Abstract
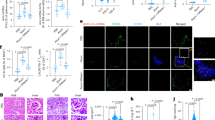
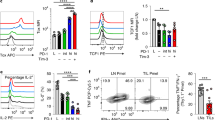
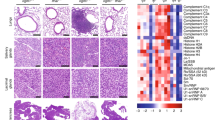
Mitochondrial DNA (mtDNA) damage and accumulation activate the cGAS-STING DNA-sensing pathway, which promotes immune clearance of tumor cells. Maintenance of the cytosolic level of mtDNA is key to sustain immune activation. T cell malignancies (T-CMs) are a general name of diseases with abnormal clonal proliferation of T lymphocytes at various stages. Immunotherapy of T-CMs is challenged by the lack of specific antigens to discriminate T-CMs from normal T cells. As intrinsic STING activation can promote the clearance of T-CMs by immune cells, we herein explored whether isoliensinine (IsoL), a natural compound from Nelumbinis Plumula could enhance NK clearance by mtDNA-mediated immune responses in tumor cells. To investigate whether IsoL modulated immune recognition and clearance of T-CMs, we pre-treated three T-CM cell lines (Jurkat, Molt4 and Hut102) with IsoL then co-cultured with NK-92MI cells. We showed that IsoL pre-treatment promoted cytosolic mtDNA accumulation by inducing ROS-dependent mitochondrial damage and inhibiting mitophagy via peroxiredoxin 1 (PRDX1), an antioxidant enzyme. Loss of PRDX1 in T-CMs also induced ROS-dependent mitochondrial DNA damage, and blocked mitophagy by preventing accumulation of mature PINK1, which was required to initiate mitophagy via recruiting Parkin to the damaged mitochondria. Remarkably, IsoL could induce expression of activating ligands in vitro, enhance NK cell infiltrations, and increase apoptosis of T-CMs. Moreover, we demonstrated that IsoL could sensitize T-CMs for NK clearance in vitro and in vivo. These results suggest that IsoL could be a potential therapeutic agent to enhance immune therapy of T-CMs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings described in this manuscript are available in the article, supplementary materials and from the corresponding author upon request. Source data about bioinformation are provided with this paper.
References
Leoncin M, La Starza R, Roti G, Pagliaro L, Bassan R, Mecucci C. Modern treatment approaches to adult acute T-lymphoblastic and myeloid/T-lymphoblastic leukemia: from current standards to precision medicine. Curr Opin Oncol. 2022;34:738–47.
Hu P, Li H, Sun W, Wang H, Yu X, Qing Y, et al. Cholesterol-associated lysosomal disorder triggers cell death of hematological malignancy: Dynamic analysis on cytotoxic effects of LW-218. Acta Pharm Sin B. 2021;11:3178–92.
Zhang L, Meng Y, Feng X, Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10:12.
Zhao X, Pan X, Wang Y, Zhang Y. Targeting neoantigens for cancer immunotherapy. Biomark Res. 2021;9:61.
Maciocia PM, Wawrzyniecka PA, Philip B, Ricciardelli I, Akarca AU, Onuoha SC, et al. Targeting the T cell receptor beta-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23:1416–23.
Chen C, Xu P. Cellular functions of cGAS-STING signaling. Trends Cell Biol. 2023;33:630–48.
Chen M, Linstra R, van Vugt M. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2022;1877:188661.
Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–17.
Oduro PK, Zheng X, Wei J, Yang Y, Wang Y, Zhang H, et al. The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy. Acta Pharm Sin B. 2022;12:50–75.
Ghosh M, Saha S, Li J, Montrose DC, Martinez LA. p53 engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression. Mol Cell. 2023;83:266–80.e6.
Pan R, Ryan J, Pan D, Wucherpfennig KW, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185:1521–1538.e18.
Harel M, Ortenberg R, Varanasi SK, Mangalhara KC, Mardamshina M, Markovits E, et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell. 2019;179:236–250.e18.
Bai R, Cui J. Mitochondrial immune regulation and anti-tumor immunotherapy strategies targeting mitochondria. Cancer Lett. 2023;564:216223.
Liang JL, Jin XK, Zhang SM, Huang QX, Ji P, Deng XC, et al. Specific activation of cGAS-STING pathway by nanotherapeutics-mediated ferroptosis evoked endogenous signaling for boosting systemic tumor immunotherapy. Sci Bull. 2023;68:622–36.
Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42.
Xie XQ, Yang Y, Wang Q, Liu HF, Fang XY, Li CL, et al. Targeting ATAD3A-PINK1-mitophagy axis overcomes chemoimmunotherapy resistance by redirecting PD-L1 to mitochondria. Cell Res. 2023;33:215–28.
Lu Y, Li Z, Zhang S, Zhang T, Liu Y, Zhang L. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics. 2023;13:736–66.
