its effect is similar to that of amitriptyline, but it does not affect spontaneous locomotor activity. In addition, chronic oral administration of the forskolin analog NKH477 at doses of 0.5–1.5 mg/kg similarly significantly shortens the duration of immobility. However, its effect has not been verified the CSDS model. Consistently, we treated both control and CSDS model mice with 0.1 mg/kg forskolin (i.p.) for 10 days and observed a significant reduction in anxiety-like behavior in the EPM test and anhedonic behavior in the SPT in CSDS model mice. Our study provides a reference for determining the effective concentration of forskolin and duration of forskolin treatment for alleviating anxiety and depression after CSDS exposure and proves that chronic forskolin treatment can effectively inhibit behavioral changes caused by CSDS, providing a basis for future research.
Overall (Fig. 5), our results demonstrated that TGR5 mediates plasticity SC–CA1 synapses in the hippocampus and behavioral changes in susceptible mice via the cAMP/PKA signaling pathway. Through the regulation of downstream signaling pathway-related molecules, it effectively alleviates anxiety- and depression-like behaviors. This study not only elucidates the mechanism underlying the specific changes induced by CSDS but also identifies possible molecular targets and their functional pathways. These findings show that the TGR5/cAMP/PKA pathway can be precisely regulated to modulate the behavior of mice and may provide insight into the mechanism underlying the antianxiety and antidepression effects of this pathway.
This figure was created in BioRender. Chen, X. (2025). https://biorender.com/e64j923.
Data availability
Data will be made available on request.
References
Kraus C, Kadriu B, Lanzenberger R, Zarate CA, Kasper S. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry. 2019;9:127.
Marx W, Penninx B, Solmi M, Furukawa TA, Firth J, Carvalho AF, et al. Major depressive disorder. Nat Rev Dis Primers. 2023;9:44.
Iob E, Kirschbaum C, Steptoe A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry. 2020;25:1130–40.
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–36.
Guo B, Zhang M, Hao W, Wang Y, Zhang T, Liu C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl Psychiatry. 2023;13:5.
Radjabzadeh D, Bosch JA, Uitterlinden AG, Zwinderman AH, Ikram MA, Van Meurs JBJ, et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun. 2022;13:7128.
Kennis M, Gerritsen L, Van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:321–38.
Lu J, Zhang Z, Yin X, Tang Y, Ji R, Chen H, et al. An entorhinal-visual cortical circuit regulates depression-like behaviors. Mol Psychiatry. 2022;27:3807–20.
Teng T, Clarke G, Maes M, Jiang Y, Wang J, Li X, et al. Biogeography of the large intestinal mucosal and luminal microbiome in cynomolgus macaques with depressive-like behavior. Mol Psychiatry. 2022;27:1059–67.
Wu J, Li Y, Huang Y, Liu L, Zhang H, Nagy C, et al. Integrating spatial and single-nucleus transcriptomic data elucidates microglial-specific responses in female cynomolgus macaques with depressive-like behaviors. Nat Neurosci. 2023;26:1352–64.
Hu X, Li Y, Wu J, Zhang H, Huang Y, Tan X, et al. Changes of gut microbiota reflect the severity of major depressive disorder: a cross sectional study. Transl Psychiatry. 2023;13:137.
Yang J, Zheng P, Li Y, Wu J, Tan X, Zhou J, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv. 2020;6:eaba8555.
Choi KW, Lee YH, Liu Z, Fatori D, Bauermeister JR, Luh RA, et al. Social support and depression during a global crisis. Nat Ment Health. 2023;1:428–35.
Lin S, Huang L, Luo ZC, Li X, Jin SY, Du ZJ, et al. The ATP level in the medial prefrontal cortex regulates depressive-like behavior via the medial prefrontal cortex-lateral habenula pathway. Biol Psychiatry. 2022;92:179–92.
Wang J, Chen HS, Li HH, Wang HJ, Zou RS, Lu XJ, et al. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav Immun. 2023;109:23–36.
Kim J, Kang S, Choi TY, Chang KA, Koo JW. Metabotropic glutamate receptor 5 in amygdala target neurons regulates susceptibility to chronic social stress. Biol Psychiatry. 2022;92:104–15.
Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062.
Wang T, Song Z, Zhao X, Wu Y, Wu L, Haghparast A, et al. Spatial transcriptomic analysis of the mouse brain following chronic social defeat stress. Exploration. 2023;3:20220133.
Wu JL, Li ZM, Chen H, Chen WJ, Hu NY, Jin SY, et al. Distinct septo-hippocampal cholinergic projections separately mediate stress-induced emotional and cognitive deficits. Sci Adv. 2024;10:eado1508.
