Abstract
Autologous transplantation remains the standard of care for eligible multiple myeloma (MM) patients, yet optimal CD34+ cell dose remains unclear. We conducted a retrospective study on MM patients undergoing upfront transplant between 2005 and 2021 and divided them into low (≤2.5 × 106 cells/kg) and high (>2.5 × 106 cells/kg) CD34+ dose groups. We included 2479 patients, 95 in the low CD34+ group and 2384 in the high CD34+ group. Patients in the low CD34+ group were older (63.2 vs 61.1 years, p = 0.013), more often had R-ISS III (19% vs 9%, p = 0.014), received plerixafor (60% vs 35%, p < 0.001) and transplanted after 2009 (88% vs 80%, p = 0.047). Time to neutrophil and platelet recovery was longer in the low CD34+ group. Median PFS and OS were lower in the low CD34+ group (31.6 vs. 43.6 months, p = 0.011 and 76.4 vs. 108.2 months, p < 0.001, respectively). Evaluation of incrementally higher CD34+ dose did not show significant improvement in survival at thresholds >2.5 × 106 cells/kg. Multivariable analysis affirmed that CD34+ >2.5 × 106 cells/kg was associated with better PFS (HR 0.71, p = 0.008) and OS (0.59, p < 0.001). After propensity score matching, a CD34+ dose >2.5 × 106 cells/kg remained a predictor of better OS (0.42, p < 0.001). In conclusion, CD34+ dose >2.5 × 106 cells/kg was associated with improved survival, without any additional benefit at incrementally higher doses.
Similar content being viewed by others
Introduction
In the dynamic landscape of treatment for multiple myeloma (MM), autologous hematopoietic stem cell transplantation (auto-HCT) remains the standard of care for eligible newly diagnosed patients. Despite its decades-long use, the optimal CD34+ cell dose to infuse during auto-HCT is still not known.
The International Myeloma Working Group (IMWG) published recommendations in 2009 for the minimum and ideal collection and infusion thresholds for patients with MM undergoing auto-HCT. They recommended a minimum dose of ≥2 × 106 CD34+ cells/kg and an optimal dose of 4–6 × 106 CD34+ cells/kg for a single transplant [1]. These recommendations were based on heterogenous data that also included non-myeloma patients, and predated modern therapies. There is considerable variability in the impact of CD34+ cell dose on neutrophil and platelet engraftment in MM patients in the published literature. One study showed that a higher CD34+ cell dose was associated with a faster platelet recovery without a beneficial effect on neutrophil recovery [2]. In contrast, another study showed more rapid hematologic engraftment with higher CD34+ cell doses [3]. Interestingly, a third study showed no positive impact of a higher CD34+ cell dose on hematologic engraftment [4].
Similarly, there are contradictory reports on the impact of CD34+ cell dose on survival in MM. This is in contrast with studies in lymphoma where there is a direct correlation between a higher CD34+ dose and improved survival in patients undergoing auto-HCT [5,6,7,8]. One study demonstrated improved hematologic recovery and median overall survival (OS) in MM patients receiving ≥5 × 106 CD34+ cells/kg [9], while a post-hoc analysis of the GOA trial demonstrated no discernable difference in progression-free survival (PFS) or OS across three CD34+ dose groups (<1.0 × 106 CD34+ cells/kg, 1–1.9 × 106 CD34+ cells/kg, and ≥2 × 106 CD34+ cells/kg) [10]. Furthermore, a recent study that included 621 patients showed shorter median PFS and OS in patients that had ≥8 × 106 CD34+ cell/kg collected for auto-HCT [11].
While there is variability in collection and infusion targets between transplant centers, clinical practice has remained largely unchanged for many years. With new therapeutic modalities for MM gaining favor, including increased use of quadruplet induction and earlier and more frequent use of CAR-T cells that can cause prolonged pancytopenia, the subject of optimal CD34+ cell dose in auto-HCT for MM has gained renewed interest [12, 13].
Given the lack of consensus on an optimal CD34+ cell dose, we sought to study the association of CD34+ cell dose on the outcomes of MM patients who underwent upfront auto-HCT.
