Abstract
This multicenter retrospective study by GETH-TC validates the prognostic value of the Allo-HCT Refined ELN 2022 risk classification in allografted AML patients. The new classification refines the ELN 2022 risk classification, dividing adverse-risk patients into two subgroups: Adv-Plus (AdvP), including those with complex karyotype, MECOM (EVI1) rearrangement, or TP53 mutations/del(17p), and an additional adverse group (Adv*). The study included 651 AML patients treated with at least one line of anthracycline-based induction therapy and in complete remission. According to the Allo-HCT Refined ELN 2022 risk classification, 19.4% (n = 126) patients were classified into the Favorable (Fav) risk, 38.1% (n = 248) into the Intermediate (Int) risk, 27.2% (n = 177) in the Adv* and 15.4% (n = 100) in the AdvP. Outcomes were significantly poorer for patients allocated in the AdvP risk group (5-year OS rate: 32.3%, 5-year LFS rate: 24.3%, both p < 0.001 with the rest of subgroups) and a higher CIR (5-year CIR: 64.3%, p < 0.001). Patients in the Adv* risk group had similar outcomes than patients in the Int risk group (5-year OS rate: 70.2% vs. 66.7%, p = 0.69, 5-year LFS rate: 63.8% vs. 55.9%, p = 0.33). Multivariate analysis confirmed the dismal outcomes for AdvP patients for OS: Hazard Ratio (HR) = 3.05, and LFS: HR = 2.66, both p < 0.001. Our findings validate the Allo-HCT Refined ELN 2022 classification as a robust prognostic tool, particularly highlighting the poor outcomes for the AdvP subgroup.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is the most common indication for allogeneic hematopoietic cell transplantation (allo-HCT), which offers a potentially curative must be considered before the procedure [1,2,3]. These risks include mortality, both short and long-term morbidity, and a significant impact on the subsequent quality of life directly linked allo-HCT adverse events [4,5,6,7]. Therefore, clinicians must balance the potential toxicities of allo-HCT and the relapse risk to determine allo-HCT eligibility.
According to the latest European LeukemiaNet AML risk classification guidelines (ELN 2022) [8], updated in 2022, allo-HCT should be considered when the relapse risk without transplantation exceeds 35–40%. This applies to a large group of patients with intermediate or adverse-risk AML in first complete response (CR1) after induction therapy and all patients who achieve a second or subsequent complete response. Additionally, treatment response and measurable residual disease (MRD) status serve as dynamic disease modifiers during follow-up, meaning that patients across all genetic risk groups may benefit from allo-HCT if the depth of response is insufficient.
Given the widespread use of the ELN 2022 guidelines in clinical practice, several studies have demonstrated the accuracy of these guidelines in predicting outcomes for patients with AML [9, 10] and more specifically in those undergoing allo-HCT consolidation [11, 12]. In this context, a recent single-center study validated the predictive ability of the ELN 2022 risk classification in patients diagnosed with AML in CR, and proposed a new AlloHCT-Refined ELN 2022 classification, introducing an additional subgroup within the adverse-risk category, termed Adverse-plus (AdvP). This subgroup included patients with complex karyotype (CK), MECOM(EVI1) rearrangements, and TP53 mutations or del (17p) (AdvP subgroup), that have a notably worse prognosis.
Considering the broad implementation of the the ELN 2022 guidelines in clinical practice, and the findings from the application of the Allo-HCT Refined ELN 2022 classification in a single institution, the Grupo Español de Transplante Hematopoyético y Terapia Celular (GETH-TC) initiated this multicenter, retrospective study to further investigate the prognostic value of the Allo-HCT Refined ELN 2022 classification in a large cohort of AML patients undergoing allo-HCT in CR.
Patients and methods
Patient selection
This retrospective, multicenter, registry-based analysis was conducted under the auspices of the Myeloid Malignancies Working Committee of the GETH-TC. GETH-TC is a non-profit, scientific society representing all HCT and cell therapy units in Spain and Portugal. All affiliates of the GETH-TC were invited to participate in the study, and sixteen institutions contributed to the project.
