Abstract
MGTA-145 or GROβT, a CXCR2 agonist, has shown promising activity for hematopoietic stem cell (HSC) mobilization with plerixafor in pre-clinical studies and healthy volunteers. Twenty-five patients with multiple myeloma enrolled in a phase 2 trial evaluating MGTA-145 and plerixafor for HSC mobilization (NCT04552743). Plerixafor was given subcutaneously followed 2 h later by MGTA-145 (0.03 mg/kg) intravenously with same day apheresis. Mobilization/apheresis could be repeated for a second day in patients who collected <6 ×106 CD34+ cells/kg. Lenalidomide and anti-CD38 antibody were part of induction therapy in 92% (n = 23) and 24% (n = 6) of patients, respectively. Median total HSC cell yield (CD34+ cells/kg × 106) was 5.0 (range: 1.1–16.2) and day 1 yield was 3.4 (range: 0.3–16.2). 88% (n = 22) of patients met the primary endpoint of collecting 2 ×106 CD34+ cells/kg in ≤ two days, 68% (n = 17) in one day. Secondary endpoints of collecting 4 and 6 × 106 CD34+ cells/kg in ≤ two days were met in 68% (n = 17) and 40% (n = 10) patients. Grade 1 or 2 adverse events (AE) were seen in 60% of patients, the most common AE being grade 1 pain, usually self-limited. All 19 patients who underwent transplant with MGTA-145 and plerixafor mobilized HSCs engrafted successfully, with durable engraftment at day 100. 74% (17 of 23) of grafts with this regimen were minimal residual disease negative by next generation flow cytometry. Graft composition for HSCs and immune cells were similar to a contemporaneous cohort mobilized with G-CSF and plerixafor.
Similar content being viewed by others
Introduction
Autologous stem cell transplant (ASCT) is an effective therapy for multiple myeloma (MM) and remains a standard of care for eligible patients [1, 2]. Collection of CD34+ hematopoietic stem cells (HSCs) to rescue the bone marrow from effects of high-dose chemotherapy in ASCT is typically done through mobilization and collection of HSCs from the peripheral blood. The two common strategies to achieve this are chemotherapy-based mobilization along with granulocyte colony stimulating factor (G-CSF) or G-CSF mobilization with or without plerixafor or other CXCR4 antagonists [3,4,5]. While these methods are efficacious, the process can be long and is associated with some adverse effects. For example, chemotherapy-based mobilization can take two to three weeks and result in complications such as neutropenic fever and significant cytopenias [6,7,8,9]. For G-CSF based methods, G-CSF injections are required for at least 4 days before apheresis can begin and the process for mobilization and apheresis can take 5 to 8 days, and these can be associated with pain for the duration of HSC mobilization [4].
The ideal stem cell mobilization and collection method should result in rapid and reliable collection of adequate CD34+ HSCs with minimal adverse effects. MGTA-145 is gro-beta truncate (GROβT), a CXCR2 agonist, which has shown promising activity for rapid mobilization of CD34+ HSCs in pre-clinical models [10]. GROβT is a synthetically manufactured naturally occurring four amino acid truncated protein variant of CXCL2. CXCL2 preferentially binds to the cell-surface chemokine receptor CXCR2, which is expressed predominantly on neutrophils and endothelial cells. GROβT binds to CXCR2 receptor and with greater potency than full length GROβ [11]. GROβT has shown synergistic activity for rapid HSC mobilization with plerixafor, a CXCR4 antagonist, in animal models and in a phase I study of healthy volunteers [12]. Given these promising data, we hypothesized that the combination of MGTA-145 and plerixafor would result in fast, effective, and safe stem cell mobilization in patients with MM. We conducted an investigator-initiated phase II study to evaluate the safety and efficacy of HSC mobilization with a combination of MGTA-145 and plerixafor in patients with MM.
Methods
Ethics approval and consent to participate
The protocol was institutional review board (IRB) approved by Stanford IRB (Protocol approval number: 57056). All patients provided written informed consent. All study procedures were performed in accordance with the relevant guidelines and regulations.
Study Design
This open-label, proof of concept phase II clinical trial was conducted at a single center (NCT04552743). Key eligibility criteria included transplant eligible patients, 18-70 years old, with a diagnosis of MM per the International Myeloma Working Group (IMWG) guidelines [13]. Patients had to be within one year of start of myeloma therapy and not received greater than 6 cumulative months of treatment with lenalidomide or other immunomodulatory agents. Creatinine Clearance of > 30 ml/minute was required, as were absolute neutrophil count > 1500 × 106/L and platelets > 100,000 × 106/L.