Materials and methods
Study design and participants
We conducted a retrospective, single-center study on newly diagnosed patients with MM who underwent upfront auto-HCT between 2005 and 2021. Data were obtained from our institution’s transplantation database and chart-based review. We included patients with available information on the infused CD34+ cell dose at auto-HCT. Primary outcomes included neutrophil and platelet engraftment, PFS, and OS. Secondary outcomes included response rates and the depth of post-transplant response. The study was conducted after approval by our Institutional Review Board and in accordance with the Declaration of Helsinki and the 1996 Health Insurance Portability and Accountability Act.
We evaluated response rates according to the criteria outlined by the IMWG [14]. Minimal residual disease (MRD) status in bone marrow samples was determined utilizing an eight-color next-generation flow cytometry (NGF) technique with a sensitivity of 1/10−5 cells (0.001%) through the acquisition and analysis of at least of two million events. Fluorescence in situ hybridization (FISH) analysis was conducted to detect high-risk cytogenetic abnormalities, specifically t(4;14), t(14;16), del(17p), and 1q21 gain or amplification, utilizing IGH::FGFR3 dual-color dual-fusion probes, IGH::MAF dual-color dual-fusion probes, TP53/CEP17 dual-color probes, and CDKN2C/CKS1B dual-color probes.
Statistical methods
Neutrophil and platelet engraftment as well as other continuous measures were summarized by medians and ranges and evaluated by the Wilcoxon rank-sum test. Categorical variables were summarized using frequencies and percentages and assessed by Fisher’s exact test or its generalizations. OS time was computed from the date of auto-HCT to the last known vital sign, with censoring for patients alive at the last follow-up. PFS time was computed from the date of auto-HCT to the date of disease progression, death (if without disease progression), or the last follow-up. Patients alive with no disease progression were censored at their last follow-up date. OS and PFS were estimated using the Kaplan–Meier method, with group differences assessed by the log-rank test. Associations between OS/PFS and measures of interest were assessed using Cox proportional hazards regression models.
The web application Cutoff Finder [15] was used to find the optimal cutoff point for CD34+ cell dose infusion with survival based on the log-rank test. Patients were then categorized into low (≤2.5 × 106 cells/kg) and high (>2.5 × 106 cells/kg) CD34+ groups based infused CD34+ cell quantity. There was considerable disparity between patient number in the low (N = 95) and high (N = 2384) CD34+ groups. To address the difference in sample sizes, 2:1 nearest-neighbor matching was employed [16, 17]. This matching method, utilizing propensity scores derived from a logistic regression model, ensured comparability for subsequent analysis. For each patient in the “low” dose group, control matches from the “high” dose group were selected one at a time based on a descending order of the distance measure. Matching variables included age at auto-HCT, year of auto-HCT (<2010, ≥2010), cytogenetic risk (standard, high, unknown), Revised International Staging System (R-ISS) stage (I, II, III, unknown), Hematopoietic Cell Transplantation (HCT)-specific Comorbidity Index (HCT-CI) (≤3, >3), induction treatment [bortezomib, lenalidomide, and dexamethasone (VRD), bortezomib, cyclophosphamide, and dexamethasone (VCD), bortezomib and dexamethasone (VD), carfilzomib, lenalidomide, and dexamethasone (KRD), immunomodulator and dexamethasone (ImiD + Dexa), bortezomib thalidomide and dexamethasone (VTD), or other], mobilization with/without chemotherapy (yes, no, unknown), conditioning regimen (melphalan, busulfan and melphalan, other), and maintenance treatment use (yes, no). Of note, we did not use plerixafor as a matching variable since it generally has not been shown to impact survival outcomes [18].
Nearest-neighbor matching was performed using the MatchIt package in R (MatchIt: Nonparametric Preprocessing for Parametric Causal Inference). All other statistical analyses were conducted using SAS 9.4 for Windows (SAS Institute Inc., Cary, NC) with a significance level of 5%. No adjustments for multiple testing were made.