Poole LP, Macleod KF. Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci. 2021;78:3817–51.
Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305.
Ding C, Fan X, Wu G. Peroxiredoxin 1 - an antioxidant enzyme in cancer. J Cell Mol Med. 2017;21:193–202.
Qing Y, Guo Y, Zhao Q, Hu P, Li H, Yu X, et al. Targeting lysosomal HSP70 induces acid sphingomyelinase-mediated disturbance of lipid metabolism and leads to cell death in T cell malignancies. Clin Transl Med. 2023;13:e1229.
Yang GM, Sun J, Pan Y, Zhang JL, Xiao M, Zhu MS. Isolation and identification of a tribenzylisoquinoline alkaloid from Nelumbo nucifera Gaertn, a novel potential smooth muscle relaxant. Fitoterapia. 2018;124:58–65.
Jiang JZ, Fang YF, Wei HY, Zhu P, Liu M, Yuan WG, et al. A remarkably diverse and well-organized virus community in a filter-feeding oyster. Microbiome. 2023;11:2.
Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, et al. Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci USA. 2009;106:21984–9.
Lin Z, Wang F, Shang Z, Lin W. Biochemical and structural analyses reveal critical residues in delta subunit affecting its bindings to beta’ subunit of Staphylococcus aureus RNA polymerase. Biochem Biophys Res Commun. 2021;545:98–104.
Goodsell DS, Sanner MF, Olson AJ, Forli S. The AutoDock suite at 30. Protein Sci. 2021;30:31–43.
Huang R, Zhou PK. DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct Target Ther. 2021;6:254.
Cheng Y, Li HL, Zhou ZW, Long HZ, Gao LCJFIP. Isoliensinine: A natural compound with “Drug-Like” potential. Front Pharmacol. 2021;12:630385.
Samson N, Ablasser A. The cGAS-STING pathway and cancer. Nat Cancer. 2022;3:1452–63.
Bushnell GG, Sharma D, Wilmot HC, Zheng M, Fashina TD, Hutchens CM, et al. Natural killer cell regulation of breast cancer stem cells mediates metastatic dormancy. Cancer Res. 2024;84:3337–53.
Lu L, Yang C, Zhou X, Wu L, Hong X, Li W, et al. STING signaling promotes NK cell antitumor immunity and maintains a reservoir of TCF-1+ NK cells. Cell Rep. 2023;42:113108.
Yu L, Liu P. Cytosolic DNA sensing by cGAS: regulation, function, and human diseases. Signal Transduct Target Ther. 2021;6:170.
Baatarjav C, Komada T, Karasawa T, Yamada N, Sampilvanjil A, Matsumura T, et al. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022;29:2487–502.
Antiochos B, Matyszewski M, Sohn J, Casciola-Rosen L, Rosen A. IFI16 filament formation in salivary epithelial cells shapes the anti-IFI16 immune response in Sjogren’s syndrome. JCI Insight. 2018;3:e120179.
Collins PL, Purman C, Porter SI, Nganga V, Saini A, Hayer KE, et al. DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner. Nat Commun. 2020;11:3158.
Teresak P, Lapao A, Subic N, Boya P, Elazar Z, Simonsen A. Regulation of PRKN-independent mitophagy. Autophagy. 2022;18:24–39.
Ren YS, Li HL, Piao XH, Yang ZY, Wang SM, Ge YW. Drug affinity responsive target stability (DARTS) accelerated small molecules target discovery: Principles and application. Biochem Pharmacol. 2021;194:114798.
Xu H, Zhao H, Ding C, Jiang D, Zhao Z, Li Y, et al. Celastrol suppresses colorectal cancer via covalent targeting peroxiredoxin 1. Signal Transduct Target Ther. 2023;8:51.
Egler RA, Fernandes E, Rothermund K, Sereika S, de Souza-Pinto N, Jaruga P, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–50.
Li J, Yang D, Li Z, Zhao M, Wang D, Sun Z, et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res Rev. 2023;84:101817.
Science, D.o.l.a. Strain introduction. 2022; Available from: https://dlas.bjmu.edu.cn/fwzn2022/gydwpx/947c90cfff3049079270d3dc60631807.htm.
Fleischer LC, Spencer HT, Raikar SS. Targeting T cell malignancies using CAR-based immunotherapy: challenges and potential solutions. J Hematol Oncol. 2019;12:141.
Toner K, Bollard CM, Dave H. T-cell therapies for T-cell lymphoma. Cytotherapy. 2019;21:935–42.
Pocock R, Farah N, Richardson SE, Mansour MR. Current and emerging therapeutic approaches for T-cell acute lymphoblastic leukaemia. Br J Haematol. 2021;194:28–43.
Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017;31:2151–60.