The trial was conducted in two sequential segments: the first segment for evaluation of safety and feasibility (n = 15), and contingent upon success of segment 1, an additional 10 patients were planned for a total sample size of 25 patients. The statistical design was based on a one-sided hypothesis test with the null hypothesis being that 70% of patients will meet the primary endpoint of collecting at least ≥ 2.0 × 106 CD34+ cells/kg with up to two apheresis sessions and the alternate hypothesis being 95% of patients will meet the primary endpoint. The sample size for this hypothesis testing was 15 patients to be enrolled in segment 1. The type I error rate was 5% and statistical power was 85%. This design featured a pre-defined futility stopping rule at the end of the segment 1. If at least 13 of 15 patients in segment 1 did not meet the primary endpoint in segment 1, the trial would be stopped for futility. Contingent upon achieving success in the first segment, 10 patients were planned to be enrolled in the second segment to gain additional safety and feasibility experience with this regimen and to evaluate efficacy in a broader patient population. As this is this first study to infuse HSCs collected with this novel regimen after myeloablative conditioning in humans, a safety run-in cohort of 6 patients was planned to ensure engraftment of these HSCs before proceeding with mobilization in a broader population of patients.
Study Treatment
Patients received subcutaneous plerixafor 0.24 mg/kg (0.16 mg/kg for creatinine clearance ≤ 50 ml/min) followed 2 hours later by MGTA-145 0.03 mg/kg intravenous infusion over 3–10 min. The dose of MGTA-145 in combination with plerixafor was determined in a phase 1 study of healthy volunteers [12, 14]. Apheresis was started on the same day within 30 min after MGTA-145 infusion. A second day of mobilization and apheresis was pursued in patients who did not collect 6.0 × 106 CD34+ cells/kg in one session. Apheresis was standardized to process 3 blood volumes, with a margin of ±10% or 4.5 h, whichever was longer. Enumeration of CD34+ cells for study endpoints was done in the clinical flow cytometry laboratory. Transplant with melphalan 140–200 mg/m2 was done per institutional guidelines, but patients (except safety run in cohort) were not required to undergo ASCT after HSC collection (Supplement).
Study Endpoints
The primary endpoint was successful collection of at least ≥ 2.0 × 106 CD34+ cells/kg with up to two apheresis sessions after MGTA-145 and plerixafor dosing. The key secondary endpoint was collection of ≥4.0 × 106 CD34+ cells/kg in up to two days of apheresis. Other secondary endpoints included collection of ≥2.0 × 106 CD34+ cells/kg on day one of apheresis and ≥6.0 × 106 CD34+ cells/kg in up to two days of apheresis, adverse events per CTCAE version 5 and achievement of engraftment. Neutrophil engraftment was defined as the first day of absolute neutrophil count (ANC) ≥ 0.5 × 109/L for 3 days following stem cell infusion and platelet engraftment was defined as first day of platelet count ≥ 20 × 109/L without transfusion in the last 7 days and with platelet count ≥20 × 109/L on 2 separate, subsequent days.
Patient Reported Outcomes (PRO)
We evaluated patient reported pain using the standardized Brief Pain Inventory (BPI) tool [15,16,17,18]. PRO assessments were done at baseline, after each day of mobilization and within 7 days after mobilization. Additional details on BPI are found in supplemental methods.
Correlative studies
Correlative studies were undertaken to evaluate graft composition and immune reconstitution in peripheral blood following ASCT at day 28 and day 100. A prospective contemporaneous comparative cohort of 15 patients who would have met key trial eligibility criteria and underwent mobilization with standard of care regimen (G-CSF and as needed plerixafor) was planned a priori with the goal of comparing data from correlative studies between the study cohort and the contemporaneous control cohort (Supplementary Data). The following correlative studies were conducted (detailed methods in supplement):
-
a.
Minimal Residual Disease Testing: Graft contamination was assessed by minimal residual disease (MRD) assessment for residual plasma cells using next generation flow cytometry (NGF) on study patients only using fresh specimens. The sensitivity of the assay is 1 in 1 × 105 to 1 × 106 cells [13, 19].
-
b.
Graft Composition: Immunophenotype of the apheresis product was evaluated by flow cytometry and CyTOF for HSC subsets and immunological characterization of the T cell subsets, B cell subsets, NK cells, neutrophils, and myeloid/monocytic cells in the product. In particular enumeration of CD90 + CD34 + CD45RA- HSCs was a key aim as CD90 + CD34+ stem cells have been shown to be true self-renewing pluripotent HSCs among the CD34+ cells, whereas CD90-CD34+ cells have been shown to be differentiated committed progenitor cells [20, 21]. This batched analysis on cryopreserved samples was done for both study patients and the control cohort
-
c.
Immune reconstitution following transplant was assessed through characterization of white blood cell population subsets (T cell subsets, NK cells) in patient’s peripheral blood at day 28 using flow cytometry and at day 100 using flow cytometry for both study patients and the control cohort on fresh specimens.
Statistical Analysis
for study endpoints and correlative studies are described in Supplementary Data.
Results
Baseline Characteristics
25 patients with MM were enrolled in the study and underwent HSC mobilization from 11/3/2020 to 7/20/2021. Table 1 shows their baseline and myeloma treatment characteristics. Median age at mobilization was 62 years (range: 35–68) and 52% of patients were female. Among the study cohort, 72% (n = 18) of patients were White and 12% (n = 3) were Black. Among patients whose cytogenetic data at baseline were available (n = 21), 57% (n = 12) had high-risk cytogenetics as defined by presence of deletion 17p, t(4;14), t(14;16) or gain 1q. Renal impairment at diagnosis was noted in 28% of patients and 24% had ISS stage 3 disease. Notably 24% (n = 6) patients had a history of prior cancer treated with systemic chemotherapy and/or radiation before their diagnosis of active myeloma, which can impact on HSC mobilization.