Results
Patient, disease, and treatment characteristics
A total of 2479 patients were included in our analysis, 95 in the low CD34+ dose group and 2384 in the high CD34+ dose group. There were 21 patients who received <2 × 106 cells/kg in the low CD34+ dose group. Median age at auto-HCT in the entire cohort was 61 years (range 25–83), and 59% were males (n = 1460). In the low CD34+ group, patients were older on average (63 vs 61 years, p = 0.013), a higher percentage had R-ISS III (19% vs 9%, p = 0.014), more often received plerixafor for mobilization (60% vs 35%, i < 0.001) and more often transplanted after 2009 (88% vs 80%, p = 0.047). In both CD34+ dose groups, VRD (28% and 31%, respectively) and KRD (18% and 15%, respectively) were the most commonly used induction regimens. After matching, only R-ISS (p = 0.048) remained significantly different between the low and high CD34+ dose groups. Patient characteristics are summarized in Table 1. Patient characteristics for the matched cohorts are presented in Supplementary Table 1.
Engraftment
In the low CD34+ dose group, neutrophil recovery (absolute neutrophil count (ANC) ≥ 500 × 108 cells/L) occurred at a median of 12 days after auto-HCT, compared to 11 days in the high CD34+ dose group (p < 0.001). Platelet recovery to ≥20 × 109 cells/L and ≥50 × 109 occurred at a median of 14 and 18 days in the low CD34+ dose group, in contrast to 11 and 14 days in the high CD34+ dose group (p < 0.001). Only one patient in the entire cohort, in the high CD34+ dose group, had engraftment failure. Individuals in the low CD34+ dose group received more red blood cell (RBC) transfusions, with a median of two units compared to one unit in the high CD34+ dose group (p < 0.001). Similarly, those in the low CD34+ dose group received more platelet transfusions, with a median of three units compared to two units in the high-dose CD34+ dose group (p < 0.001). Engraftment outcomes are summarized in Supplementary Table 2.
Patients in the high CD34+ group had a shorter median duration of hospitalization for auto-HCT compared to the low CD34+ group [16 (range 0–94) days and 17 (range 0–38) days, respectively; p = 0.007]. This translates to an estimated saving of 2700 US dollars per patient (i.e., average daily cost of hospital stay) in the high CD34+ group.
Response and MRD assessment
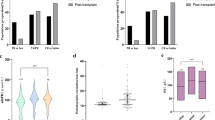
There were no significant differences in day-100 and best post-auto-HCT responses based on CD34+ cell dose (Fig. 1). In the low CD34+ dose group, day-100 ≥complete response (CR) and ≥very good partial response (VGPR) response rates were 34% and 80%, respectively, and at best post-transplant response ≥CR and ≥VGPR response rates improved to 49% and 89%, respectively. In the high CD34+ group, day-100 ≥ CR and ≥VGPR response rates were 35% and 78%, respectively, and at best post-transplant response ≥CR and ≥VGPR response rates improved to 55% and 86%, respectively. MRD negative ≥VGPR was seen in 72% and 65% of evaluable patients in the low and high CD34+ dose groups, respectively. Similarly, there was no significant difference in post-transplant response or MRD rates in the propensity-matched patients (Supplementary Table 3).
Survival outcomes
Median follow-up for the entire cohort was 51.9 months (range 0.2–201.6). Median PFS was 31.6 and 43.6 months (p = 0.011) in the low and high-dose CD34+ dose groups, respectively (Fig. 2A). The median OS was 76.4 and 108.2 months (p < 0.001) months in the low and high CD34+ dose groups, respectively (Fig. 2B). Five-year OS rate was 61% and 74% for low and high CD34+ dose groups, respectively. We also assessed the impact of incremental CD34+ cell dose on PFS and OS, up to 6 × 106 cells/kg. As shown in Supplementary Table 4, in univariate analysis there was no significant correlation between doses above 2.5 × 106 cells/kg and either PFS or OS. Day-100 non-relapse mortality (NRM) was 0% and 1% (p = 0.47) in the low and high CD34+ dose groups, respectively.
