Abstract
The treatment of multiple myeloma has changed dramatically in recent years, with huge strides forward made in the field. Chimeric antigen receptor T-cell therapy targeting the B cell maturation antigen (BCMA) is now widely approved in relapsed refractory patients and is moving into earlier treatment lines. In this review, we discuss the evidence underpinning current regulatory approvals and consider mechanisms through which CAR-T cell efficacy could be improved. These include tackling BCMA-loss, harnessing the immunosuppressive tumour microenvironment, manufacturing concerns including the potential role of other cellular sources, safety issues such as cytokine release syndrome and neurotoxicity, and optimal patient selection.
Similar content being viewed by others
Background
Multiple myeloma (MM) is the second most common haematological malignancy of adults in the Western world [1]. Well-characterised manifestations of this malignancy, involving proliferation of clonal plasma cells, include anaemia, renal impairment, bone disease and immunoparesis [2, 3]. Median overall survival (OS) has more than doubled in recent years following incorporation of proteosome inhibitors (PIs), immunomodulatory agents (IMiDs) and monoclonal antibodies into standard-of-care treatment pathways [4]. However, patients with adverse cytogenetics, extra-medullary myeloma, or high-risk disease by the Revised International Staging System (R-ISS) have far less favourable outcomes, and the majority of patients eventually develop treatment-resistant disease [5,6,7]. Prior to the advent of T-cell engagers and cellular therapies, triple-class refractory patients, who are refractory to a PI, IMiD and CD38-directed monoclonal antibody, have had reported survival rates of less than 1 year, and penta-refractory patients (refractory to 2 IMiDs, 2 PIs and a monoclonal antibody) usually survive less than half that [8]. Given this significant unmet need, development of novel therapies has remained a priority in MM.
Chimeric antigen receptors are engineered T-cell receptors designed to recognise a specific tumour antigen without the need for antigen presentation by major histocompatibility class (MCH) molecules. The most common CAR-T construct comprises an extra-cellular antigen-recognition domain connected by a transmembrane domain to a co-stimulatory molecule, and then to the T-cell activating CD3-zeta domain [9]. The optimal tumour antigen should be consistently expressed in high concentrations by MM cells, but not expressed by the normal haematopoietic counterpoint or other tissues. The antigen most closely meeting these criteria is B-cell maturation antigen (BCMA), which is expressed by MM cells, late memory B-cells, plasmablasts and mature plasma cells, but not haematopoietic stem cells or non-haematopoietic cells [10]. BCMA has thus been at the forefront of CAR-T cell development in MM, with 2 products receiving regulatory approval in recent years.
BCMA-directed CAR-T cells: approved products
US National Cancer Institute
The US National Cancer Institute (NCI) performed the first-in-human study of a BCMA-targeting CAR-T with a murine single chain variable fragment (scFv) and a CD28 co-stimulatory domain. Twenty-four RRMM patients with a median 7.5 prior lines of therapy received lymphodepleting chemotherapy followed by 0.3 × 103–9 × 106 CAR-T cells/kg. Of the 16 patients who received the highest dose, overall response rate (ORR) was 81% with complete remission (CR) achieved by 13% [11, 12].
Idecabtagene vicleucel
Idecabtagene vicleucel (Ide-cel, previously bb2121) uses the same murine scFv as the NCI product alongside a 4-1BB co-stimulatory domain. A phase 1 study, initially in 33 RRMM patients showed promising results which led to development of the phase 2 KarMMa study [13], in which 128 triple class-exposed patients, who had received at least 3 prior lines of therapy, were infused 150–450 × 106 CAR-T cells. In the 54 patients who received the highest CAR-T cell dose, ORR was 81%, ≥CR rate 39%, median PFS 12.1 months and OS was 19.4 months. Of patients in CR/stringent CR (sCR), 76% were measurable residual disease (MRD) negative to a level of 10-5 by next generation sequencing (NGS) and 59% of those had sustained MRD negativity after 12 months. Cytokine release syndrome (CRS) occurred in 84% and neurotoxicity in 18% [14,15,16]. Based on these results, the US Food and Drug Administration (FDA) granted approval for the use of Ide-cel in patients with RRMM after 4 or more prior lines of therapy, in March 2021.
The phase 2 KarMMa-2 study assessed Ide-cel in RRMM in a number of different cohorts. Cohort 2a enroled patients with progressive disease within 18 months of induction therapy, autologous stem cell transplantation (ASCT) and lenalidomide maintenance. Thirty-nine patients were enroled, of which 37 were successfully infused 150–450 × 106 CAR-Ts. With a median follow-up of 21.5 months, ORR was 83.8%, CR rate was 45.9%, and 11 of 13 evaluable patients were MRD negative to a level of 10-5 at 6 months. Median PFS was 11.4 months and median OS was not reached. CRS occurred in 81% and neurotoxicity in 22% [17]. Cohort 2c assessed Ide-cel in patients with less than a very good partial remission (VGPR) after ASCT. With a median follow-up of 39.4 months, ORR was 87.1%, CR rate 77.4%, and median PFS and OS have not been reached in the 31 patients who received Ide-cel. After 36 months, 64.3% of evaluable patients were MRD negative. Fifty-eight percent had CRS and 7% developed neurotoxicity [18].
The phase 3 KarMMA-3 study compared Ide-cel with standard of care regimens in triple-class exposed (TCE) RRMM after 2-4 prior regimens. Three hundred and eighty-six patients were enroled, 254 randomised to the Ide-cel arm, of which 225 received the investigational product. After a median follow-up of 30.9 months, median PFS was significantly improved in the Ide-cel cohort (13.8 vs. 4.4 months) with a 51% reduced risk of PD or death. ORR, CR and MRD negativity were all also significantly in favour of Ide-cel (ORR 71% vs 42%, CR 44% vs. 5%). CRS occurred in 88% of treated patients and neurotoxicity in 15% [19]. Following these results, the FDA broadened the indication for Ide-cel to TCE RRMM after ≥2 prior lines. A more recent analysis of results failed to demonstrate a benefit in OS (median OS 41.4 vs. 37.9 months; HR 1.01, 95% CI 0.73–1.40). This is likely partly explained by the cross-over design, as 56% of patients in the standard of care arm received subsequent Ide-cel. After adjustment for cross-over, there was a trend to improvement seen with Ide-cel, however this did not meet statistical significance [20]. Results from the phase 1 KarMMa-4 (NCT04196491) study of frontline Ide-cel in high-risk MM are awaited.
LCAR-B38M
LCAR-B38M is an anti-BCMA CAR-T which incorporates 2 camelid (derived from llama) variable heavy-chain-only domains, which are highly specific for BCMA, conferring high avidity binding. The phase 1/2 LEGEND-2 study was conducted in 4 sites in China. Of 57 patients treated in 1 site, reported ORR was 88%, with a CR rate of 74% and PFS of 20 months. 82% of patients developed CRS [21, 22]. At 5 years follow-up, 74 patients had been treated, with an ORR of 88%, CR rate 73% and an MRD negative CR rate of 67%. Median PFS was 18 months and OS was 55.8 months in this heavily pre-treated cohort [23].
Ciltacabtagene autoleucel
Ciltacabtagene autoleucel (Cilta-cel, previously JNJ-4528) utilizes the same BCMA binding domain as the LCAR-B38M construct. In the phase 1b CARTITUDE-1 study, patients were administered 0.75 ×106 CAR-T cells/kg following lymphodepletion. Ninety-seven RRMM patients, of whom 87% were triple-refractory and 42% were penta-refractory received Cilta-cel, with an ORR of 98% and sCR of 80% reported. Ninety-two percent of evaluable patients were MRD negative to a level of 10-5. The US prescribing information from the FDA reports the development of CRS in 95% of patients receiving Cilta-cel in CARTITUDE-1 (Grade ≥3 in 4%), neurotoxicity in 23% (Grade ≥3 in 3%), including movement and neurocognitive treatment-emergent adverse events (MNTs) in 6% [24]. FDA approval in RRMM after 4 or more prior lines of therapy was granted in February 2021 [25,26,27]. Updated results after 33 months follow-up reported an impressive median PFS of 34.9 months with an estimated 63% OS at 36 months [28].