Myeloma Treatment History
the median duration of induction chemotherapy was 4 months (range: 3–6). Twelve percent patients had more than one line of induction therapy. The most common induction regimen was bortezomib, lenalidomide and dexamethasone (VRD) in 68% of patients, followed by daratumumab-VRD in 24% of patients. Ninety two percent of patients were exposed to lenalidomide with a median of 5 cycles (range:1–8), 24% (n = 6) were exposed to anti-CD38 antibodies, and 20% patients received cyclophosphamide with 16% receiving more than two cycles of cyclophosphamide before mobilization. In this cohort, 20% of patients received radiation for MM as part of upfront treatment before mobilization with median dose of 2000 cGy (range: 800–3000). (Table 1).
Hematopoietic stem cell mobilization
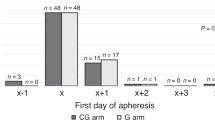
All 15 patients in segment 1 met the primary endpoint and therefore the study proceeded to enrolling additional 10 patients in segment 2. All 25 patients completed one day of mobilization with MGTA-145 and plerixafor and 22 patients underwent a second day of mobilization. (Table 2, Fig. 1) Plerixafor was dose reduced in one patient due to renal impairment. Median time for MGTA-145 infusion was 4 min (range: 3–10). There were no dose interruptions. The median total HSC cell yield (CD34+ cells/kg × 106) was 5.0 (range: 1.1–16.2), day 1 yield was 3.4 (range: 0.3–16.2) and day 2 yield was 1.9 (range: 0.5–4.6). 88% (n = 22) of patients met the primary endpoint of collecting ≥ 2 × 106 CD34+ cells/kg in one or two days of mobilization + apheresis, and 68% (n = 17) in 1 day. Three patients who did not meet the primary endpoint successfully collected HSCs with standard GCSF + plerixafor dosing and 2–3 apheresis sessions as shown in Table S5. Secondary endpoints of collecting ≥ 4 and ≥ 6 × 106 CD34+ cells/kg in up to 2 days were met in 68% (n = 17) and 40% (n = 10) patients, respectively.
Median CD34+ cell count in peripheral blood on day 1 following plerixafor and MGTA-145 administration, but before starting apheresis was 24/µl (range: 3–99, N = 25) and after apheresis completion was 30/µl (range: 6–452, N = 25). On day 2, median CD34+ cell count before start of apheresis was 15/µl (range: 5-46, N = 22) and following apheresis was 18/µl (range: 3–49, N = 22).
We explored HSC yields in sub-groups of patients based on exposure to myeloma directed therapies known to impact HSC yield. Patients receiving anti-CD38 antibody (n = 6) or more than two months of cyclophosphamide (n = 4) during induction therapy had lower HSC yields compared to patients who did not have these exposures, although these differences were not statistically significant (p = 0.07). The total median HSC collection (CD34+ cells × 106/kg) in the three groups was 3.7 (range: 1.1–16.2), 3.6 (range: 1.2–4.7) and 6.7 (range: 2.9–10.4), respectively.
Of the three patients who did not meet the primary endpoint, two had exposure to antiCD38 antibody during induction and one had received more than two cycles of cyclophosphamide.
Safety and Adverse Events
The combination of MGTA-145 and plerixafor was well tolerated. At least one treatment emergent adverse events (TEAE) with MGTA-145+plerixafor was seen in 60% of patients (grade 1, n = 13, grade 2, n = 2) (Table 3). The most common adverse event at least possibly related to investigational agent was grade 1 pain seen in 44% (n = 11) of patients, of whom 9 patients experienced acute onset, transient pain with MGTA-145 infusion (locations: back, n = 5, hip, n = 1, sternum, n = 1 and generalized pain, n = 1). The median time to onset of pain after MGTA-145 infusion was 5 minutes (range: 3–10) and median duration of pain was 7 min (range: 3–28). Only one patient required analgesic medication. Pain recurred in 2 of 22 patients on day 2 of mobilization. There were two grade 2 AEs, including vomiting and poor graft function. Poor graft function is described under engraftment. Transplant related AEs were not captured unless directly related to the study drug. AEs deemed to be unrelated/unlikely to be related to investigational therapy are summarized in supplementary Table S6.
Patient Reported Outcomes
Using a linear mixed effects model on patient reported data from the BPI questionnaire, 56% (n = 14) of patients self-reported pain with mobilization vs 40% (n = 10) at baseline. Seven out of 15 patients (47%) without baseline pain reported pain with mobilization. Amongst patients who reported pain, the worst pain was reported on mobilization day 1, and returned to baseline after that time. There was no difference in aggregate score of pain severity or interference in day-to-day activities compared to baseline, likely as the pain was very short lasting as described above.
Outcomes with ASCT
Overall, 23 of the 25 patients underwent ASCT. Amongst this group, 19 patients underwent ASCT with MGTA-145 and plerixafor mobilized HSCs. The remaining 4 patients underwent ASCT with HSCs collected with subsequent mobilization with GCSF and plerixafor (n = 3 due to failure to collect adequate HSCs for ASCT and n = 1 due to clotting of the HSCs during cryopreservation, unrelated to the mobilization regimen).