In multivariable analysis for PFS, CD34+ cell dose >2.5 × 106 cells/kg (hazard ratio [95% CI] 0.71 [0.55–0.91], p = 0.008) was associated with superior PFS. Other variables associated with superior PFS included auto-HCT after 2009 (0.79 [0.68–0.91], p = 0.002), use of KRD as the induction regimen (0.76 [0.61–0.94], p = 0.013) and achieving MRD negative ≥VGPR prior to auto-HCT (0.59 [0.50–0.69, p < 0.001)]. In contrast, R-ISS stage III (1.62 [1.25–2.11], p < 0.001) compared to R-ISS stage I, lambda light chain type (1.24 [1.11–1.39], p < 0.001), high-risk cytogenetics (1.93 [1.67–2.23], p < 0.001) and the use of chemotherapy for mobilization (1.24 [1.07–1.45], p = 0.005) were associated with inferior PFS (Table 2). Univariate analysis for PFS is shown in Supplementary Table 5.
In multivariable analysis for OS, CD34+ cell dose >2.5 × 106 cells/kg (0.59 [0.44–0.79], p < 0.001) was associated with superior OS. Other variables associated with superior OS were achieving a CR at the best response (0.50 [0.43–0.59], p < 0.001), and the use of maintenance therapy (0.69 [0.59–0.81], p < 0.001). In contrast, R-ISS stage III (2.08 [1.46–2.97, p < 0.001)] compared to R-ISS stage I, lambda light chain type (1.27 [1.10–1.47], p < 0.001), high-risk cytogenetics (2.15 [1.78–2.60], p < 0.001) and HCT-CI > 3 (1.41 [1.20–1.65], p < 0.001) were associated with worse OS (Table 3). After propensity score matching, a CD34+ dose of >2.5 × 106 cells/kg remained associated with better OS (0.42 [0.28–0.63, p < 0.001)] (Table 4). Univariate analysis for OS is shown in Supplementary Table 6.
Discussion
Despite the widespread use of auto-HCT in patients with MM, the optimal CD34+ cell dose to infuse during transplant remains unclear. Current recommendations for CD34+ dosing were developed prior to the use of contemporary therapeutic agents and also applied to non-MM patients. In this large cohort of MM patients who received upfront auto-HCT, we showed that a CD34+ cell dose of >2.5 × 106 cells/kg is associated with faster hematologic recovery and decreased length of hospitalization, as well as improved PFS and OS, compared to a CD34+ cell dose of ≤2.5 × 106 cells/kg. Incremental increase in CD34+ dose beyond 2.5 × 106 cells/kg was not associated with any additional benefit. The OS benefit was confirmed by a separate analysis using propensity score matching.
The IMWG consensus statement recommends that a minimum and ideal target of 4 × 106 CD34+ cells/kg and 8–10 × 106 CD34+ cells/kg, respectively, be collected. This allows most patients to undergo at least two autografts if needed [1]. They advocate for a minimum acceptable dose of 2 × 106 CD34+ cells/kg and an optimal dose of 4–6 × 106 CD34+ cells/kg for a single transplant. These recommendations were based on older retrospective studies with significant heterogeneity in conditioning regimens and optimal CD34+ cell cutoff [2, 19, 20]. They also largely focused on hematologic recovery, rather than survival. Studies have shown that a higher CD34+ cell dose was associated with improved PFS and OS after auto-HCT in non-Hodgkin lymphoma and Hodgkin disease [5,6,7,8]. However, the role of infused CD34+ cell dose on survival in patients with MM is not clear. Moreover, some studies in MM have focused on optimal CD34+ cell mobilization rather than optimal CD34+ cell dose for infusion, with varying outcomes [21,22,23].
