Abstract
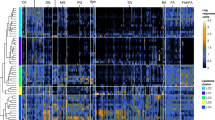
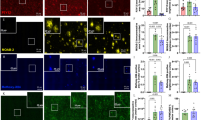
Mild cognitive impairment (MCI) is an early stage in the progression toward dementia. Lipids are central to neurodegeneration, yet the biomarker potential of lipidomics from saliva, plasma, and feces remains underexplored. As part of the Microbiome in Aging Gut and Brain (MiaGB) consortium, saliva, plasma, and fecal samples were collected from older adults with MCI and healthy controls. Samples were analyzed by high-performance liquid chromatography coupled with high-resolution mass spectrometry (LC/MS), to profile lipidomic alterations and identify candidate biomarkers. Lipidomic profiling annotated over 200 molecular species spanning five major lipid classes. Compared with controls, MCI patients exhibited increased oxidized triacylglycerols (oxTGs) in saliva, reduced cholesteryl linoleate (CE 18:2) in plasma, and decreased fatty acid esters of hydroxy fatty acids (FAHFAs) in feces. Receiver operating characteristic (ROC) analysis identified α-linolenic acid (FA 18:3), docosapentaenoic acid (FA 22:5), and CE 18:2 as discriminatory metabolites with notable diagnostic performance. Moreover, elevated fecal triacylglycerols containing medium-chain fatty acids (TG-MCFAs) were observed in MCI, suggesting impaired lipid absorption or altered metabolism. This multi-sample lipidomics strategy highlights TG-MCFAs as fecal biomarkers for MCI detection, supporting further mechanistic and longitudinal validation.
Similar content being viewed by others
Data availability
All data relevant to the study are contained within the article and supplementary information.
References
DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14:32.
Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–8.
Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of early alzheimer’s disease: clinical practice in 2021. J Prev Alzheimers Dis. 2021;8:371–86.
Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–55.
Bai W, Chen P, Cai H, Zhang Q, Su Z, Cheung T, et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies. Age Ageing. 2022;51:afac173. https://doi.org/10.1093/ageing/afac173.
Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer’s disease: challenging but feasible. Biomark Med. 2010;4:65–79.
Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 2018;14:981–8.
Hane FT, Robinson M, Lee BY, Bai O, Leonenko Z, Albert MS. Recent progress in alzheimer’s disease research, part 3: diagnosis and treatment. J Alzheimers Dis. 2017;57:645–65.
Zhang X, Liu W, Zan J, Wu C, Tan W. Untargeted lipidomics reveals progression of early Alzheimer’s disease in APP/PS1 transgenic mice. Sci Rep. 2020;10:14509.
O’Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6:537–44.
Byeon SK, Madugundu AK, Jain AP, Bhat FA, Jung JH, Renuse S, et al. Cerebrospinal fluid lipidomics for biomarkers of Alzheimer’s disease. Mol Omics. 2021;17:454–63.
Kao Y-C, Ho P-C, Tu Y-K, Jou I-M, Tsai K-J Lipids and Alzheimer’s disease. Int J Mol Sci 2020; 21. https://doi.org/10.3390/ijms21041505.
Huynh K, Lim WLF, Giles C, Jayawardana KS, Salim A, Mellett NA, et al. Concordant peripheral lipidome signatures in two large clinical studies of Alzheimer’s disease. Nat Commun. 2020;11:5698.
Walter A, Korth U, Hilgert M, Hartmann J, Weichel O, Hilgert M, et al. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol Aging. 2004;25:1299–303.
Lefèvre-Arbogast S, Hejblum BP, Helmer C, Klose C, Manach C, Low DY, et al. Early signature in the blood lipidome associated with subsequent cognitive decline in the elderly: A case-control analysis nested within the Three-City cohort study. EBioMedicine. 2021;64:103216.
Wood PL. Lipidomics of Alzheimer’s disease: current status. Alzheimers Res Ther. 2012;4:5.
Ferré-González L, Lloret A, Cháfer-Pericás C. Systematic review of brain and blood lipidomics in Alzheimer’s disease mouse models. Prog Lipid Res. 2023;90:101223.
Peña-Bautista C, Álvarez-Sánchez L, Roca M, García-Vallés L, Baquero M, Cháfer-Pericás C Plasma lipidomics approach in early and specific alzheimer’s disease diagnosis. J Clin Med 2022; 11. https://doi.org/10.3390/jcm11175030.
Liu Y, Thalamuthu A, Mather KA, Crawford J, Ulanova M, Wong MWK, et al. Plasma lipidome is dysregulated in Alzheimer’s disease and is associated with disease risk genes. Transl Psychiatry. 2021;11:344.