Amongst 19 patients undergoing ASCT with MGTA-145+plerixafor mobilized HSCs, 84% (n = 16) received melphalan 200 mg/m2 (Table 4). The median time from start of mobilization to start of conditioning chemotherapy for these patients was 10 days (range 4–46 days). A median of 3.4 × 106 CD34+ cells/kg (range: 2.2–8.1) were infused following high dose melphalan chemotherapy. All patients engrafted successfully, with durable engraftment at day 100. Median time to neutrophil engraftment was 12 days (range: 11-15). Median time to platelet engraftment (platelets ≥ 20,000) was 18 days (range 15–33). After achieving initial engraftment, one patient had slow count recovery and was deemed to have poor graft function. Primary neutrophil engraftment in this patient was on day 14 and primary platelet engraftment was on day 33 post stem cell transplant. However, due to lack of adequate hematological recovery, this patient received back-up stem cells at day 36 with 1.9 million CD34+ cells/kg collected with MGTA-145+plerixafor and had good hematological recovery and durable engraftment at day 100. At day 100 follow-up, only one patient had progressed. Day 100 response per International Myeloma Working Group (IMWG) response criteria was complete response in 32% (n = 6), VGPR in 63% (n = 12) and progression in 5% (n = 1) of patients, respectively.
The 4 patients who underwent transplant with HSCs collected with alternate mobilization after MGTA-145+plerixafor had timely and durable engraftment as shown in supplementary Table S7.
Control Cohort
A contemporaneous control cohort of 15 patients undergoing standard of care mobilization was identified as described in Methods. These patients underwent HSC mobilization with G-CSF and as needed plerixafor from 2/8/2021 to 7/19/2021. Baseline characteristics of these 15 patients are shown in supplemental results and Table S8 and mobilization/transplant outcomes are shown in Table S9.
Correlative Studies
Graft Contamination/Minimal Residual Disease testing
Graft contamination by clonal plasma cells was assessed by MRD testing by NGF. Seventy four percent (17 of 23) of grafts were MRD negative. Median number of events analyzed were 2.5 × 106 (range: 1 × 106 - 4.8 × 106). In the MRD positive grafts, median clonal plasma cells burden was low at 0.0002% (range: 0.0001-0.0004). Polyclonal plasma cells were present in 71% of MRD negative grafts (12 of 17) and 50% (3 of 6) MRD positive grafts.
Graft Composition by Flow Cytometry
Graft composition of HSCs and lymphocyte subsets were comparable in MGTA-145 + plerixafor mobilized grafts and standard of care mobilization (Fig. 2).
A HSCs (CD34 + CD90+ and CD34 + CD90 + CD45RA-) shown as % of CD34+ cells assessed by FACS. B T, B and NK cells shown as % of the lymphocytes assessed by FACS. C Frequency of total, memory and naïve CD4 + T cells as assessed by FACS. D Frequency of total, memory and naive CD8 + T cells as assessed by flow cytometry. Each figure shows individual datapoints as circles or squares and the bar represents the median.
HSC Phenotype
We observed high enrichment for CD34 + CD90+ and CD34 + CD90 + CD45RA- among CD34+ cells, subsets of long-term engrafting HSCs. In the MGTA-145 mobilized study cohort (N = 25), CD34 + CD90+ cells comprised median of 50% (range: 23–85%) and CD34 + CD90 + CD45RA- cells comprised 36% (range: 13–66%) of CD34+ cells. Evaluation of HSC subsets in the prospective contemporaneous cohort (N = 15) revealed similar findings: CD34 + CD90+ cells comprised median of 49% (range: 26–70%) and CD34 + CD90 + CD45RA- cells comprised 42% (range: 23–65%t) of CD34+ cells in patients mobilized with G-CSF and as needed plerixafor (Fig. 2A).
Immune Cell Subsets
Similarly, the proportion of immune cells and their subsets was similar across the study and control cohort, including lymphocytes and their subsets (T cells, B cells, NK cells) (Fig. 2B), and different T cell subsets (CD4, CD4 memory, CD4 naïve, CD8, CD8 memory, CD8 naïve cells) (Fig. 2C, D).
Graft Composition by CyTOF
CyTOF can evaluate additional subsets of cell populations compared with flow cytometry. Here we observed differences in some of these subsets in the apheresis grafts mobilized with MGTA-145+plerixafor vs the control cohort. Although CD90 + CD34+ cells were similar in both groups on CyTOF analysis, Supplementary Fig. 1 shows immunophenotypic populations that were significantly different in the apheresis grafts from study patients compared to the control cohort. Higher proportion of T cells, particularly Tregs and their subsets were observed in the study cohort. CD161 + , CD94 + , PD-1 + , and CXCR2+ subsets of both CD4 and CD8 T cells were higher in the study population. Several CXCR3+ (Type 1 T Helper cells) and CXCR5+ (T follicular helper cells) populations were also higher in the study population, as were activated CD4 populations (HLA-DR+ and/or CD38 + ) and plasmacytoid dendritic cells. Myeloid dendritic cells were lower in the study population compared to controls.