In cohort A of the phase 1 CARTITUDE-2 study, 20 patients with lenalidomide-refractory MM, after 1-3 prior lines of therapy, were infused 0.75 ×106 Cilta-cel CAR-T cells. After a median follow-up of nearly 30 months, ORR and rate of ≥CR were 95% and 80% respectively, with 24-month PFS and OS of 75%. CRS occurred in 95%, neurotoxicity in 30%, ICANS in 15%, and there have been no reported MNTs to date. In cohort B, 19 patients with early relapse after frontline therapy (within 12 months of ASCT, or 12 months from commencement of a non-transplant-containing regimen), have been treated. After a median follow-up of 28 months, ORR and CR were 100% and 74% respectively, with a 24-month PFS of 73% and OS of 84%. CRS was reported in 84%, neurotoxicity in 32% and MNTs in 5% [29]. Cohort C enroled 20 triple class-exposed RRMM patients. All patients had received prior non-cellular BCMA-directed treatment (bispecific antibodies in 40%, BCMA-antibody drug conjugates in 65%) and the majority had refractory disease to such therapies. After a median follow-up of 11 months, ORR was 95% with a median PFS of 9.1 months. CRS occurred in 60%, ICANS in 20% and no patients had MNTs [30].
The phase 3 CARTITUDE-4 study in lenalidomide-refractory RRMM after 1-3 lines of therapy compared Cilta-cel with pomalidomide, bortezomib and dexamethasone, or daratumumab, pomalidomide and dexamethasone. Four hundred and nineteen patients were enroled, 208 were randomised to Cilta-cel, of which, 176 received a CAR-T infusion. Twenty-six percent of each cohort had triple-exposed RRMM. After a median 16 months follow-up, ORR, ≥CR rate and MRD negativity were significantly higher in the Cilta-cel arm (ORR 85% vs. 67%, ≥CR rate 73% vs. 22%, MRD negative status 61% vs. 16%). Median PFS has not been reached in the Cilta-cel arm versus 11.8 months in the standard of care cohort, with a 12-month PFS of 76% in the Cilta-cel arm and 49% in the control. Seventy-six percent of Cilta-cel-treated patients developed CRS, 4.5% had ICANS and 9% had cranial nerve (CN) palsy [31]. The FDA subsequently expanded approval for Cilta-cel to include patients with lenalidomide-refractory disease after 1-3 prior lines. Updated results were presented at the International Myeloma Society Annual meeting, 2024, reporting an OS benefit in the Cilta-cel arm (30 month OS 76.4% vs. 63.8%; HR 0.55, 95% CI 0.39–0.79) [32]. The phase 3 CARTITUDE-5 and CARTITUDE-6 studies in newly diagnosed MM (transplant-ineligible or transplant-eligible respectively) are ongoing [33].
At the time of writing, Cilta-cel appears to be the more efficacious of the two approved agents, with fairly similar toxicity profiles reported. Choice of drug will likely be significantly impacted by availability at a local level. BCMA-directed CAR-T studies are summarised in Table 1, and Table 2 summarises the adverse events reported within the CARTITUDE and KarMMa studies to date.
Means of improving CAR-T cell efficacy
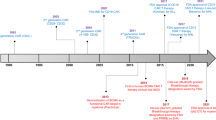
Despite the dramatic responses produced by CAR-T cell therapy in RRMM, the majority of patients will eventually progress. The reasons for this are multifactorial, including loss of BCMA expression, high levels of sBCMA, the immunosuppressive MM microenvironment, timing of CAR-T administration within the treatment pathway, suboptimal CAR-T cell function and manufacturing issues [34]. Potential approaches to improve CAR-T outcomes are summarised in Fig. 1.
Reducing CAR-T immunogenicity: Fully human BCMA-binding domains
Demonstration of T-cell reactivity against the murine BCMA-binding scFv used in Ide-cel has generated interest in producing fully human BCMA CAR-Ts in order to reduce anti-CAR-T immune responses and improve persistence [35].
A number of fully humanized anti-BCMA CAR-Ts have been designed. These include CT053 (zevorcabtagene-autoleucel, Zevor-cel), which has a human BCMA-directed scFv. In the phase 1 Lummicar 1 study, 14 RRMM patients received CT053, with an ORR and ≥CR after 38 months follow-up of 100% and 79% respectively, and a median PFS of 25 months [36].
JCARH125 (Orva-cel, orvacabtagene-autoleucel; no longer under development), was investigated in the phase 1/2 EVOLVE clinical study. Fourty-four RRMM patients received 300–600 × 106 CAR-Ts. ORR and ≥CR were 91% and 39%, respectively [37, 38].
Eighteen RRMM patients were infused with escalating doses of the fully human anti-BCMA CAR-T CT103A in a phase 1 study. After a median 41 months, ORR was 100% and 78% achieved ≥CR. At data cut-off, 7 patients remained in MRD negative sCR. Median PFS and OS were 23 and 42 months respectively. Median CAR-T persistence was 419 days, with transgenes detectable in 39% at the time of reporting [39]. In comparison, the majority of patients treated with Cilta-cel in CARTITUDE-1 did not have demonstrable CAR-T transgene persistence at 6 months [25]. In the phase 1b/2 FUMANBA-1 study, 103 RRMM patients with prior PI and IMiD treatment received CT103A (now called Equecabtagene Autoleucel, Eque-cel) at a dose of 1 × 106 cells. After a median follow-up of 14 months, ORR was 96%, ≥CR 74%, and 12-month PFS was 79%. A small number of patients with prior BCMA CAR-T treatment achieved sCR (5 of 89 patients). Eque-cel could still be detected in 40% after 24 months, with anti-drug antibodies identified in 19% of evaluable samples [40]. Eighty-eight patients within FUMANBA-1 were MRD negative, which was sustained over 6 months in 78% and over 12 months in 74% of evaluable patients [41]. This agent has been granted orphan drug designation by the FDA and appears promising. Longer follow-up will be required to determine whether improved CAR-T persistence translates to longer remission duration.
Studies of human BCMA CAR-Ts are shown in Table 1.
Approaches to BCMA loss
Reversible or partial loss: Enhance BCMA expression
Evidence of reversible or partial loss of BCMA expression has been identified in a small number of patients following BCMA-directed CAR-T cell therapy. In one study of 18 patients, 12 demonstrated decreased BCMA expression which subsequently recovered [42]. BCMA is cleaved from the MM cell surface by gamma-secretases leading to reduced cellular expression and increased levels of serum BCMA, a known adverse prognostic feature [43, 44]. High levels of circulating soluble BCMA (sBCMA) impair binding of anti-BCMA antibodies to MM cells in vitro, in addition to the impact of reduced target antigen density on the cell surface [45]. Additional mechanisms which may lead to partial BCMA loss include trogocytosis, in which the target antigen is transferred to the CAR-T cells themselves, leading to CAR-T fratricide alongside impaired MM killing, and potential epigenetic mechanisms observed in other haematological malignancies [46, 47].
Inhibition of gamma-secretase was shown to enhance MM cell BCMA expression and reduce sBCMA in vitro [48]. A phase 1 study of CRB402 (bb21217), the Ide-cel CAR-T construct treated with a phosphoinositide-3-kinase inhibitor during ex vivo culture to enrich for memory-like T cells, incorporated JSMD194, a gamma-secretase inhibitor (GSI), pre- and post-CART-T infusion in 18 RRMM patients. Reported ORR was 89% with 44% achieving ≥CR. In comparison, CRB402 without GSI in 72 RRMM patients achieved an ORR of 69% and ≥CR rate of 28% [49,50,51,52]. This possible increase in efficacy came at the expense of high levels of severe ICANS (grade ≥3 in 50%), frequent electrolyte disturbance and grade 3 diarrhoea in 17% [52]. Despite initial interest, bb21217 is no longer being pursued. Recent work has shown that BCMA is degraded by the ubiquitin proteosome system, and that in vitro treatment with a proteosome inhibitor increases surface BCMA expression and improves BCMA CAR-T efficacy, providing a rationale for combining PIs and BCMA-CAR-Ts in future studies [53].
Complete BCMA loss: Target other tumour epitopes
Complete BCMA loss may also occur in patients following BCMA CAR-T therapy. Homozygous gene deletion or heterozygous deletion and mutation have been identified by a number of authors in a small proportion of refractory patients [54,55,56]. Heterozygous BCMA gene deletion is found in approximately 20% of patients prior to BCMA-directed therapy [55]. These patients may therefore be at higher risk of selective expansion of BCMA-negative MM cells, in response to BCMA CAR-T cell therapy [57]. Patients with complete BCMA loss will not respond to BCMA-directed approaches, and consideration of other target antigens may be required. There are numerous clinical and pre-clinical trials ongoing assessing a variety of antigens, such as CD38, CD138, SLAMF7, GPRC5D, NKG2D, APRIL, CD44v6 and FcHR5.