Wang T, Arnold M, Huynh K, Weinisch P, Giles C, Mellett NA, et al. Trajectory of plasma lipidome associated with the risk of late-onset Alzheimer’s disease: a longitudinal cohort study. EBioMedicine. 2025;118:105826.
Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–8.
James AS, Adil NA, Goltz D, Tangudu D, Chaudhari DS, Shukla R, et al. Abnormalities in gut virome signatures linked with cognitive impairment in older adults. Gut Microbes. 2024;16:2431648.
Walters KF, Shukla R, Kumar V, Schueren S, Yadav H, Schilaty ND et al. Resting-State EEG power spectral density analysis between healthy and cognitively impaired subjects. Brain Sci 2025; 15. https://doi.org/10.3390/brainsci15020173.
Jayaprakash J, Gowda D, M Gangadhara R, Jain S, Yadav H, et al. Discovering novel short- and medium-chain esters of hydroxy fatty acids in human fecal samples using untargeted liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2025;39:e10032.
Chaudhari DS, Jain S, Yata VK, Mishra SP, Kumar A, Fraser A, et al. Unique trans-kingdom microbiome structural and functional signatures predict cognitive decline in older adults. Geroscience. 2023;45:2819–34.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
Minami Y, B Gowda SG, Gowda D, Chiba H, Hui S-P. Regio-specific lipid fingerprinting of edible sea cucumbers using LC/MS. Food Res Int. 2024;184:114253.
Jayaprakash J, B Gowda SG, K Shukla P, Gowda D, et al. Sex-Specific effect of ethanol on colon content lipidome in a mice model using nontargeted LC/MS. ACS Omega. 2024;9:16044–54.
Nath LR, B Gowda SG, Roberts TH, Gowda D, Khoddami A, Hui SP. Nontargeted lipidomics of sorghum grain reveals novel fatty acid esters of hydroxy fatty acids and cultivar differences in lipid profiles. J Agric Food Chem. 2024;72:20690–703.
Zhao L, Duan X, Liu H. Novel grade classification tool with lipidomics for indica rice eating quality evaluation. Foods. 2023;12:944 https://doi.org/10.3390/foods12050944.
Sakr F, Dyrba M, Bräuer A, Teipel S. Alzheimer’s disease neuroimaging initiative. association of lipidomics signatures in blood with clinical progression in preclinical and prodromal alzheimer’s disease. J Alzheimers Dis. 2022;85:1115–27.
Barupal DK, Baillie R, Fan S, Saykin AJ, Meikle PJ, Arnold M, et al. Sets of coregulated serum lipids are associated with Alzheimer’s disease pathophysiology. Alzheimers Dement. 2019;11:619–27.
Wang X, Bui H, Vemuri P, Graff-Radford J, Jack CR, Petersen RC, et al. Lipidomic network of mild cognitive impairment from the mayo clinic study of aging. J Alzheimers Dis. 2021;81:533–43.
Proitsi P, Kim M, Whiley L, Simmons A, Sattlecker M, Velayudhan L, et al. Association of blood lipids with alzheimer’s disease: a comprehensive lipidomics analysis. Alzheimers Dement. 2017;13:140–51.
Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18:383–99.
Hosseini M, Poljak A, Braidy N, Crawford J, Sachdev P. Blood fatty acids in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res Rev. 2020;60:101043.
Nien S-W, Lin I-H, Wu H-C, Chen Y-H, Yang S-C Evaluation of dietary intake in individuals with mild cognitive impairment. Nutrients 2023; 15. https://doi.org/10.3390/nu15173694.
Baierle M, Vencato P, Oldenburg L, Bordignon S, Zibetti M, Trentini C, et al. Fatty acid status and its relationship to cognitive decline and homocysteine levels in the elderly. Nutrients. 2014;6:3624–40.
Dakterzada F, Jové M, Cantero JL, Mota-Martorell N, Pamplona R, Piñoll-Ripoll G. The shift in the fatty acid composition of the circulating lipidome in Alzheimer’s disease. Alzheimers Dement. 2024;20:3322–33.
Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr Health. 2006;18:249–59.
Park YH, Shin SJ, Kim HS, Hong SB, Kim S, Nam Y et al. Omega-3 fatty acid-type docosahexaenoic acid protects against aβ-mediated mitochondrial deficits and pathomechanisms in alzheimer’s disease-related animal model. Int J Mol Sci 2020; 21. https://doi.org/10.3390/ijms21113879.