Immune Reconstitution
Immune reconstitution of different immune subsets (T cells and subsets including memory and naïve CD4+ and CD8 + T cells, and NK cells) was measured at baseline and on days 28 and 100 post-transplant. Figure 3 shows data from the study cohort and Supplementary Fig. 2 shows data for control cohort and Supplementary Fig. 3 for study vs control cohort. Trends were evaluated over time within each cohort and the two groups compared at individual timepoints.
The graph represents absolute cell counts/μl shown as median and interquartile range of (A). T cells (CD3 + ), (B) NK cells (CD3-CD56 + ), C CD4 T cells (CD3 + CD4 + ), (D) CD8 T cells (CD3 + CD8 + ), (E) CD4 naive T cells (CD45RA + ), (F) CD8 naive T cells (CD45RA + ), (G) CD4 memory T cells (CD45RO + ), and (H) CD8 memory T cells (CD45RO + ) as assessed by flow cytometry at baseline and at day 28 and 100 after autologous stem cell transplantation with MGTA-145 and plerixafor mobilized HSCs. P values at each timepoint represent comparison vs. baseline.
In the study cohort, most immune subsets at day 28 and day 100 post-transplant were not significantly different compared to baseline, except for lower CD4. T cells post-transplant at day 28, while CD4 counts at day 100 were not significantly different compared to baseline. Interestingly NK cells were higher at day 28 compared to baseline, but levels by day 100 were comparable to baseline. In the control cohort, there were no significant differences in reconstitution of immune subsets compared to baseline. However, we noted a pattern of decline for total T cells including CD4 T cells and CD8 T cells at day 28 vs baseline, that improved to levels comparable to baseline or better by day 100. (Supplementary Fig. 2) There were no significant differences in different immune subsets at day 28 and day 100 post-transplant in the study vs control cohort patients. (Supplementary Fig. 3).
Discussion
This trial is the first to evaluate MGTA-145 or GROβT, a CXCR2 agonist and plerixafor, a CXCR4 antagonist for HSC mobilization in patients with MM. Our cohort of 25 patients was representative of a broad population of transplant eligible patients with MM, including 92% of patients being exposed to lenalidomide and 24% being exposed to anti-CD38 antibodies and 16% with prolonged treatment with cyclophosphamide. With this novel regimen, 88% of patients met the primary endpoint of collecting ≥ 2 × 106 CD34+ cells/kg in one or two days of mobilization and apheresis. This regimen was well-tolerated and MGTA-145 + plerixafor mobilized HSCs resulted in timely and durable engraftment. We also performed extensive correlative studies to understand graft composition and immune reconstitution in these patients, and compared them to a contemporaneous, demographically similar cohort of patients being mobilized with standard of care G-CSF and as needed plerixafor.
The combination of MGTA-145 and plerixafor evaluated in this study is a unique mobilization regimen as patients can begin apheresis on the same day as the start of mobilization offering patient convenience and the potential to decrease health care resource utilization. In contrast, G-CSF with or without CXCR4 antagonists typically require at least 4-5 days G-CSF before apheresis can begin [3, 4]. Historically, plerixafor has been the only CXCR4 antagonist used in clinical practice for HSC mobilization.(4) In 2023, motixafortide, another CXCR4 antagonist was FDA approved based on the results of a phase 3 trial [3] and given the high efficacy seen with motixafortide in combination with G-CSF, it would interesting to evaluate motixafortide in combination with this novel CXCR2 agonist in future studies. Chemotherapy based mobilization is another very effective mobilization method, but is associated with more adverse effects and is less cost effective [6, 9].
Overall, MGTA-145+plerixafor was well tolerated, with grade 1 AEs noted in 52% and grade 2 AEs in 8% of patients, and no grade 3 or 4 events. The most common investigator reported AE was acute-onset musculoskeletal pain in 44% of patients that started within minutes of MGTA-145 infusion and lasted less than half-hour in all patients. Using BPI questionnaire, a validated patient reported pain questionnaire, 56% patients reported pain with mobilization, which is lower than 95% of patients who reported pain with G-CSF based HSC mobilization in another study with the same questionnaire [22].
Optimal targets for HSC collection vary widely across centers, but guidelines generally agree that a minimum of ≥ 2 × 106 CD34+ cells/kg are needed for successful ASCT [5, 23], which was the primary endpoint of this study. In our study, 67% of patients collected ≥ 2 × 106 CD34+ cells/kg in one day. This CD34+ cell yield compares favorably to G-CSF only mobilization. In a recent randomized trial, 64.3% patients in G-CSF arm collected ≥ 2 × 106 CD34+ cells/kg in one apheresis session [3]. However, a higher rate success rate for collecting ≥ 2 × 106 CD34+ cells/kg in one day is observed with G-CSF and CXCR4 antagonist-based mobilization [3, 4, 24]. Collection of ≥ 6 × 106 CD34+ cells/kg has been used as an endpoint in prior phase 3 clinical trials and at some centers [3,4,5, 23]. With MGTA-145+plerixafor, 40% of patients collected of ≥ 6 × 106 CD34+ cells/kg in one or two apheresis sessions. With G-CSF and plerixafor, 54.2% patients met this target in one apheresis session and 77.9% with two apheresis sessions [4]. With G-CSF and motixafortide, 88% patients met this target in one apheresis session and 92.5% met this target in 2 apheresis sessions [3]. In-contrast, in the G-CSF+placebo group in the GENESIS trial of motixafortide, only 9.5% patients and 26.2% patients met this target in 1 and 2 apheresis sessions, respectively [3]. Clinical trials of G-CSF with plerixafor or motixafortide based mobilization did not have a significant proportion of patients with anti-CD38 antibody (daratumumab or isatuximab) exposure in the study population. Both lenalidomide and anti-CD38 antibodies are known to adversely impact HSC mobilization [25, 26] and these drugs are now being widely used in induction therapy given superior outcomes [27, 28]. This could potentially result in a lower HSC yield in current clinical care than observed in the phase III trials that led to approval of plerixafor and motixaoforatide based regimens and that historically seen in clinical practice. Indeed, exposure to antiCD38 antibody was associated with numerically lower HSC yield in our cohort as well.