G protein-coupled receptor class C group 5 member D (GPRC5D) is one of the more promising targets, with efficacy already demonstrated by the bispecific antibody talquetamab [58]. The phase 1 MCARH109 study enroled 17 patients, almost half of whom had received prior BCMA CAR-T cell therapy. ORR was 71%, ≥CR 35% and median duration of response was 7.8 months [59]. The phase 1 POLARIS study administered OriCAR-017 to 10 patients, with 50% prior BCMA CAR-T exposure. ORR was 100%, with 60% attaining a sCR [60]. The phase 1, BMS-986393 study enroled 33 patients, with 39% having received a BCMA CAR-T. ORR was 90%, with ≥CR in 47% [61]. As with the GPRC5D bispecific antibodies, on-target, off-tumour adverse events were common across these studies, including dysgeusia, nail disorders and dysphagia. The majority were mild and resolved without intervention [62]. Fc receptor-like 5 (FcHR5), a membrane protein that regulates B-cell receptor signalling [63] has shown encouraging results when targeted by the bispecific antibody, cevostamab [64]. Pre-clinical work has demonstrated efficacy of FcRH5 CAR-Ts in murine MM models, including those lacking BCMA expression [65, 66], however there are no clinical trials available at the time of writing.
Clinical studies of non-BCMA CAR-Ts in MM are shown in Table 3.
Reducing the likelihood for BCMA loss: dual antigen targeting
Targeting multiple tumour epitopes may be achieved by administering multiple CAR-T products or through use of bispecific CAR-T cells. This approach may improve specificity and reduce the selective pressure that promotes BCMA downregulation or loss.
Only a very small proportion of myeloma plasma cells express CD19, with some groups suggesting these may represent so-called myeloma stem cells [67, 68]. Despite expression in only 0.05% of the dominant plasma cell population, administration of CD19-directed CAR-T cell therapy alone has demonstrated efficacy in MM, providing a rationale for combined approaches with BCMA [69]. GC012F, a BCMA/CD19 dual-targeting CAR-T was tested in escalating doses in 22 newly diagnosed high-risk MM patients in a phase 1 study. After a median follow-up of nearly 14 months, ORR was 100%, sCR 96% and 100% attained MRD negativity to a sensitivity of 10-6. CRS occurred in only 27%, with no ICANS reported [70]. One group gave a combination of BCMA and CD19-directed CAR-Ts to 62 of 69 enroled participants. After a median follow-up of 21 months, ORR was 92%, ≥CR occurred in 60%, and 77% of assessed patients were MRD negative. Median PFS was 18 months, with CRS and neurotoxicity in 95% and 11% respectively [71]. Another study administered sequential anti-CD19 and BCMA CAR-T cell therapy with lenalidomide maintenance following ASCT in 10 high-risk NDMM patients. Seventy percent of the cohort have remained MRD negative after 2 years with this strategy [72].
APRIL (a proliferation-inducing ligand) recognises both BMCA and TACI (transmembrane activator, and calcium-modulator and cyclophilin ligand interactor), another MM epitope. AUTO2 is a CAR-T cell construct incorporating a truncated form of APRIL as it’s antigen-binding domain, enabling dual-targeting of BCMA and TACI. In a phase 1 study, ORR in 11 patients was 43% [73]. Similarly, a bispecific BCMA/CD38 CAR-T cell construct was tested in 16 RRMM patients in a single-centre phase 1 study (ChiCTR1900026286). ORR was 87.5%, 81% achieved sCR, and 12 month PFS and OS were 69% and 75% respectively [74]. Other bispecific CAR-Ts are under pre-clinical development, including a BCMA/CD24 CAR-T [75] and a CAR-T directed against BCMA and MICA (human MHC class 1-related chain gene A), which is upregulated by MM cells as a means of immune-evasion [76].
There are numerous methods of generating CAR-T products with specificity for more than one tumour associated antigen, with advantages and disadvantages unique to each. Additional information may be found in some excellent reviews on this rapidly developing area [77, 78]. A combination approach utilising CAR-Ts and/or bispecific antibodies to target multiple tumour epitopes may provide a highly efficacious future model of therapy. Studies of dual-targeting BCMA CAR-Ts are shown in Table 3.
Targeting the TME
One of the hallmarks of MM is the tumour-permissive microenvironment (TME). A complex interplay between MM cells, immune cells and bone marrow stromal cells leads to progressive immunoparesis, protecting and facilitating MM growth and survival. Peripheral blood and bone marrow samples were analysed from 11 and 6 Ide-cel-treated patients, respectively. Durable responses have been observed in patients with upregulation of genes expressing pro-inflammatory cytokines, Nuclear factor kappa B (NFKB) signalling genes and anti-apoptotic genes such as MCL-1 [79]. IMiDs stimulate upregulation of NFKB expression [80] and increase CD8 positive T-cells and memory T-cell subsets [81]. The addition of a cereblon E3 ligase modulator (CelMod), mezigdomide, to a BCMA-bispecific antibody led to improved T-cell activation and cell killing in a preclinical model [82], and IMiDs have been shown to enhance BCMA CAR-T function in vitro [83]. Lenalidomide or mezigdomide maintenance strategies post CAR-T cell therapy are being tested in ongoing clinical studies (NCT05032820, NCT06048250), as is the combination of lenalidomide with a BCMA CAR-T at the time of infusion (NCT03070327).
The checkpoint inhibitor programmed death axis (PD-1/PD-L1) is a well-studied mechanism of immune evasion in myeloma [84, 85]. High levels of PD-1 expressing T-cells have been shown to be associated with inferior in vitro MM cell lysis following treatment with Cilta-cel [86]. Combining checkpoint inhibitors (CPI) with IMiDs in MM was associated with increased mortality in KEYNOTE-185 [87], however studies are ongoing to investigate the role of CPI after CAR-T failure in MM (NCT04205409, NCT05204160, NCT06523621).
Analysis of samples from Cilta-cel treated patients identified increased expression of genes involved in T-cell activation and pro-inflammatory cytokines, such as interleukin-15 (IL-15), in patients with superior outcomes [88]. A fourth generation armoured BCMA CAR-T, engineered to release soluble IL-15, has demonstrated improved MM cell killing in vitro, with further investigation underway [89]. Another pre-clinical study identified increased levels of terminally exhausted T-cells with low levels of BCL2L1 (the gene encoding the anti-apoptotic protein BCL-XL). The authors generated a BCMA-BCL2L1 armoured CAR-T, with promising results shown in a murine MM model [90]. Cancer associated fibroblasts (CAFs) are a part of the immunosuppressive stromal compartment, which impair CAR-T activity and promote MM cell survival. Dual-targeting CAR-Ts, directed against both BCMA and CAFs were shown to improve CAR-T functionality compared with BCMA-CAR-Ts alone in a preclinical model [91].
The CAR-T construct itself can be modified to improve persistence within the immune microenvironment. One factor that may limit efficacy is tonic T-cell signalling in the absence of the target tumour epitope, leading to T-cell exhaustion and anergy. One means of reducing this is to use d-domain proteins instead of scFVs, which are small synthetic proteins that do not induce tonic signalling [92]. A novel BCMA-CAR-T with a completely synthetic d-domain-based antigen binding site (CART-ddBCMA; now called anitocabtagene autoleucel; Anito-cel) has been trialled in 13 patients in a phase 1 study to date, with an ORR of 100% and 89% MRD negativity in evaluable patients [93]. A phase 3 study, iMMagine-3, is enroling patients with RRMM who have received a prior 1-3 lines of therapy, with the view to comparing Anito-cel with pomalidomide, daratumumab or carfilzomib-based regimens. Results are highly anticipated using this novel CAR-T construct.