Lee AY, Lee MH, Lee S, Cho EJ. Alpha-Linolenic Acid from Perilla frutescens var. japonica Oil Protects Aβ-Induced Cognitive Impairment through Regulation of APP Processing and Aβ Degradation. J Agric Food Chem. 2017;65:10719–29.
Wei B-Z, Li L, Dong C-W, Tan C-C. Alzheimer’s disease neuroimaging initiative, xu w. the relationship of omega-3 fatty acids with dementia and cognitive decline: evidence from prospective cohort studies of supplementation, dietary intake, and blood markers. Am J Clin Nutr. 2023;117:1096–109.
Lázaro I, Brugulat-Serrat A, Suárez-Calvet M, Fauria K, Minguillon C, Gispert J-D, et al. Red blood cell ω-3 status and longitudinal cognition in individuals at risk of Alzheimer disease. J Nutr. 2025;155:4514–22. https://doi.org/10.1016/j.tjnut.2025.09.032.
Naaman RK, Alashmali S, Bakhsh MA, Muqaibil AA, Ghunaim FM, Alattas AH. Association of omega-3 polyunsaturated fatty acids intake and cognitive function in middle-aged and older adults. Nutr Neurosci. 2025;28:649–58.
Grimm MOW, Kuchenbecker J, Grösgen S, Burg VK, Hundsdörfer B, Rothhaar TL, et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem. 2011;286:14028–39.
Barros MI, Brandão T, Irving SC, Alves P, Gomes F, Correia M. Omega-3 polyunsaturated fatty acids and cognitive decline in adults with non-dementia or mild cognitive impairment: an overview of systematic reviews. Nutrients. 2025;17:3002 https://doi.org/10.3390/nu17183002.
Snowden SG, Ebshiana AA, Hye A, An Y, Pletnikova O, O’Brien R, et al. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: a nontargeted metabolomic study. PLoS Med. 2017;14:e1002266.
de Oliveira Otto MC, Wu JHY, Thacker EL, Lai HTM, Lemaitre RN, Padhye N, et al. Circulating omega-3 and omega-6 fatty acids, cognitive decline, and dementia in older adults. J Alzheimers Dis. 2023;95:965–79.
Hwangbo N, Zhang X, Raftery D, Gu H, Hu S-C, Montine TJ, et al. Predictive modeling of alzheimer’s and parkinson’s disease using metabolomic and lipidomic profiles from cerebrospinal fluid. Metabolites. 2022;12:277.
Macias S, Yilmaz A, Kirma J, Moore SE, Woodside JV, Graham SF, et al. Non-targeted LC–MS/MS metabolomic profiling of human plasma uncovers a novel Mediterranean diet biomarker panel. Metabolomics. 2023;20:3.
Nitsch R, Pittas A, Blusztajn JK, Slack BE, Growdon JH, Wurtman RJ. Alterations of phospholipid metabolites in postmortem brain from patients with Alzheimer’s disease. Ann N Y Acad Sci. 1991;640:110–3.
Xu S, Zhu Z, Delafield DG, Rigby MJ, Lu G, Braun M, et al. Spatially and temporally probing distinctive glycerophospholipid alterations in Alzheimer’s disease mouse brain via high-resolution ion mobility-enabled sn-position resolved lipidomics. Nat Commun. 2024;15:6252.
Peña-Bautista C, Roca M, López-Cuevas R, Baquero M, Vento M, Cháfer-Pericás C. Metabolomics study to identify plasma biomarkers in alzheimer disease: ApoE genotype effect. J Pharm Biomed Anal. 2020;180:113088.
Ahsanul Haque M, Omori N, Md Sheikh A, Yano S, Osago H, Mitaki S, et al. Analysis of the time-dependent changes of phospholipids in the brain regions of a mouse model of Alzheimer’s disease. Brain Res. 2023;1800:148197.
Chalbot S, Zetterberg H, Blennow K, Fladby T, Grundke-Iqbal I, Iqbal K. Cerebrospinal fluid secretory Ca2+-dependent phospholipase A2 activity is increased in Alzheimer disease. Clin Chem. 2009;55:2171–9.
Yin F. Lipid metabolism and Alzheimer’s disease: clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2023;290:1420–53.
Iqbal G, Braidy N, Ahmed T. Blood-Based biomarkers for predictive diagnosis of cognitive impairment in a pakistani population. Front Aging Neurosci. 2020;12:223.
Castillo-Mendieta T, Arana-Lechuga Y, Campos-Peña V, Sosa AL, Orozco-Suarez S, Pinto-Almazán R, et al. Plasma levels of amyloid-β peptides and tau protein in mexican patients with alzheimer’s disease. J Alzheimers Dis. 2021;82:S271–S281.