Based on the findings of our study, there are two potential approaches for future development of CXCR2 agonists for HSC mobilization. One approach is to continue to pursue a G-CSF free approach to limit side effects of G-CSF and the days needed for mobilization with combination of a CXCR2 agonist with plerixafor or motixafortide. This may be suitable for a small population of patients. However, as the targets for HSC collection at most centers are high, and patients are now increasingly exposed to drugs that adversely impact HSC collection, a combination approach with G-CSF and CXCR4 antagonist-based regimens can be considered in future studies as MGTA-145 is well tolerated in combination with plerixafor. Such a combination may further optimize HSC collection and decrease the apheresis sessions needed to meet the optimal HSC collection targets, particularly in patients at higher risk for not meeting the HSC mobilization target in one day. This would optimize resource utilization with increasing use of apheresis for other cellular therapies like CAR-T cell therapy, and the ongoing need to decrease the number of apheresis sessions needed to collect HSCs for ASCT.
This study is the first to assess HSC mobilization with a G-CSF free regimen in patients with hematologic malignancies. MGTA-145 mobilized HSCs successfully engrafted. Except for one patient with poor graft function, all patients had timely and durable engraftment. In the patient with poor graft function, graft function subsequently improved after infusion of backup HSCs collected with MGTA-145 and plerixafor. The median time to neutrophil engraftment was 12 days and median time to platelet engraftment was 18 days, which is in range for that reported in a large retrospective study from our center [9], and that seen in clinical trials of HSCs collected with G-CSF and G-CSF and CXCR4 antagonists [3, 4].
Graft composition, including HSCs and other immune subsets impacts immune reconstitution and disease related outcomes [21, 29,30,31,32,33,34,35,36,37,38,39,40,41]. We observed a similar graft composition in the study and control cohort for CD34 + CD90 + CD45RA- cells, a pre-defined population of interest, which are widely regarded as the true pluripotent HSCs [20]. Other immune subsets including T cells and NK cells were comparable by flow cytometry. CyTOF based assessment demonstrated higher proportion of T cells in patients mobilized with MGTA-145+plerixafor, particularly Tregs and their subsets, and a bias towards plasmacytoid dendritic cells and away from myeloid dendritic cells. These are exploratory analyses, and these data can serve as a framework for future work on analyzing immune subsets in mobilized grafts, both autologous and allogeneic. We observed robust and comparable lymphocyte recovery in the study cohort and control cohort, including different immune subsets including NK cells, T cells, B cells, CD4 and CD8 + T cells, and memory and naïve subsets of these cells. Three-fourths of the grafts mobilized with MGTA-145 and plerixafor were MRD negative using NGF, similar recent findings with HSC mobilization with G-CSF and plerixafor [42].
Strengths of our study include the use of an innovative mobilization regimen for same day mobilization and apheresis. This clinical study is the first to demonstrate timely and durable engraftment with HSCs mobilized with this novel regimen. We also included a contemporaneous control cohort mobilized with G-CSF and as needed plerixafor for robust correlative studies. As this study is a proof of concept, phase 2 trial, limitations include small patient cohort and single center design.
In summary, this novel regimen of a CXCR2 agonist and plerixafor has the potential to safely mobilize HSCs and future studies should evaluate this CXCR2 agonist in combination with standard of care approaches to further optimize HSC collection.
Data availability
The data generated for this study is available upon reasonable request from the corresponding author.
References
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl J Med. 2022;387:132–47.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl J Med. 2017;376:1311–20.
Crees ZD, Rettig MP, Jayasinghe RG, Stockerl-Goldstein K, Larson SM, Arpad I, et al. Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial. Nat Med. 2023;29:869–79.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–6.
Giralt S, Costa L, Schriber J, DiPersio J, Maziarz R, McCarty J, et al. Optimizing Autologous Stem Cell Mobilization Strategies to Improve Patient Outcomes: Consensus Guidelines and Recommendations. Biol Blood Marrow Transplant. 2014;20:295–308.
Afifi S, Adel NG, Devlin S, Duck E, Vanak J, Landau H, et al. Upfront plerixafor plus G-CSF versus cyclophosphamide plus G-CSF for stem cell mobilization in multiple myeloma: efficacy and cost analysis study. Bone marrow Transplant. 2016;51:546–52.