Improving manufacture of CAR-Ts
Production of CAR-T cells is a costly and often lengthy process. The patient undergoes leucopheresis to obtain peripheral blood mononuclear cells which are then enriched for T-cells. The CAR is introduced, usually via a viral vector, to generate CAR-T cells which are expanded ex vivo before being re-infused into the patient after lymphodepletion. This process may take weeks, during which time patients may become ineligible for treatment. Within KarMMA, 9% of enroled subjects did not receive their planned infusion of Ide-cel [14], nor did 14% of patients on CARTITUDE-1 [25]. The French Descar-T registry of commercial Ide-cel, reported that 9% of participants were not infused due to either disease progression or manufacturing failure [94]. Earlier use of CAR-Ts within the treatment paradigm may alleviate this issue as patients will have treatment options available whilst awaiting product manufacture. Additionally, given the progressive immune dysfunction that occurs in MM, CAR-Ts from less heavily pre-treated individuals are likely to be more efficacious than those obtained from RRMM patients.
High speed CAR-T generation is starting to enter clinical practice. The FasTCAR platform boasts next day CAR-T manufacture, by concurrently transducing and activating resting T-cells. This technology demonstrated feasibility in acute lymphoblastic leukaemia, and now in the GC012F BCMA/CD19 CAR-T product [70]. T-charge is another process in which CAR-Ts may be produced within 2 days of leucopheresis by removing the requirement for ex vivo expansion. A phase 1 study of PHE885, a BCMA-directed CAR-T product produced using the T-charge platform reported an average 16 day timeline from apheresis to lymphodepletion in 46 RRMM patients, with an ORR of 100% at the highest dose, and MRD negativity in 60% [95]. Furthermore, analysis of the CAR-T product showed preservation of early memory T-cells in the final product [95]. Analysis of Cilta-cel delivered to patients in CARTITUDE-1 demonstrated that a high CD8 positive stem-like phenotype correlated with improved duration of response [96]. Such rapid manufacturing techniques may therefore provide benefits in addition to faster CAR-T generation.
Allogeneic CAR-T cells offer another potential solution to the manufacturing problem. The use of allogeneic T-cells promises an ‘off-the shelf’ product, and potentially also a more active product. T-cells from patients with monoclonal gammopathy of uncertain significance mount a more robust immune response against MM cells than those from patients with symptomatic MM [97]. CAR-Ts manufactured from healthy donor T-cells, lacking the humoral and cellular immunoparesis typical of MM, may therefore be expected to produce more efficacious cellular therapy products. On the other hand, these products provide their own unique challenges [98].
Graft versus host disease (GvHD) may be prevented by disrupting expression of T cell receptor (TCR) alpha to prevent the patient’s immune system from recognizing the CAR-T cell product as foreign. Higher intensity lymphodepletion is also required to improve CAR-T persistence, which confers a higher risk of serious infection. ALLO-715 is a first-in-class allogeneic BCMA-directed CAR-T, with disrupted CD52 expression. In the phase 1 UNIVERSAL trial, 43 patients were treated with escalating doses of ALLO-715 alongside fludarabine at 90 mg/m2, cyclophosphamide at 900 mg/m2 and an anti-CD52 antibody, to prevent rejection of the infused product. ORR was 71%, 25% achieved ≥CR and there were no reported cases of GvHD. Overall infection rate was 54%, which is in keeping with Cilta-cel and Ide-cel, however there were 3 deaths out of 43 infused patients due to grade 5 infections. Furthermore, despite the intensive lymphodepletion, only 67% of patients had evidence of CAR-T persistence at 28 days, which correlated with efficacy [99]. This product is not being taken forward in clinical development.
P-BCMA-ALLO1 is an allogeneic BCMA CAR-T manufactured using a non-viral transposon-based integration system (piggyBac). Such systems are less complex and may be cheaper than traditional viral vector-based approaches [100]. Thirty-four patients have been treated to date, with no dose-limiting toxicities or GvHD observed. Grade ≥3 febrile neutropenia occurred in 24%, with a low rate of CRS reported (29%, all Grade 1-2) [101, 102].
Interest in CAR-NK (natural killer) cells is growing as an alternative ‘off-the-shelf’ product, with preclinical studies of constructs targeting BCMA, CD38 and CD138 reported [103,104,105]. NK cells act independently of MHC expression, reducing the risk of GvHD compared with allogeneic T-cell products. They also have a different cytokine profile, leading to a reduced likelihood of CRS [106]. Challenges associated with CAR-NK therapies include their inferior persistence [107], and the complex series of activating and inhibitory signalling that controls activity. Strategies to improve persistence include the addition of an IL-15 payload to promote cellular expansion [108, 109]. Technology, such as CRISPR/Cas9-based genome editing offers a means of manipulating effector function, by knocking out multiple inhibitory genes including CD16a and TIGIT, to augment NK-mediated antibody-dependent cellular cytotoxicity [110]. There are various approaches being tested in pre-clinical models, which have yet to fully translate to clinical outcomes. For example, FT576 is a BCMA CAR-NK with a CD38 knock-out to prevent NK fratricide by CD38-directed monoclonal antibodies, such as Daratumumab. Interim results from a phase 1 dose-escalation study have reported no GvHD and no CRS or ICANS of any grade in 9 RRMM patients, however responses have been modest, with VGPR reported in one third of evaluable patients and no further studies in RRMM planned [111]. Other studies are ongoing (eg. NCT05652530, NCT06045091) with results eagerly awaited.
Universal CAR-T or CAR-NK studies are summarised in Table 4. Allogeneic T-cell and NK-based approaches are an ongoing area of interest and development, but require significant improvement before they reach the clinic.
Improving safety of CAR-T cell therapy in MM: CRS, neurotoxicity, IEC-HS and cytopenias
Cytokine release syndrome (CRS) is common side-effect of CAR-T cell therapy. Rates reported following BCMA CAR-T in MM are high, but generally grade 1-2, with high tumour burden and rapidly progressive disease identified as major risk factors [14, 19, 25, 31, 112]. Increasing clinician familiarity alongside consensus guidelines, may lead to earlier recognition and prompt treatment with the interleukin-6 (IL-6) receptor antagonist, tocilizumab [113]. Other approaches include use of the IL-1 inhibitor anakinra, the JAK1/2 inhibitor ruxolitinib and cyclophosphamide [114, 115]. Safety switches may be incorporated into CAR-T designs to mitigate refractory cases, by choosing to selectively destroy circulating CAR-T cells. For example, expression of the endothelial growth factor receptor (EGFR) enables used of EGFR inhibitors, and herpesvirus thymidine kinase confers sensitivity to ganciclovir [116, 117]. Another approach being tested in several ongoing clinical trials is the provision of a fractionated CAR-T product. Administering the CAR-T over 2-3 days could stagger the rise in cytokines, tempering the peak levels reached, which may translate to less severe CRS. No grade ≥3 CRS was experienced by the first 30 patients who received the ARI0002 product, however updated results reported an overall CRS rate of 90% with 5% developing grade 3 [118, 119]. A recent meta-analysis has failed to identify significant evidence of improved safety with this approach, however a number of studies are ongoing [120].
Immune effector cell-associated HLH-like syndrome (IEC-HS), a poorly understood hyperinflammatory syndrome stimulated by CAR-T therapy, may be seen in up to one fifth of patients receiving BCMA-directed CAR-T cells [121]. Risks are enriched in patients with high tumour burden, especially with concomitant or preceding severe CRS or ICANS, and fatalities have been reported in the literature [122]. Treatments include high-dose steroids and anakinra, alongside supportive care [112].
Neurotoxicity, in the form of ICANS was another anticipated consequence of these therapies. Rates of overall neurotoxicity were similar in the early phase KarMMa and CARTITUDE-1 studies (18% and 21% respectively), however 5 patients in CARTITUDE-1 developed unexplained parkinsonian-like symptoms, termed MNTs. Development of MNTs was shown to be associated with high baseline tumour burden, high baseline IL-6 levels, grade 2 or higher CRS, ICANS, high absolutely lymphocyte count post-Cilta-cel infusion and strong CAR-T expansion and persistence. Use of bridging chemotherapy and prompt treatment of CRS and ICANS successfully reduced the incidence of MNTs from 5% to <1% in subsequent CARTITUDE patients [123]. Movement disorders have also been reported post Ide-cel, suggesting this may be a class effect rather than specific to Cilta-cel [124,125,126]. A potential hypothesis involves damage to the striatum, which receives dopaminergic innervation from the substantia nigra. Reduced uptake was demonstrated by PET-CT in some affected patients [124, 127]. Conflicting results have been obtained regarding CNS BCMA expression. Analysis of RNA sequencing has demonstrated low levels of BCMA expression within the striatum of children and young adults only, with no convincing evidence demonstrated by immunohistochemistry [128], whereas another group identified localised RNA expression in the caudate nucleus within the basal ganglia in adults [127].