Dakterzada F, Benítez ID, Targa A, Carnes A, Pujol M, Jové M, et al. Cerebrospinal fluid lipidomic fingerprint of obstructive sleep apnoea in Alzheimer’s disease. Alzheimers Res Ther. 2023;15:134.
Dakterzada F, Benítez ID, Targa A, Carnes A, Pujol M, Jové M, et al. Blood-based lipidomic signature of severe obstructive sleep apnoea in Alzheimer’s disease. Alzheimers Res Ther. 2022;14:163.
Prakash P, Manchanda P, Paouri E, Bisht K, Sharma K, Rajpoot J, et al. Amyloid-β induces lipid droplet-mediated microglial dysfunction via the enzyme DGAT2 in Alzheimer’s disease. Immunity. 2025;58:1536–1552.e8.
Dunn E, Zhang B, Sahota VK, Augustin H. Potential benefits of medium chain fatty acids in aging and neurodegenerative disease. Front Aging Neurosci. 2023;15:1230467 https://doi.org/10.3389/fnagi.2023.1230467.
Espina A, Mendoza E, Lao A Modelling the effects of medium-chain triglycerides on cerebral ketone body metabolism. Front Syst Biol 2022; 2. https://doi.org/10.3389/fsysb.2022.907957.
Juby AG, Blackburn TE, Mager DR Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: a randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimers Dement: TRCI. 2022;8. https://doi.org/10.1002/trc2.12259.
Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18:437–46.
Jayaprakash J, Gowda SGB, Gowda D, Ikeda A, Bamai YA, Ketema RM, et al. Plasma lipidomics of preadolescent children: a hokkaido study. J Lipids. 2025;2025:3106145.
Wood PL Fatty acyl esters of hydroxy fatty acid (FAHFA) lipid families. Metabolites 2020; 10. https://doi.org/10.3390/metabo10120512.
Obis E, Sol J, Andres-Benito P, Martín-Gari M, Mota-Martorell N, Galo-Licona JD, et al. Lipidomic alterations in the cerebral cortex and white matter in sporadic alzheimer’s disease. Aging Dis. 2023;14:1887–916.
Proitsi P, Kim M, Whiley L, Pritchard M, Leung R, Soininen H, et al. Plasma lipidomics analysis finds long chain cholesteryl esters to be associated with alzheimer’s disease. Transl Psychiatry. 2015;5:e494.
Acknowledgements
We would like to acknowledge the resources provided by the University of South Florida (USF) Center for Microbiome Research, the Microbiomes Institute, the Center for Excellence in Aging and Brain Repair, the Department of Neurosurgery and Brain Repair, and the USF Morsani College of Medicine.
Funding
This work was supported by the JST SPRING (Grant Number JPMJSP2119) and the Japan Society for the Promotion of Science KAKENHI Grants (25K00258 and 23K06861). Additional support was provided by the Ed and Ethel Moore Alzheimer’s Disease Research Program of the Florida Department of Health (Grant Number 22A17), as well as the U.S. National Institutes of Health, the National Institute on Aging (Grant Numbers RF1AG071762, R21AG072379, U01AG076928, and R21AG085881).
Author information
Authors and Affiliations
Consortia
Contributions
Jayashankar Jayaprakash: methodology, data curation, visualization, writing – original draft. Siddabasave Gowda B. Gowda: conceptualization, methodology, funding acquisition, resources, visualization, supervision, writing –review and editing. Divyavani Gowda: data curation, visualization, writing – original draft. Shalini Jain: resources, writing – review and editing. Hariom Yadav: samples, resources, funding acquisition, writing – review and editing. Shu-Ping Hui: resources, writing –review and editing.
Corresponding authors
Ethics declarations
Competing interests
Dr. Hariom Yadav is the cofounder and chief scientific officer of Postbiotics Inc., and BiomAge Inc. He is also cofounder of MusB LLC., MusB Research LLC., and MeraBiome Inc., with Dr. Shalini Jain. However, they have no conflict of interest with respect to the work/literature reviewed and presented in this manuscript. Other authors have no conflicts of interest to declare.
Ethics approval
Ethical approval was obtained from the Institutional Review Board of the University of South Florida (approval no. 002365), USA and the Ethics Committee of the Department of Health Sciences, Hokkaido University (approval no. 22–87), Japan. All methods were performed in accordance with the relevant guidelines and regulations.
Informed consent
All participants involved in this study provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jayaprakash, J., B. Gowda, S.G., Gowda, D. et al. Lipidomic signatures reveal biomarkers of mild cognitive impairment. Transl Psychiatry (2026). https://doi.org/10.1038/s41398-026-03893-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-026-03893-y