Antar A, Otrock ZK, Kharfan-Dabaja MA, Ghaddara HA, Kreidieh N, Mahfouz R, et al. G-CSF plus preemptive plerixafor vs hyperfractionated CY plus G-CSF for autologous stem cell mobilization in multiple myeloma: effectiveness, safety and cost analysis. Bone marrow Transplant. 2015;50:813–7.
Jagasia MH, Savani BN, Neff A, Dixon S, Chen H, Pickard AS. Outcome, toxicity profile and cost analysis of autologous stem cell mobilization. Bone marrow Transplant. 2011;46:1084–8.
Johnsrud A, Ladha A, Muffly L, Shiraz P, Goldstein G, Osgood V, et al. Stem Cell Mobilization in Multiple Myeloma: Comparing Safety and Efficacy of Cyclophosphamide +/- Plerixafor versus Granulocyte Colony-Stimulating Factor +/- Plerixafor in the Lenalidomide Era. Transpl Cell Ther. 2021;27:590.e1–e8.
Hoggatt J, Singh P, Tate TA, Chou BK, Datari SR, Fukuda S, et al. Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell. 2018;172:191–204.e10.
King AG, Johanson K, Frey CL, DeMarsh PL, White JR, McDevitt P, et al. Identification of unique truncated KC/GRO beta chemokines with potent hematopoietic and anti-infective activities. J Immunol (Baltim, Md : 1950). 2000;164:3774–82.
DiPersio JF, Hoggatt J, Devine S, Biernat L, Howell H, Schmelmer V, et al. Rapid and Robust Mobilization of CD34+ HSCs without G-CSF Following Administration of Mgta-145 Alone or in Combination with Plerixafor. Blood. 2019;134:1961.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
Goncalves KA, Hyzy SL, Hammond KJ, Falahee PC, Howell H, Pinkas J, et al. Mgta-145, in Combination with Plerixafor in a Phase 1 Clinical Trial, Mobilizes Large Numbers of Human Hematopoietic Stem Cells and a Graft with Immunosuppressive Effects for Allogeneic Transplant. Blood. 2020;136:31–2.
Vadhan-Raj S, von Moos R, Fallowfield LJ, Patrick DL, Goldwasser F, Cleeland CS, et al. Clinical benefit in patients with metastatic bone disease: results of a phase 3 study of denosumab versus zoledronic acid. Ann Oncol : Off J Eur Soc Med Oncol. 2012;23:3045–51.
Mendoza TR, Koyyalagunta D, Burton AW, Thomas SK, Phan MH, Giralt SA, et al. Changes in pain and other symptoms in patients with painful multiple myeloma-related vertebral fracture treated with kyphoplasty or vertebroplasty. J Pain. 2012;13:564–70.
Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J pain symptom Manag. 2011;41:558–65.
Atkinson TM, Mendoza TR, Sit L, Passik S, Scher HI, Cleeland C, et al. The Brief Pain Inventory and its “pain at its worst in the last 24 h” item: clinical trial endpoint considerations. Pain Med. 2010;11:337–46.
Sidana S, Muchtar E, Sidiqi MH, Jevremovic D, Dispenzieri A, Gonsalves W, et al. Impact of minimal residual negativity using next generation flow cytometry on outcomes in light chain amyloidosis. Am J Hematol. 2020;95:497–502.
Radtke S, Adair JE, Giese MA, Chan YY, Norgaard ZK, Enstrom M, et al. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Science translational medicine. 2017;9.
Shimazaki C, Sumikuma T, Inaba T. CD34+ CD90+ cells and late hematopoietic reconstitution after autologous peripheral blood stem cell transplantation. Leuk Lymphoma. 2004;45:661–8.
Anaka M, Cox-Kennett N, Crisp N, Peters A, Ravisankar S, Venner CP, et al. 457 - Filgrastim-Induced Bone Pain Is More Prevalent and Severe during Stem Cell Mobilization. Transplant Cell Ther. 2021;27:S374–S5.
Duong HK, Savani BN, Copelan E, Devine S, Costa LJ, Wingard JR, et al. Peripheral Blood Progenitor Cell Mobilization for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:1262–73.
Kumar SK, Mikhael J, Laplant B, Lacy MQ, Buadi FK, Dingli D, et al. Phase 2 trial of intravenously administered plerixafor for stem cell mobilization in patients with multiple myeloma following lenalidomide-based initial therapy. Bone marrow Transplant. 2014;49:201–5.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–42.
Chhabra S, Callander N, Watts NL, Costa LJ, Thapa B, Kaufman JL, et al. Stem Cell Mobilization Yields with Daratumumab- and Lenalidomide-Containing Quadruplet Induction Therapy in Newly Diagnosed Multiple Myeloma: Findings from the MASTER and GRIFFIN Trials. Transpl Cell Ther. 2023;29:174.e1–e10.
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl J Med. 2024;390:301–13.
Voorhees PM, Sborov DW, Laubach J, Kaufman JL, Reeves B, Rodriguez C, et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023;10:e825–e37.
Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–55.