In addition to ICANS and MNTs, cranial nerve (CN) palsies were observed in 6% of Cilta-cel-treated patients within CARTITUDE-1, -2 and -4, with no clear predictive factors identified. In all cases, CN VII was involved, and the majority responded to corticosteroid treatment [129]. Further research is required to understand the aetiology of MNTs following BCMA CAR-T cell therapy, and how to optimize risk in patients pre-treatment.
Although certain features are known to increase the risk of CRS and ICANS, there are currently no widely recognized prediction models for these side effects. The Glasgow prognostic score (GPS), is a simple metric comprised of serum albumin and C-Reactive Protein (CRP) on day of pre-CAR-T lymphodepletion. In a cohort of 139 RRMM patients, of which 83% received Ide-cel and 13% Cilta-cel, high-risk patients by the GPS were significantly more likely to develop severe CRS, any grade ICANS, and have inferior 6-month PFS and OS [130]. Identifying patients more likely to develop sustained cytopenias should also be considered. The CAR-HEMATOTOX score was developed in the setting of anti-CD19 CAR-T cell therapy for B-cell malignancies, and has recently been validated in MM. In a cohort of 113 patients, high-risk individuals, with evidence of pre-lymphodepletion cytopenias and inflammation (raised CRP or ferritin), had significantly longer durations of severe neutropenia (9 vs. 3 days) and higher rates of severe infection (40% vs. 5%). Moreover, one-year non-relapse mortality was 13% compared with 2% in the low-risk group [131].
Patient selection for CAR-T therapy
Alongside identifying and proactively managing patients at increased risk of toxicity, it is important to consider which subgroups are likely to glean the greatest efficacy benefit. The recently published MyCARe (Myeloma CAR-T Relapse) model can be used to identify RRMM patients at risk of early progression, based on the presence of extramedullary disease, plasma cell leukaemia, lenalidomide-refractory disease and elevated serum ferritin at the time of lymphodepletion. Patients with none of these risk factors had a 5-month incidence of relapse/progression of 7%, compared with 53% for those meeting all 4 criteria. This score was derived and validated in a heavily pre-treated, high-risk population, with a median 6 prior lines of therapy. Around 75% of the cohort had adverse cytogenetics, 45% had received previous BCMA-directed therapy and 86% were triple-class refractory. Whether this score can predict outcomes in NDMM is therefore not known [132].
A recent meta-analysis also reviewed 17 trials of 723 RRMM patients receiving a variety of commercial and academic CAR-Ts. The target was BCMA in the majority of cases, with dual BCMA-CD19 and BCMA-CD38 also included. This study again confirmed the poor prognostic impact of the presence of extramedullary disease, which conferred a 44% increase in the risk of relapse/progression or death after treatment. High-risk cytogenetics were also associated with reduced ORR (risk ratio 0.86; 95% CI 0.76-0.97) and a 70% increased risk of progression/relapse or death [133].
The impact of high tumour burden on outcomes is less certain. In CARTITUDE-1, bone marrow plasma cell (BMPC) percentage of 60 or higher was associated with reduced PFS and OS compared with BMPC < 30% [28, 134]. These results have not been recreated within subsequent CARTITUDE trials or the KarMMa studies. Increasing disease burden is known to impair T cell function in vitro and has been demonstrated to adversely impact responses to BCMA-directed bispecific antibodies in preclinical studies [135]. Its relevance to CAR-T outcomes may become clearer with future research.
Determining whether upfront CAR-T therapy can counteract the adverse effects of high-risk features is a crucial question. Optimising bridging therapies may also be a means of improving outcomes. Use of high-doses of alkylators has been shown to produce inferior results [136], whereas there is recent evidence to suggest that pre-CAR-T bispecific antibody treatment may alter subsequent CAR-T expansion dynamics and improve early responses [137]. Radiotherapy, which has immunomodulatory effects alongside cytotoxicity, may also have a beneficial role within bridging therapy, as shown in a number of cases [138, 139].
Currently, patient selection necessitates careful consideration of both patient and disease-related factors. Ultimately, a patient-centred approach should be prioritised.
Conclusions
CAR-T therapy in MM offers the promise of inducing meaningful remissions in patients with relapsed, high-risk disease, for whom effective treatments are lacking. Cilta-cel and Ide-cel are approved in Europe, the USA and elsewhere in RRMM, with Cilta-cel now an option in lenalidomide-refractory patients after one prior line of therapy in a number of countries. Results are eagerly awaited from upfront studies in NDMM, where these therapies could potentially lead to long-term deep remissions, and possibly even the hope for cure.
However, MM is a complex disease, characterised by numerous subclones with different levels of target antigen expression and different potential mechanisms of treatment resistance. Patients not infrequently succumb to their disease while awaiting CAR-T manufacture, and after successful infusion, these treatments can also confer the risk of significant morbidity, the aetiology of which is not fully understood. In order to bring CAR-T cell therapy into routine clinical practice, these challenges need to be optimized.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33.
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Avet-Loiseau H. Ultra high-risk myeloma. Hematol Am Soc Hematol Educ Program. 2010;2010:489–93.
Weinhold N, Ashby C, Rasche L, Chavan SS, Stein C, Stephens OW, et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood 2016;128:1735–44.
Weinhold N, Heuck CJ, Rosenthal A, Thanendrarajan S, Stein CK, Van Rhee F, et al. Clinical value of molecular subtyping multiple myeloma using gene expression profiling. Leukemia 2016;30:423–30.
Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019;33:2266–75.
Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 2017;130:2594–602.
Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–60.
Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016;128:1688–700.
Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36:2267–80.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl J Med. 2019;380:1726–37.
Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl J Med. 2021;384:705–16.
Anderson JLD, Munshi NC, Shah N, Jagannath S, Berdeja JG, Lonial S, et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: updated KarMMa results. J Clin Oncol. 2021;39:8016.
Manier S, Kansagra AJ, Anderson JLD, Berdeja JG, Jagannath S, Lin Y, et al. Characteristics of neurotoxicity associated with idecabtagene vicleucel (ide-cel, bb2121) in patients with relapsed and refractory multiple myeloma (RRMM) in the pivotal phase II KarMMa study. J Clin Oncol. 2021;39:8036.
Usmani S, Patel K, Hari P, Berdeja J, Alsina M, Vij R, et al. KarMMa-2 Cohort 2a: Efficacy and Safety of Idecabtagene Vicleucel in Clinical High-Risk Multiple Myeloma Patients with Early Relapse after Frontline Autologous Stem Cell Transplantation. Blood 2022;140:875–7.
Dhodapkar MV, Alsina M, Berdeja JG, Patel KK, Richard S, Vij R, et al. Efficacy and Safety of Idecabtagene Vicleucel (ide-cel) in Patients with Clinical High-Risk Newly Diagnosed Multiple Myeloma (NDMM) with an Inadequate Response to Frontline Autologous Stem Cell Transplantation (ASCT): KarMMa-2 Cohort 2c Extended Follow-up. Blood 2023;142:2101.
Rodríguez Otero P, Ailawadhi S, Arnulf B, Patel KK, Cavo M, Nooka AK, et al. Idecabtagene Vicleucel (ide-cel) Versus Standard (std) Regimens in Patients (pts) with Triple-Class-Exposed (TCE) Relapsed and Refractory Multiple Myeloma (RRMM): Updated Analysis from KarMMa-3. Blood 2023;142:1028.
Ailawadhi S, Arnulf B, Patel KK, Cavo M, Nooka AK, Manier S, et al. Ide-cel vs standard regimens in triple-class-exposed relapsed and refractory multiple myeloma: updated KarMMa-3 analyses. Blood. 2024.
Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141.
Xu J, Chen L-J, Yang S-S, Sun Y, Wu W, Liu Y-F, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA. 2019;116:9543–51.
Mi J-Q, Zhao W-H, Chen L-J, Fu W-J, Wang B-Y, Xu J, et al. Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment of LCAR-B38M CAR-T: At least 5-year follow-up in LEGEND-2. J Clin Oncol. 2023;41:8010.
03.08.2024 hwfgmda.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24.
Martin T, Usmani SZ, Berdeja JG, Jakubowiak A, Agha M, Cohen AD, et al. Updated results from CARTITUDE-1: phase 1b/2 study of ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T cell therapy, in patients with relapsed/refractory multiple myeloma. Blood 2021;138:549.