In a study that included 117 MM patients who underwent auto-HCT, the infused CD34+ cell dose showed no impact on neutrophil recovery. However, a significant association was seen between a CD34+ cell dose of ≥1.5 × 106 cells/kg and platelet recovery [2]. Conversely, a study with 508 MM patients showed a faster hematologic recovery and reduced hospitalization with CD34+ cell dose of ≥6.55 × 106 cells/kg with CD34+ cell selection, and ≥ 7.50 × 106 cells/kg without CD34+ cell selection. Interestingly, OS, transplant-related mortality, and day-100 response rates were not correlated with CD34+ cell dose [3]. A prospective trial at MD Anderson Cancer Center assessed the impact of CD34+ cell dose on the outcomes of patients with MM (73%) or light chain amyloidosis who underwent auto-HCT and received either a standard (4–6 × 106 CD34+ cells/kg) or high-dose (10–15 × 106 CD34+ cells/kg). The trial revealed no significant difference in symptom burden, hematologic recovery, or survival between the two dose groups [4]. This aligns with our results, showing no benefit in survival outcomes beyond an infused dose of 2.5 × 106 CD34+ cells/kg. It also highlights that a faster hematologic recovery does not necessarily correlate with better survival. In contrast, a retrospective study from Turkey with 271 patients enrolled between 2003 and 2019, revealed that a CD34+ cell dose of ≥5 × 106 CD34+ cells/kg was associated with a faster neutrophil and platelet recovery and superior median OS (145 months vs. 103 months; p = 0.009) when compared to a dose of <5 × 106 CD34+ cells/kg [9]. These contradictory results underscore the need for larger studies in the era of novel therapies. We believe that our current study, which included a large cohort that was treated with contemporary regimens is a step in the right direction.
Previous studies have suggested that the composition and quality of the graft is as important as the dose of CD34+ cells [24,25,26]. In a recent study by our group, the presence of clonal plasma cells (CPC) in the autograft was associated with worse survival outcomes [26]. Furthermore, infusion of CPC-contaminated autografts was associated with inferior PFS in multivariable analysis. Myeloid-derived suppressor cells (MDSC) are also possible contaminants of autologous grafts, and studies have observed a correlation between pre-transplant MDSC and adverse outcomes in MM [27, 28]. Setting lower thresholds for CD34+ cell collection and shortening the collection process could potentially reduce the amount of collected and infused detrimental MDSC.
In the present study, patients who received KRD as induction had superior PFS in multivariable analysis compared with recipients of other regimens. Although VRD was the most commonly used induction regimen, several other regimens were also used. Similar to our current study, another single-center study observed improved 5-year PFS (67% vs. 56%, p = 0.027) and a trend toward improved OS (90% vs. 80%; p = 0.053) in patients who received KRD vs. VRD in newly diagnosed MM patients. This benefit was notable in the high-risk subgroup, which accounted for 49% and 37% of the KRD and VRD groups, respectively [29].
Increasing use of daratumumab in the frontline setting for transplant-eligible MM patients [30,31,32] has also raised concerns about its impact on stem cell mobilization and collection [33, 34]. Moreover, with greater use of CAR-T cells and ongoing sporadic use of tandem auto-HCT, it is important to define optimal CD34+ cell collection and infusion targets in MM. Immune Effector Cell Associated Hematotoxicity (ICAHT) is a common toxicity following anti-BMCA CAR-T cell therapy. In a recent study of 108 MM patients who underwent anti-BMCA CAR-T cell therapy, 60% experienced ICAHT at day 21, 28% of whom received a stem cell boost at a median of 116 days post-CAR-T infusion. Stem cell boost resulted in neutrophil recovery in all patients and improvements in hemoglobin and platelets in the majority [12]. Based on these data, the European Society for Blood and Marrow Transplantation (EBMT) and the European Hematology Association (EHA) jointly recommended best practices for managing ICAHT, suggesting autologous stem cell boosts for ≥grade 3 ICAHT beyond day 14 if readily available. Furthermore, there have been several reports of using allogeneic stem cell boosts to mitigate prolonged cytopenias after CAR-T therapy [35, 36]. These factors underscore a future broader use of CD34+ cells and their optimal dose, and some of the collected cells for auto-HCT could be set aside for potential ICAHT in the future.
Our study has inherent limitations of a retrospective analysis, including selection bias, treatment heterogeneity, a relatively small number of patients in the low CD34+ dose cohort, and missing data. Notwithstanding these limitations, this is one of the largest studies to evaluate the association between CD34+ cell dose and survival outcomes in patients with MM undergoing auto-HCT. Bottom of Form
In summary, our study showed that a CD34+ cell dose of >2.5 × 106 CD34+ cells/kg, compared to ≤2.5 × 106 CD34+ cells/kg, is associated with optimal hematologic engraftment and better survival.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia. 2009;23:1904–12.