Xia F, Fascianelli V, Vishwakarma N, Ghinger FG, Kwon A, Gergues MM, et al. Understanding the neural code of stress to control anhedonia. Nature. 2024. https://doi.org/10.1038/s41586-024-08241-y.
Liu Y, Deng SL, Li LX, Zhou ZX, Lv Q, Wang ZY, et al. A circuit from dorsal hippocampal CA3 to parvafox nucleus mediates chronic social defeat stress-induced deficits in preference for social novelty. Sci Adv. 2022;8:eabe8828.
Li HH, Liu Y, Chen HS, Wang J, Li YK, Zhao Y, et al. PDGF-BB-dependent neurogenesis buffers depressive-like behaviors by inhibition of GABAergic projection from medial septum to dentate gyrus. Adv Sci. 2023;10:e2301110.
Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry. 2019;85:487–97.
Song Y, Cho JH, Kim H, Eum YJ, Cheong EN, Choi S, et al. Association between taurine level in the hippocampus and major depressive disorder in young women: a proton magnetic resonance spectroscopy study at 7T. Biol Psychiatry. 2024;95:465–72.
Borsini A, Nicolaou A, Camacho-Muñoz D, Kendall AC, Di Benedetto MG, Giacobbe J, et al. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry. 2021;26:6773–88.
Liu L, Tang J, Liang X, Li Y, Zhu P, Zhou M, et al. Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress. Mol Psychiatry. 2024;29:2031–42.
Deutschmann K, Reich M, Klindt C, Droge C, Spomer L, Haussinger D, et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1319–25.
Castellanos-Jankiewicz A, Guzman-Quevedo O, Fenelon VS, Zizzari P, Quarta C, Bellocchio L, et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021;33:1483–92.e10.
Jin P, Deng S, Tian M, Lenahan C, Wei P, Wang Y, et al. INT-777 prevents cognitive impairment by activating Takeda G protein-coupled receptor 5 (TGR5) and attenuating neuroinflammation via cAMP/ PKA/ CREB signaling axis in a rat model of sepsis. Exp Neurol. 2021;335:113504.
Wu X, Lv YG, Du YF, Hu M, Reed MN, Long Y, et al. Inhibitory effect of INT-777 on lipopolysaccharide-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Prog Neuro Psychopharmacol Biol Psychiatry. 2019;88:360–74.
Huang R, Gao Y, Chen J, Duan Q, He P, Zhang J, et al. TGR5 agonist INT-777 alleviates inflammatory neurodegeneration in Parkinson’s disease mouse model by modulating mitochondrial dynamics in microglia. Neuroscience. 2022;490:100–19.
Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–30.
Hu X, Yan J, Huang L, Araujo C, Peng J, Gao L, et al. INT-777 attenuates NLRP3-ASC inflammasome-mediated neuroinflammation via TGR5/cAMP/PKA signaling pathway after subarachnoid hemorrhage in rats. Brain Behav Immun. 2021;91:587–600.
Wang H, Tan YZ, Mu RH, Tang SS, Liu X, Xing SY, et al. Takeda G protein-coupled receptor 5 modulates depression-like behaviors via hippocampal CA3 pyramidal neurons afferent to dorsolateral septum. Biol Psychiatry. 2021;89:1084–95.
Li XY, Zhang SY, Hong YZ, Chen ZG, Long Y, Yuan DH, et al. TGR5-mediated lateral hypothalamus-dCA3-dorsolateral septum circuit regulates depressive-like behavior in male mice. Neuron. 2024;112:1795–814.e10.
Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91.
Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–28.
Tu H, Okamoto AY, Shan B. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc Med. 2000;10:30–35.
Kwong E, Li Y, Hylemon PB, Zhou H. Bile acids and sphingosine-1-phosphate receptor 2 in hepatic lipid metabolism. Acta Pharm Sin B. 2015;5:151–7.
Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002;43:359–64.
Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–41.
Blazev R, Hussain M, Bakker AJ, Head SI, Lamb GD. Effects of the PKA inhibitor H-89 on excitation-contraction coupling in skinned and intact skeletal muscle fibres. J Muscle Res Cell Motil. 2001;22:277–86.
Robbins JD, Boring DL, Tang WJ, Shank R, Seamon KB. Forskolin carbamates: binding and activation studies with type I adenylyl cyclase. J Med Chem. 1996;39:2745–52.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Almulla AF, Thipakorn Y, Algon AAA, Tunvirachaisakul C, Al-Hakeim HK, Maes M. Reverse cholesterol transport and lipid peroxidation biomarkers in major depression and bipolar disorder: a systematic review and meta-analysis. Brain Behav Immun. 2023;113:374–88.