Jakubowiak A, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Efficacy and Safety of Ciltacabtagene Autoleucel in Patients With Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 Subgroup Analysis. Blood 2021;138:3938.
Lin Y, Martin TG, Usmani SZ, Berdeja JG, Jakubowiak AJ, Agha ME, et al. CARTITUDE-1 final results: Phase 1b/2 study of ciltacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2023;41:8009.
Hillengass J, Cohen AD, Agha ME, Delforge M, Kerre T, Roeloffzen W, et al. The Phase 2 CARTITUDE-2 Trial: Updated Efficacy and Safety of Ciltacabtagene Autoleucel in Patients with Multiple Myeloma and 1-3 Prior Lines of Therapy (Cohort A) and with Early Relapse after First Line Treatment (Cohort B). Blood 2023;142:1021.
Cohen AD, Mateos MV, Cohen YC, Rodriguez-Otero P, Paiva B, van de Donk N, et al. Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to other BCMA-targeting agents. Blood 2023;141:219–30.
San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl J Med. 2023;389:335–47.
Mateos M-VS-MJ, San-Miguel J, Dhakal B, Touzeau C, Leleu X, van de Donk N et al. Overall survival (OS) with ciltacabtagene autoleucel (cilta-cel) versus standard of care (SoC) in lenalidomide (len)-refractory multiple myeloma (MM): phase 3 CARTITUDE-4 study update. Presented at: 2024 International Myeloma Society Annual Meeting; September 25-28, 2024; Rio de Janeiro, Brazil Abstract OA – 65.
Dytfeld D, Dhakal B, Agha M, Manier S, Delforge M, Kuppens S, et al. Bortezomib, Lenalidomide and Dexamethasone (VRd) Followed By Ciltacabtagene Autoleucel Versus Vrd Followed By Lenalidomide and Dexamethasone (Rd) Maintenance in Patients with Newly Diagnosed Multiple Myeloma Not Intended for Transplant: A Randomized, Phase 3 Study (CARTITUDE-5). Blood 2021;138:1835.
van de Donk N, Usmani SZ, Yong K. CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. 2021;8:e446–e61.
Lam N, Trinklein ND, Buelow B, Patterson GH, Ojha N, Kochenderfer JN. Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nat Commun. 2020;11:283.
Fu C, Chen W, Cai Z, Yan L, Wang H, Shang J, et al. Three-year follow-up on efficacy and safety results from phase 1 lummicar study 1 of Zevorcabtagene Autoleucel in Chinese patients with relapsed or refractory multiple myeloma. Blood 2023;142:4845.
Mailankody S, Htut M, Lee KP, Bensinger W, Devries T, Piasecki J, et al. JCARH125, anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: initial proof of concept results from a phase 1/2 multicenter study (EVOLVE). Blood 2018;132:957.
Mailankody S, Jakubowiak AJ, Htut M, Costa LJ, Lee K, Ganguly S, et al. Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): update of the phase 1/2 EVOLVE study (NCT03430011). J Clin Oncol. 2020;38:8504.
Li C, Wang D, Yu Q, Li Z, Wang W, Hu G, et al. Long-Term Follow-up of Fully Human BCMA-Targeting CAR (CT103A) in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2023;142:4854.
Li C, Wang D, Song Y, Huang H, Li J, Chen B, et al. CT103A, a novel fully human BCMA-targeting CAR-T cells, in patients with relapsed/refractory multiple myeloma: Updated results of phase 1b/2 study (FUMANBA-1). J Clin Oncol. 2023;41:8025.
Li C, Qiu L-G, Wang D, Song Y, Huang H, Li J, et al. Efficacy Outcomes and Characteristics of Patients with Multiple Myeloma (MM) Who Achieved Sustained Minimal Residual Disease Negativity after Treatment with Equecabtagene Autoleucel (Eque-cel, CT103A) in Fumanba-1. Blood 2023;142:761.
Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129:2210–21.
Ghermezi M, Li M, Vardanyan S, Harutyunyan NM, Gottlieb J, Berenson A, et al. Serum B-cell maturation antigen: a novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2017;102:785–95.
Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158:727–38.
Chen H, Li M, Xu N, Ng N, Sanchez E, Soof CM, et al. Serum B-cell maturation antigen (BCMA) reduces binding of anti-BCMA antibody to multiple myeloma cells. Leuk Res. 2019;81:62–6.
Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019;568:112–6.
Hiraga J, Tomita A, Sugimoto T, Shimada K, Ito M, Nakamura S, et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood 2009;113:4885–93.
Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, et al. Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019;134:1585–97.
Cowan AJ, Pont M, Sather BD, Turtle CJ, Till BG, Libby E, et al. Safety and Efficacy of Fully Human BCMA CAR T Cells in Combination with a Gamma Secretase Inhibitor to Increase BCMA Surface Expression in Patients with Relapsed or Refractory Multiple Myeloma. Blood 2021;138:551.
Raje NS, Shah N, Jagannath S, Kaufman JL, Siegel DS, Munshi NC, et al. Updated Clinical and Correlative Results from the Phase I CRB-402 Study of the BCMA-Targeted CAR T Cell Therapy bb21217 in Patients with Relapsed and Refractory Multiple Myeloma. Blood 2021;138:548.
Shah N, Alsina M, Siegel DS, Jagannath S, Madduri D, Kaufman JL, et al. Initial Results from a Phase 1 Clinical Study of bb21217, a Next-Generation Anti Bcma CAR T Therapy. Blood 2018;132:488.
Cowan AJ, Pont MJ, Sather BD, Turtle CJ, Till BG, Libby EN 3rd, et al. Secretase inhibitor in combination with BCMA chimeric antigen receptor T-cell immunotherapy for individuals with relapsed or refractory multiple myeloma: a phase 1, first-in-human trial. Lancet Oncol. 2023;24:811–22.
Rieger L, Irlinger K, Fuechsl F, Barbian N, Tietje M, Faber M, et al. Regulation of BCMA by the ubiquitin proteasome system enables optimization of BCMA-targeting therapies in multiple myeloma. Blood 2023;142:3310.
Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai Y-T, Prabhala R, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. 2021;12:868.
Da Vià MC, Dietrich O, Truger M, Arampatzi P, Duell J, Heidemeier A, et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat Med. 2021;27:616–9.
Leblay N, Maity R, Barakat E, McCulloch S, Duggan P, Jimenez-Zepeda V, et al. Cite-seq profiling of T cells in multiple myeloma patients undergoing BCMA targeting CAR-T or bites immunotherapy. Blood 2020;136:11–2.
Zhou X, Rasche L, Kortüm KM, Mersi J, Einsele H. BCMA loss in the epoch of novel immunotherapy for multiple myeloma: from biology to clinical practice. Haematologica 2023;108:958–68.
Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk NWCJ, Rodríguez-Otero P, et al. Talquetamab, a T-Cell–Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl J Med. 2022;387:2232–44.
Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med. 2022;387:1196–206.
Zhang M, Wei G, Zhou L, Zhou J, Chen S, Zhang W, et al. GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): a first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol. 2023;10:e107–e16.
Bal S, Htut M, Nadeem O, Anderson LD, Koçoğlu H, Gregory T, et al. BMS-986393 (CC-95266), a G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from a Phase 1 Study. Blood 2023;142:219.
Rodriguez-Otero P, van de Donk NWCJ, Pillarisetti K, Cornax I, Vishwamitra D, Gray K, et al. GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review. Blood Cancer J. 2024;14:24.
Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, et al. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J Immunol. 2013;190:5739–46.
Lesokhin AM, Richter J, Trudel S, Cohen AD, Spencer A, Forsberg PA, et al. Enduring responses after 1-Year, fixed-duration cevostamab therapy in patients with relapsed/refractory multiple myeloma: early experience from a phase I study. Blood 2022;140:4415–7.
Jiang D, Huang H, Qin H, Tang K, Shi X, Zhu T, et al. Chimeric antigen receptor T cells targeting FcRH5 provide robust tumour-specific responses in murine xenograft models of multiple myeloma. Nat Commun. 2023;14:3642.
Yu Z, Li H, Lu Q, Zhang Z, Tong A, Niu T. Fc receptor-like 5 (FCRL5)-directed CAR-T cells exhibit antitumor activity against multiple myeloma. Signal Transduct Target Ther. 2024;9:16.
Hajek R, Okubote SA, Svachova H. Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol. 2013;163:551–64.
Gao M, Kong Y, Yang G, Gao L, Shi J. Multiple myeloma cancer stem cells. Oncotarget 2016;7:35466–77.
Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. 2018;3.
Du J, Qiang W, Lu J, Jia Y, He H, Liu J, et al. Updated results of a phase I open-label single-arm study of dual targeting BCMA and CD19 Fastcar-T cells (GC012F) as first-line therapy for transplant-eligible newly diagnosed high-risk multiple myeloma. Blood 2023;142:1022.
Wang Y, Cao J, Gu W, Shi M, Lan J, Yan Z, et al. Long-term follow-up of combination of B-cell maturation antigen and CD19 chimeric antigen receptor T cells in multiple myeloma. J Clin Oncol. 2022;40:2246–56.
Shi X, Yan L, Shang J, Kang L, Yan Z, Jin S, et al. Anti-CD19 and anti-BCMA CAR T cell therapy followed by lenalidomide maintenance after autologous stem-cell transplantation for high-risk newly diagnosed multiple myeloma. Am J Hematol. 2022;97:537–47.
Popat R, Zweegman S, Cavet J, Yong K, Lee L, Faulkner J, et al. Phase 1 first-in-human study of AUTO2, the first chimeric antigen receptor (CAR) T cell targeting APRIL for patients with relapsed/refractory multiple myeloma (RRMM). Blood 2019;134:3112.
Tang Y, Yin H, Zhao X, Jin D, Liang Y, Xiong T, et al. High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma. J Exp Clin Cancer Res. 2022;41:2.
Sun F, Cheng Y, Peng B, Xu H, van Rhee F, Janz S, et al. Bispecific CAR-T cells targeting both BCMA and CD24: a potentially treatment approach for multiple myeloma. Blood 2021;138:2802.
Reiser J, Mathavan K, Mahmood S, Pan Y, Hancock B, Blum R, et al. Dual chimeric antigen receptor approach combining novel tumor targeting strategies circumvents antigen escape in multiple myeloma. Blood 2021;138:1718.
Xie B, Li Z, Zhou J, Wang W. Current status and perspectives of dual-targeting chimeric antigen receptor T-cell therapy for the treatment of hematological malignancies. Cancers (Basel). 2022;14.
van der Schans JJ, van de Donk NWCJ, Mutis T. Dual targeting to overcome current challenges in multiple myeloma CAR T-cell treatment. Frontiers in Oncology. 2020;10.
Melnekoff DT, Ghodke-Puranik Y, Van Oekelen O, Aleman A, Upadhyaya B, Sebra R, et al. Single-cell profiling reveals contribution of tumor extrinsic and intrinsic factors to BCMA-targeted CAR-T cell efficacy in multiple myeloma. Blood 2021;138:326.
LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 2004;103:1787–90.
Fostier K, Caers J, Meuleman N, Broos K, Corthals J, Thielemans K, et al. Impact of lenalidomide maintenance on the immune environment of multiple myeloma patients with low tumor burden after autologous stem cell transplantation. Oncotarget. 2018;9.
Paiva B, Gaffney B, Burnett K, Castiglioni P, Angelo M, Pierce DW, et al. Synergistic antitumor activity of alnuctamab (ALNUC; BMS-986349; CC-93269), a BCMA 2 + 1 T cell engager (TCE), and celmod agents in multiple myeloma (MM) preclinical models. Blood 2022;140:7054–5.
Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, et al. Lenalidomide enhances the function of CS1 chimeric antigen receptor-redirected T cells against multiple myeloma. Clin Cancer Res. 2018;24:106–19.
Tamura H, Ishibashi M, Sunakawa-Kii M, Inokuchi K. PD-L1-PD-1 pathway in the pathophysiology of multiple myeloma. Cancers 2020;12:924.
Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010;116:1291–8.
Verkleij CBM, Wong A, Zweegman S, Verona R, Adams H, Mutis T et al. Mechanisms of resistance and determinants of response of the GPRC5D-targeting T-cell redirecting bispecific antibody JNJ-7564 in multiple myeloma. ASH abstract. 2020.
Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6:e448–e58.
Vieira dos Santos J, Melnekoff D, Aleman A, Bhalla S, Mouhieddine TH, Van Oekelen O, et al. Multimodal single-cell transcriptomic and proteomic correlatives of patients outcomes following anti-BCMA cellular therapy with ciltacabtagene autoleucel (Cilta-cel) in relapsed multiple myeloma. Blood 2023;142:93.
Mitsugi T, Suzuki C, Andrade GP, Vidal EKS, Barros CCC, Azevedo JTC, et al. Enhancing anti-BCMA CAR-T cell activity with sIL-15 cytokine: evaluation of its impact on in vitro cytotoxicity, cytokine production, and T cell exhaustion. Transplant Cell Ther. 2024;30:S371–S2.
Maity R, Benaoudia S, Zemp F, Lee H, Barakat E, Leblay N, et al. A BCL2L1 armoured BCMA targeting CAR T Cell to overcome exhaustion and enhance persistence in multiple myeloma. Blood 2021;138:327.
Sakemura R, Hefazi M, Siegler EL, Cox MJ, Larson DP, Hansen MJ, et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood. 2022.
Walsh STR, Cheng H, Bryson JW, Roder H, DeGrado WF. Solution structure and dynamics of a de novo designed three-helix bundle protein. Proc Natl Acad Sci. 1999;96:5486–91.
Frigault MJ, Bishop MR, Rosenblatt J, O’Donnell EK, Raje N, Cook D, et al. Phase 1 study of CART-ddBCMA for the treatment of subjects with relapsed and refractory multiple myeloma. Blood Adv. 2023;7:768–77.
Ferment B, Lambert J, Caillot D, Lafon I, Karlin L, Lazareth A, et al. French Early Nationwide Idecabtagene Vicleucel (Ide-Cel) Chimeric Antigen Receptor (CAR) T-Cell Therapy Experience in Patients with Relapsed/Refractory Multiple Myeloma (FENIX): An IFM Study from the Descar-T Registry. Blood 2022;140:4668–70.
Sperling A, Derman B, Nikiforow S, Im S-Y, Ikegawa S, Prabhala R, et al. Updated phase I study results of PHE885, a T-Charge manufactured BCMA-directed CAR-T cell therapy, for patients (pts) with r/r multiple myeloma (RRMM). J Clin Oncol. 2023;41:8004.
Montes de Oca R, Gu J, Zhao H, Zelinsky K, Wu D, Davis C, et al. Biomarker correlates of response to ciltacabtagene autoleucel in patients with relapsed or refractory multiple myeloma from CARTITUDE-1, a phase 1b/2 open-label study, at the ~3 year follow-up. Blood 2023;142:2099.
Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198:1753–7.
Lonez C, Breman E. Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells. 2024;13.
Mailankody S, Matous JV, Chhabra S, Liedtke M, Sidana S, Oluwole OO, et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat Med. 2023;29:422–9.
Scheller L, Tebuka E, Rambau P, Einsele H, Hudecek M, Prommersberger S, et al. BCMA CAR-T cells in multiple myeloma–ready for take-off? Leuk Lymphoma. 2023;65:1–15.
Dholaria B, Kocoglu MH, Kin A, Asch AS, Ramakrishnan A, Bachier C, et al. Early Safety Results of P-BCMA-ALLO1, a Fully Allogeneic Chimeric Antigen Receptor T-Cell (CAR-T), in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2023;142:3479.
Dholaria B, Kocoglu M, Kin A, Ramakrishnan A, Shune L, Ganguly S, et al. A Phase 1 Study of P-BCMA-ALLO-1, a non-viral, allogeneic BCMA directed CAR-T in relapsed/refractory Multiple Myeloma (RRMM). Presented at the International Myeloma Society Meeting, 2024, Abstract OA-04. 2024.
Rezvani K. Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transpl. 2019;54:785–8.
Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014;28:917–27.
Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl J Med. 2020;382:545–53.
Lowry LE, Zehring WA. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front Immunol. 2017;8:1061.
Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32:520–31.
Li L, Mohanty V, Dou J, Huang Y, Banerjee PP, Miao Q, et al. Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering. Sci Adv. 2023;9:eadd6997.
Wang M, Kluesner M, Vázquez PC, Webber B, Moriarity B. 216 Multiplex base editing of NK cell to enhance cancer immunotherapy. J Immunother Cancer. 2021;9:A229-A.