Desikan KR, Tricot G, Munshi NC, Anaissie E, Spoon D, Fassas A, et al. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112:242–7.
Klaus J, Herrmann D, Breitkreutz I, Hegenbart U, Mazitschek U, Egerer G, et al. Effect of CD34 cell dose on hematopoietic reconstitution and outcome in 508 patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Eur J Haematol. 2007;78:21–8.
Shah N, Shi Q, Williams LA, Mendoza TR, Wang XS, Reuben JM, et al. Higher stem cell dose infusion after intensive chemotherapy does not improve symptom burden in older patients with multiple myeloma and amyloidosis. Biol Blood Marrow Transpl. 2016;22:226–31.
Pavone V, Gaudio F, Console G, Vitolo U, Iacopino P, Guarini A, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transpl. 2006;37:719–24.
Bolwell BJ, Pohlman B, Rybicki L, Sobecks R, Dean R, Curtis J, et al. Patients mobilizing large numbers of CD34+ cells (‘super mobilizers’) have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transpl. 2007;40:437–41.
Yoon DH, Sohn BS, Jang G, Kim EK, Kang BW, Kim C, et al. Higher infused CD34+ hematopoietic stem cell dose correlates with earlier lymphocyte recovery and better clinical outcome after autologous stem cell transplantation in non-Hodgkin’s lymphoma. Transfusion. 2009;49:1890–900.
Blystad AK, Delabie J, Kvaløy S, Holte H, Vålerhaugen H, Ikonomou I, et al. Infused CD34 cell dose, but not tumour cell content of peripheral blood progenitor cell grafts, predicts clinical outcome in patients with diffuse large B-cell lymphoma and follicular lymphoma grade 3 treated with high-dose therapy. Br J Haematol. 2004;125:605–12.
Aladağ KarakulakE, Demiroğlu H, Büyükaşik Y, Turgut M, Aksu S, Sayinalp N, et al. CD34+ hematopoietic progenitor cell dose as a predictor of engraftment and survival in multiple myeloma patients undergoing autologous stem cell transplantation. Turk J Med Sci. 2020;50:1851–6.
Partanen A, Turunen A, Silvennoinen R, Valtola J, Pyörälä M, Siitonen T, et al. Impact of the number of cryopreserved CD34(+) cells in the infused blood grafts on hematologic recovery and survival in myeloma patients after autologous stem cell transplantation: experience from the GOA study. J Clin Apher. 2023;38:33–44.
Lebel E, Lajkosz K, Masih-Khan E, Reece D, Trudel S, Tiedemann R, et al. The impact of CD34(+) cell collection yields for autologous transplant on survival outcomes in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2023;23:850–6.
Mohan M, Szabo A, Patwari A, Esselmann J, Patel T, Bachu R, et al. Autologous stem cell boost improves persistent immune effector cell associated hematotoxicity following BCMA directed chimeric antigen receptor T (CAR T) cell therapy in multiple myeloma. Bone Marrow Transplant. 2024;59:647–652.
Davis JA, Sborov DW, Wesson W, Julian K, Abdallah AO, McGuirk JP, et al. Efficacy and safety of CD34+ stem cell boost for delayed hematopoietic recovery after BCMA directed CAR T-cell therapy. Transpl Cell Ther. 2023;29:567–71.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE. 2012;7:e51862.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–8.
Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Anal. 2007;15:199–236.
Jantunen E, Turunen A, Varmavuo V, Partanen A. Impact of plerixafor use in the mobilization of blood grafts for autologous hematopoietic cell transplantation. Transfusion. 2024;64:742–50.
Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9.
Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–55.
Raschle J, Ratschiller D, Mans S, Mueller BU, Pabst T. High levels of circulating CD34+ cells at autologous stem cell collection are associated with favourable prognosis in multiple myeloma. Br J Cancer. 2011;105:970–4.
Brioli A, Perrone G, Patriarca F, Pezzi A, Nobile F, Ballerini F, et al. Successful mobilization of PBSCs predicts favorable outcomes in multiple myeloma patients treated with novel agents and autologous transplantation. Bone Marrow Transpl. 2015;50:673–8.