Milaneschi Y, Simmons WK, Van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24:18–33.
Pan LA, Naviaux JC, Wang L, Li K, Monk JM, Lingampelly SS, et al. Metabolic features of treatment-refractory major depressive disorder with suicidal ideation. Transl Psychiatry. 2023;13:393.
Chuang JC, Cui H, Mason BL, Mahgoub M, Bookout AL, Yu HG, et al. Chronic social defeat stress disrupts regulation of lipid synthesis. J Lipid Res. 2010;51:1344–53.
Wu L, Zhao M, Li M, Guo Q, Ren Z, Zheng X, et al. The clinical and mechanistic roles of bile acids in depression, Alzheimer’s disease, and stroke. Proteomics. 2022;22:e2100324.
Sun N, Zhang J, Wang J, Liu Z, Wang X, Kang P, et al. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin Neurosci. 2022;76:321–8.
Morito K, Yamagata M, Naka F, Kobayashi K, Ueda H, Morimoto H, et al. Sub-chronic and mild social defeat stress exposure to C57BL/6J mice increases visceral fat mass and causes accumulation of cholesterol and bile acids in the liver. Biochem Biophys Res Commun. 2024;702:149631.
Yang Y, Eguchi A, Mori C, Hashimoto K. Depression-like phenotypes in mice following common bile duct ligation: insights into the gut-liver-brain axis via the vagus nerve. Neurobiol Dis. 2024;192:106433.
Cheng L, Huang C, Chen Z. Tauroursodeoxycholic acid ameliorates lipopolysaccharide-induced depression like behavior in mice via the inhibition of neuroinflammation and oxido-nitrosative stress. Pharmacology. 2019;103:93–100.
Jia W, Li Y, Cheung KCP, Zheng X. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis. Sci China Life Sci. 2023;67:865–78.
Yanguas-Casas N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramirez L. TUDCA: an agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. J Cell Physiol. 2017;232:2231–45.
Zuo G, Zhang T, Huang L, Araujo C, Peng J, Travis Z, et al. Activation of TGR5 with INT-777 attenuates oxidative stress and neuronal apoptosis via cAMP/PKCε/ALDH2 pathway after subarachnoid hemorrhage in rats. Free Radic Biol Med. 2019;143:441–53.
Liang H, Matei N, McBride DW, Xu Y, Tang J, Luo B, et al. Activation of TGR5 protects blood brain barrier via the BRCA1/Sirt1 pathway after middle cerebral artery occlusion in rats. J Biomed Sci. 2020;27:61.
Tang XH, Zhang GF, Xu N, Duan GF, Jia M, Liu R, et al. Extrasynaptic CaMKIIα is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model. J Neuroinflammation. 2020;17:181.
Popova D, Castren E, Taira T. Chronic fluoxetine administration enhances synaptic plasticity and increases functional dynamics in hippocampal CA3-CA1 synapses. Neuropharmacology. 2017;126:250–6.
Reiach JS, Li PP, Warsh JJ, Kish SJ, Young LT. Reduced adenylyl cyclase immunolabeling and activity in postmortem temporal cortex of depressed suicide victims. J Affect Disord. 1999;56:141–51.
Maeda H, Ozawa H, Saito T, Irie T, Takahata N. Potential antidepressant properties of forskolin and a novel water-soluble forskolin (NKH477) in the forced swimming test. Life Sci. 1997;61:2435–42.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2017YFA0505700), The Chongqing Medical Scientific Research Project (Joint Project of Chongqing Health Commission and Science and Technology Bureau) (No. 2023CCXM003), and the Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-BHX0725).
Author information
Authors and Affiliations
Contributions
PX and XYC conceived the study and designed experiments; XYC, QJZ, and YH performed the experiments; XYC, YYJ, YR, CC, YW, JCC, HMY and YKR contributed new reagents or analytic tools; XYC, QJZ, YH and KC interpreted the data; XYC edited the manuscript. All authors contributed to the revisions of the manuscript, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experimental steps in this study were conducted in strict accordance with the animal testing guidelines of Chongqing Medical University and approved by the Ethics Committee of Chongqing Medical University. The experimental process, animal care and treatments were in compliance with the guide for the Care and Use of laboratory animals published by the National Institutes of Health (NIH). Animal operations were approved by the experimental Animal Ethics Committee of Chongqing Medical University(Approval NO.2017013).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, X., Zhou, Q., He, Y. et al. TGR5 dysfunction underlies chronic social defeat stress via cAMP/PKA signaling pathway in the hippocampus. Transl Psychiatry 15, 366 (2025). https://doi.org/10.1038/s41398-025-03599-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03599-7