Dhakal B, Berdeja JG, Gregory T, Ly T, Bickers C, Zong X, et al. Interim phase I clinical data of FT576 as monotherapy and in combination with daratumumab in subjects with relapsed/refractory multiple myeloma. Blood 2022;140:4586–7.
Anderson LD, Dhakal B, Jain T, Oluwole OO, Shah GL, Sidana S, et al. Chimeric antigen receptor T cell therapy for myeloma: where are we now and what is needed to move chimeric antigen receptor T cells forward to earlier lines of therapy? Expert panel opinion from the American Society for transplantation and cellular therapy. Transplant Cell Ther. 2024;30:17–37.
Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;39:3978–92.
Pan J, Deng B, Ling Z, Song W, Xu J, Duan J, et al. Ruxolitinib mitigates steroid-refractory CRS during CAR T therapy. J Cell Mol Med. 2021;25:1089–99.
Gazeau N, Liang EC, Wu QV, Voutsinas JM, Barba P, Iacoboni G, et al. Anakinra for refractory cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T cell therapy. Transpl Cell Ther. 2023;29:430–7.
Green DJ, Pont M, Cowan AJ, Cole GO, Sather BD, Nagengast AM, et al. Response to Bcma CAR-T cells correlates with pretreatment target antigen density and is improved by small molecule inhibition of gamma secretase. Blood 2019;134:1856.
Mailankody S, Ghosh A, Staehr M, Purdon TJ, Roshal M, Halton E, et al. Clinical responses and pharmacokinetics of MCARH171, a human-derived Bcma targeted CAR T cell therapy in relapsed/refractory multiple myeloma: final results of a phase I clinical trial. Blood 2018;132:959.
Oliver-Caldés A, González-Calle V, Cabañas V, Español-Rego M, Rodríguez-Otero P, Reguera JL, et al. Fractionated initial infusion and booster dose of ARI0002h, a humanised, BCMA-directed CAR T-cell therapy, for patients with relapsed or refractory multiple myeloma (CARTBCMA-HCB-01): a single-arm, multicentre, academic pilot study. Lancet Oncol. 2023;24:913–24.
Larrea CFF, Oliver-Caldés A, Calle VGDL, Cabañas V, López-Muñoz N, Rodríguez-Otero P, et al. Long-term follow-up of ARI0002h (cesnicabtagene autoleucel), an academic point-of-care B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell strategy: Activity and safety after fractionated initial therapy and booster dose in 60 patients with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2024;42:7544.
Frigault M, Rotte A, Ansari A, Gliner B, Heery C, Shah B. Dose fractionation of CAR-T cells. A systematic review of clinical outcomes. J Exp Clin Cancer Res. 2023;42:11.
Kennedy VE, Wong C, Huang C-Y, Kambhampati S, Wolf J, Martin TG, et al. Macrophage activation syndrome-like (MAS-L) manifestations following BCMA-directed CAR T cells in multiple myeloma. Blood Adv. 2021;5:5344–8.
Swan D, Thachil J. Management of haemostatic complications of chimaeric antigen receptor T-cell therapy. Br J Haematol. 2022;197:250–9.
Cohen AD, Parekh S, Santomasso BD, Gállego Pérez-Larraya J, van de Donk N, Arnulf B, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12:32.
Karschnia P, Miller KC, Yee AJ, Rejeski K, Johnson PC, Raje N, et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood 2023;142:1243–8.
Gong Z, Umoru G, Monge J, Shah N, Mohyuddin GR, Radhakrishnan SV, et al. Adverse effects and non-relapse mortality of BCMA directed T cell therapies in multiple myeloma: an FAERS database study. Blood Cancer J. 2024;14:36.
Couturier A, Escoffre M, Leh F, Villoteau A-S, Palard X, Le Jeune F, et al. Parkinson-like neurotoxicity in female patients treated with idecabtagene-vicleucel. HemaSphere. 2024;8:e131.
Van Oekelen O, Aleman A, Upadhyaya B, Schnakenberg S, Madduri D, Gavane S, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27:2099–103.
Marella M, Yao X, Carreira V, Bustamante MF, Clark HB, Jackson CC, et al. Comprehensive BCMA expression profiling in adult normal human brain suggests a low risk of on-target neurotoxicity in BCMA-targeting multiple myeloma therapy. J Histochem Cytochem. 2022;70:273–87.
Van De Donk NWCJ, Sidana S, Schecter JM, Jackson CC, Lendvai N, De Braganca KC, et al. Clinical experience with cranial nerve impairment in the CARTITUDE-1, CARTITUDE-2 cohorts A, B, and C, and cartitude-4 studies of ciltacabtagene autoleucel (Cilta-cel). Blood 2023;142:3501.
Akhtar OS, Modi K, Kim J, Skelson L, Smith E, Al-Jumayli MA, et al. Simple score of albumin and CRP predicts high-grade toxicity in patients with multiple myeloma receiving CAR-T therapy. Transplant Cell Ther Off Publ Am Soc Transplant Cell Ther. 2024;30:283.e1–e10.
Rejeski K, Hansen DK, Bansal R, Sesques P, Ailawadhi S, Logue JM, et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J Hematol Oncol. 2023;16:88.
Gagelmann N, Dima D, Merz M, Hashmi H, Ahmed N, Tovar N, et al. Development and validation of a prediction model of outcome after B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in relapsed/refractory multiple myeloma. J Clin Oncol. 2024;42:1665–75.
Gagelmann N, Ayuk FA, Klyuchnikov E, Wolschke C, Berger SC, Kröger N. Impact of high-risk disease on the efficacy of chimeric antigen receptor T-cell therapy for multiple myeloma: a meta-analysis of 723 patients. Haematologica 2023;108:2799–802.
Madduri DBJ, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart K et al. CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen–Directed Chimeric Antigen Receptor T Cell Therapy, in Relapsed/Refractory Multiple Myeloma. ASH abstract. 2020.
Meermeier EW, Welsh SJ, Sharik ME, Du MT, Garbitt VM, Riggs DL, et al. Tumor burden limits bispecific antibody efficacy through T cell exhaustion averted by concurrent cytotoxic therapy. Blood Cancer Discov. 2021;2:354–69.
Zafar A, Huang C-Y, Lo M, Arora S, Chung A, Wong SW, et al. Intensity of cyclophosphamide-based bridging therapy before chimeric antigen receptor T cell therapy in myeloma. Transplant Cell Ther. 2023;29:504.e1–e7.
Fandrei D, Seiffert S, Rade M, Gagelmann N, Kreuz M, Born P et al. Sequential administration of bispecific antibodies and anti-BCMA CAR-T cell therapy in relapsed/refractory multiple myeloma is associated with expansion of CD8 effector clones and high response rates. Abstract S194. Presented at the European Hematology Association (EHA) 2024 Congress. 2024.
Manjunath SH, Cohen AD, Lacey SF, Davis MM, Garfall AL, Melenhorst JJ, et al. The safety of bridging radiation with anti-BCMA CAR T-cell therapy for multiple myeloma. Clin Cancer Res. 2021;27:6580–90.
Smith EL, Mailankody S, Staehr M, Wang X, Senechal B, Purdon TJ, et al. BCMA-targeted CAR T-cell therapy plus radiotherapy for the treatment of refractory myeloma reveals potential synergy. Cancer Immunol Res. 2019;7:1047–53.
Author information
Authors and Affiliations
Contributions
DS wrote the manuscript, DM provided expert review, JH supervised the project and provided critical appraisal.
Corresponding author
Ethics declarations
Competing interests
DS: none; DM: Employment: Johnson and Johnson; JH: none.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Swan, D., Madduri, D. & Hocking, J. CAR-T cell therapy in Multiple Myeloma: current status and future challenges. Blood Cancer J. 14, 206 (2024). https://doi.org/10.1038/s41408-024-01191-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-024-01191-8
This article is cited by
-
In vivo chimeric antigen receptor (CAR)-T cell therapy
Nature Reviews Drug Discovery (2026)
-
Investigating the anti-proliferative effect of Trypanosoma cruzi: the saga of developing concomitant immunity against cancer
Journal of Parasitic Diseases (2026)
-
BCMA-directed mRNA CAR T cell therapy for myasthenia gravis: a randomized, double-blind, placebo-controlled phase 2b trial
Nature Medicine (2026)
-
Immunotherapy using CAR T cells shows promising long-term outcomes for people with the blood cancer myeloma
Nature (2025)
-
Fifty years of monoclonals: the past, present and future of antibody therapeutics
Nature Reviews Immunology (2025)