Bayon-Calderon F, Toribio ML, Gonzalez-Garcia S. Facts and challenges in immunotherapy for T-cell acute lymphoblastic leukemia. Int J Mol Sci. 2020;21:1.
Guan X, Ruan Y, Che X, Feng W. Dual role of PRDX1 in redox-regulation and tumorigenesis: Past and future. Free Radic Biol Med. 2024;210:120–9.
Lv C, Huang Y, Wang Q, Wang C, Hu H, Zhang H, et al. Ainsliadimer A induces ROS-mediated apoptosis in colorectal cancer cells via directly targeting peroxiredoxin 1 and 2. Cell Chem Biol. 2023;30:295–307.e5.
Jin K, Shi Y, Zhang H, Zhangyuan G, Wang F, Li S, et al. A TNFalpha/Miz1-positive feedback loop inhibits mitophagy in hepatocytes and propagates non-alcoholic steatohepatitis. J Hepatol. 2023;79:403–16.
Patin EC, Dillon MT, Nenclares P, Grove L, Soliman H, Leslie I, et al. Harnessing radiotherapy-induced NK-cell activity by combining DNA damage-response inhibition and immune checkpoint blockade. J Immunother Cancer. 2022;10:1.
Berger G, Knelson EH, Jimenez-Macias JL, Nowicki MO, Han S, Panagioti E, et al. STING activation promotes robust immune response and NK cell-mediated tumor regression in glioblastoma models. Proc Natl Acad Sci USA. 2022;119:e2111003119.
Lee LJ, Hassan N, Idris SZ, Subbiah SK, Seow HF, Mohtaruddin N, et al. Differential regulation of NK cell receptors in acute lymphoblastic leukemia. J Immunol Res. 2022;2022:7972039.
Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14:7.
Acebes-Huerta A, Lorenzo-Herrero S, Folgueras AR, Huergo-Zapico L, Lopez-Larrea C, Lopez-Soto A, et al. Drug-induced hyperploidy stimulates an antitumor NK cell response mediated by NKG2D and DNAM-1 receptors. Oncoimmunology. 2016;5:e1074378.
Schmiedel D, Mandelboim O. NKG2D ligands-critical targets for cancer immune escape and therapy. Front Immunol. 2018;9:2040.
Bachiller M, Perez-Amill L, Battram AM, Carne SC, Najjar A, Verhoeyen E, et al. NK cells enhance CAR-T cell antitumor efficacy by enhancing immune/tumor cells cluster formation and improving CAR-T cell fitness. J Immunother Cancer. 2021;9:e002866.
Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73.
Tiwari RK, Rawat SG, Gupta VK, Jaiswara PK, Sonker P, Kumar S, et al. Epinephrine facilitates the growth of T cell lymphoma by altering cell proliferation, apoptosis, and glucose metabolism. Chem Biol Interact. 2023;369:110278.
Silic-Benussi M, Sharova E, Ciccarese F, Cavallari I, Raimondi V, Urso L, et al. mTOR inhibition downregulates glucose-6-phosphate dehydrogenase and induces ROS-dependent death in T-cell acute lymphoblastic leukemia cells. Redox Biol. 2022;51:102268.
Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11:11.
Acknowledgements
The authors would like to gratefully acknowledge the kind support we received from Prof. Qing-long Guo, Prof. Hui Hui, and Dr. Hui Li from China Pharmaceutical University, and Wan-yu Gan and De-hui Wang from NJUCM. This work was supported by the National Natural Science Foundation of China (82474123, 82204420, 82004039, 82404924), Natural Science Foundation of Jiangsu province (BK20220474, BK20230459), National excellent youth cultivation project of NJUCM (RC202408), Chinese medicine first-class scientific research and cultivation project of NJUCM (ZYXPY2024-007, ZYXYL2024-006), and 2023 Supported by Jiangsu Science and Technology Association Youth Science and Technology Talent Lifting Project (TJ-2023-060).
Author information
Authors and Affiliations
Contributions
PH, YP, and GMY (conceptualization, methodology, formal analysis, investigation, visualization, data curation, funding acquisition and writing of original draft and revision); XG and XLZ (investigation); SBS (writing of original draft); YJQ (investigation, writing of original draft, and funding support); JC (funding support, revision).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental protocols of animals were strictly conformed to the guidelines for the Guide of the Care and Use of Laboratory Animals and were approved by the Ethical Committee of Nanjing University of Chinese Medicine (202401A050, 202412A009). The tumor volume in Fig. 6 was not more than 10% of the original body weight of the mice. These tumors usually have a hemispherical appearance. In the survival statistics, the weight loss level of mice did not exceed 20% of the original body weight.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, X., Yang, Gm., Zhang, Xl. et al. Isoliensinine inhibits mitophagy and sensitizes T cell malignancies for STING-mediated NK clearance. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01636-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01636-1