Kakihana K, Ohashi K, Akiyama H, Sakamaki H. Correlation between survival and number of mobilized CD34+ cells in patients with multiple myeloma or Waldenström macroglobulinemia. Pathol Oncol Res. 2010;16:583–7.
Vogel W, Kopp HG, Kanz L, Einsele H. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J Cancer Res Clin Oncol. 2005;131:214–8.
Kopp HG, Yildirim S, Weisel KC, Kanz L, Vogel W. Contamination of autologous peripheral blood progenitor cell grafts predicts overall survival after high-dose chemotherapy in multiple myeloma. J Cancer Res Clin Oncol. 2009;135:637–42.
Pasvolsky O, Milton DR, Rauf M, Ghanem S, Masood A, Mohamedi AH, et al. Impact of clonal plasma cells in autografts on outcomes in high-risk multiple myeloma patients. Blood Cancer J. 2023;13:68.
Lee SE, Lim JY, Kim TW, Ryu DB, Park SS, Jeon YW, et al. Different role of circulating myeloid-derived suppressor cells in patients with multiple myeloma undergoing autologous stem cell transplantation. J Immunother Cancer. 2019;7:35.
Lim JY, Kim TW, Ryu DB, Park SS, Lee SE, Kim BS, et al. Myeloma-secreted galectin-1 potently interacts with CD304 on monocytic myeloid-derived suppressor cells. Cancer Immunol Res. 2021;9:503–13.
Tan CR, Derkach A, Nemirovsky D, Ciardiello A, Diamond B, Hultcrantz M, et al. Bortezomib, lenalidomide and dexamethasone (VRd) vs carfilzomib, lenalidomide and dexamethasone (KRd) as induction therapy in newly diagnosed multiple myeloma. Blood Cancer J. 2023;13:112.
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390:301–13.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936–45.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38.
Lemonakis K, Tatting L, Lisak M, Carlson K, Crafoord J, Blimark CH, et al. Impact of daratumumab-based induction on stem cell collection parameters in Swedish myeloma patients. Haematologica. 2023;108:610–4.
Hulin C, Offner F, Moreau P, Roussel M, Belhadj K, Benboubker L, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/dexamethasone in the phase 3 CASSIOPEIA study. Haematologica. 2021;106:2257–60.
De Tena PS, Bailen R, Oarbeascoa G, Gomez-Centurion I, Perez-Corral A, Carbonell D, et al. Allogeneic CD34-selected stem cell boost as salvage treatment of life-threatening infection and severe cytopenias after CAR-T cell therapy. Transfusion. 2022;62:2143–7.
Mullanfiroze K, Lazareva A, Chu J, Williams L, Burridge S, Silva J, et al. CD34+-selected stem cell boost can safely improve cytopenias following CAR T-cell therapy. Blood Adv. 2022;6:4715–8.
Funding
This work was supported in part by Cancer Center Support Grant P30 CA016672. This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672). RZO, the Florence Maude Thomas Cancer Research Professor, would like to acknowledge support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Riney Family Multiple Myeloma Research Fund at MD Anderson from the Paula and Rodger Riney Foundation, the Leukemia & Lymphoma Society (SCOR-12206-17), and the MD Anderson Cancer Center High-Risk Multiple Myeloma Moon Shot.
Author information
Authors and Affiliations
Contributions
OP and MHQ conceived and designed the study; OP, CM, BP, and JL collected and assembled the data; OP, MHQ, and DRM analyzed and verified the data; All authors interpreted the data, wrote and approved the article, and are accountable for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted after approval by the Institutional Review Board at the University of Texas MD Anderson Cancer Center (protocol number PA17-0450). Approval was obtained by this Institutional Review Board to waive informed consent for this retrospective chart review. The study was conducted in accordance with the Declaration of Helsinki and the 1996 Health Insurance Portability and Accountability Act.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pasvolsky, O., Marcoux, C., Milton, D.R. et al. Optimal infused CD34+ cell dose in multiple myeloma patients undergoing upfront autologous hematopoietic stem cell transplantation. Blood Cancer J. 14, 189 (2024). https://doi.org/10.1038/s41408-024-01165-w
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-024-01165-w