Porrata LF, Gertz MA, Geyer SM, Litzow MR, Gastineau DA, Moore SB, et al. The dose of infused lymphocytes in the autograft directly correlates with clinical outcome after autologous peripheral blood hematopoietic stem cell transplantation in multiple myeloma. Leukemia. 2004;18:1085–92.
Condomines M, Quittet P, Lu ZY, Nadal L, Latry P, Lopez E, et al. Functional regulatory T cells are collected in stem cell autografts by mobilization with high-dose cyclophosphamide and granulocyte colony-stimulating factor. J Immunol (Baltim, Md : 1950). 2006;176:6631–9.
Holtan SG, Porrata LF, Micallef IN, Padley DJ, Inwards DJ, Ansell SA, et al. AMD3100 affects autograft lymphocyte collection and progression-free survival after autologous stem cell transplantation in non-Hodgkin lymphoma. Clin lymphoma myeloma. 2007;7:315–8.
Hiwase DK, Hiwase S, Bailey M, Bollard G, Schwarer AP. Higher infused lymphocyte dose predicts higher lymphocyte recovery, which in turn, predicts superior overall survival following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant : J Am Soc Blood Marrow Transplant. 2008;14:116–24.
Kansagra A, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Hogan WJ, et al. Infusion of autograft natural killer cell/CD14(+)HLA-DR(DIM) cell ratio predicts survival in lymphoma post autologous stem cell transplantation. Bone marrow Transplant. 2018;53:146–54.
Porrata LF. Autograft immune effector cells and survival in autologous peripheral blood hematopoietic stem cell transplantation. J Clin Apher. 2018;33:324–30.
Valtola J, Silvennoinen R, Ropponen A, Siitonen T, Säily M, Sankelo M, et al. Blood graft composition and post-transplant recovery in myeloma patients mobilized with plerixafor: a prospective multicenter study. Leuk Lymphoma. 2019;60:453–61.
Varmavuo V, Mantymaa P, Silvennoinen R, Nousiainen T, Kuittinen T, Jantunen E. CD34+ cell subclasses and lymphocyte subsets in blood grafts collected after various mobilization methods in myeloma patients. Transfusion. 2013;53:1024–32.
Good Z, Borges L, Vivanco Gonzalez N, Sahaf B, Samusik N, Tibshirani R, et al. Proliferation tracing with single-cell mass cytometry optimizes generation of stem cell memory-like T cells. Nat Biotechnol. 2019;37:259–66.
Stern L, McGuire H, Avdic S, Rizzetto S, Fazekas de St Groth B, Luciani F, et al. Mass Cytometry for the Assessment of Immune Reconstitution After Hematopoietic Stem Cell Transplantation. Front Immunol. 2018;9:1672.
Smets T, Stevenaert F, Adams H 3rd, Vanhoof G. Deep Profiling of the Immune System of Multiple Myeloma Patients Using Cytometry by Time-of-Flight (CyTOF). Methods Mol Biol (Clifton, NJ). 2018;1792:47–54.
Sahaf B, Rahman A, Maecker HT, Bendall SC. High-Parameter Immune Profiling with CyTOF. Methods Mol Biol (Clifton, NJ). 2020;2055:351–68.
Bal S, Landau HJ, Shah GL, Scordo M, Dahi P, Lahoud OB, et al. Stem Cell Mobilization and Autograft Minimal Residual Disease Negativity with Novel Induction Regimens in Multiple Myeloma. Biol Blood Marrow Transplant : J Am Soc Blood Marrow Transplant. 2020;26:1394–401.
Acknowledgements
Ayesha Fraser, Khanh Nguyen and Kimberly Xie.
Funding
Magenta Therapeutics (now known as Dianthus Therapeutics) provided drug support and research funding for this investigator-initiated clinical trial. Surbhi Sidana was supported by Stanford Clinical and Translational Science KL2 Career Development Award program, Award Number KL2 TR003143, Stanford Cancer Institute/American Cancer Society Pilot Grant 2022 and Doris Duke Charitable Foundation. S10RR027582 from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
SS designed the study, enrolled patients, oversaw the clinical trial and analysis and wrote the manuscript. AKB, THH, SMA, AL, TH performed correlative studies and/or analysis of correlative studies. YL and JT were study biostatisticians and designed the trial and conducted analysis. HH was involved with trial management. S.K.K. advised on study design and oversaw MRD analysis. LSM, LJ, SA, RL, EM, AR, WW, MJF, PS, DBM were involved in trial enrollment and clinical care of patients. JAS oversaw correlative study design, analysis and interpretation and clinical care. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
SS (Consultancy: Magenta Therapeutics, now known as Dianthus therapeutics).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sidana, S., Bankova, A.K., Hosoya, H. et al. Phase II study of novel CXCR2 agonist and Plerixafor for rapid stem cell mobilization in patients with multiple myeloma. Blood Cancer J. 14, 173 (2024). https://doi.org/10.1038/s41408-024-01152-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-024-01152-1
This article is cited by
-
Metabolic crosstalk among cancer-associated fibroblasts, adipocytes and immune cells as an immunosuppressive tumor microenvironment driver
Experimental & Molecular Medicine (2026)