Inclusion criteria were as follows: adults aged 18 or older with AML treated with at least one line of anthracycline-based induction therapy who underwent their first allo-HCT in complete remission (CR1/CR2 or beyond) between January 2015 and June 2023. Only patients with information at diagnosis that permitted the retrospective risk classification into the ELN 2022 risk categories were included.
All patients signed informed consent for retrospective data collection. The study received ethical approval from the Ethics Committee of the Hospital Clínic de Barcelona and the GETH-TC, and was conducted following the standards set forth by the Declaration of Helsinki.
AML diagnosis, biological risk assessment (Allo-HCT refined ELN 2022 risk classification), and treatment
AML risk was defined after data collection according to the updated 2022 AML classifications [13, 14]. All patients included were retrospectively classified into favorable (Fav), Intermediate (Int), and adverse (Adv) risk groups according to the ELN 2022 risk classification [13, 14] and based on cytogenetic and mutational information documented at diagnosis. Subsequently, patients classified into the Adv risk group, were redistributed in the AdvP risk or the Adv* risk categories, according to the Allo-HCT Refined ELN 2022 classification proposed by Jimenez-Vicente et al. [12]. Specifically, patients with a complex karyotype, inv(3)/t(3;3)/MECOM (EVI1) rearrangement, or TP53 mutations and/or loss of the 17p region at diagnosis were included in the newly defined AdvP, while the rest of the adverse-risk genetic subgroups were reclassified in the Adv* group.
Biological abnormalities at diagnosis were defined through cytogenetic analyses (G-Banding Karyotype) and mutational analysis based on standard polymerase chain reaction (PCR)-based analysis (NPM1, FLT3-ITD,…) and targeted next-generation sequencing (NGS). MRD analysis was assessed before allo-HCT by every individual center according to the ELN-DAVID MRD 2021 updated recommendations [15].
All patients were treated with at least one line of anthracycline-based induction therapy, undergoing their first allo-HCT in a morphological CR (CR1/CR2 or beyond). Response criteria were defined according to the ELN 2022 Guidelines and assessed by the participant institutions.
Allo-HCT information and main definitions
Eligibility criteria for allo-HCT, donor selection and conditioning regimen adhered to standard practices and internal protocols from the participant institutions. Conditioning regimen intensity was defined using classical criteria and was tailored to chronological age and comorbidities. Most patients older than 55 years and those with clinically relevant comorbidities received reduced-intensity conditioning (RIC) regimens. Peripheral blood stem cell sources were infused into all patients. Grading of acute and chronic GVHD (aGVHD and cGVHD) followed established criteria [16,17,18,19]. Main definitions are detailed in the Supplementary Methods.
Endpoint and statistical analysis
The primary endpoint was the validation of the prognostic value of the Allo-HCT Refined ELN 2022 classification for overall survival (OS) prediction. Secondary outcome variables included leukemia-free survival (LFS) and cumulative incidence of relapse (CIR).
Categorical variables were presented as counts and percentages and continuous variables as medians with interquartile range (IQR). χ2, Fisher’s exact and analysis of variance tests were used to compare categorical and continuous variables.
OS and LFS were estimated using the Kaplan-Meier method. LFS was calculated until the date of first relapse, death from any cause, or the last follow-up for patients in morphological CR. CIR and non-relapse mortality were estimated using cumulative incidence and competing risk analyses. Death was used as a competing risk for CIR and relapse was used for NRM. Long-rank test was used for univariate analysis of LFS and OS and OS and Gray’s test for cumulative incidence. The impact of the main variable of interest (Allo-HCT Refined ELN 2022 risk groups) on transplant outcomes was explored using univariate (UVA) and multivariate (MVA) Cox regression analyses. The baseline risk factors included in each of the multivariable models were selected based on clinical judgment prior to the analysis and variables with a p value lower than 0.1 in the UVA. All p values were two-sided with statistical significance evaluated at the 0.05 alpha level. All statistical analyses were performed with R statistics version 4.3.2 (R core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
AML and allo-HCT baseline information
All baseline characteristics are displayed in Table 1. Overall, 651 patients were included in the study, with a median age of 55 years (interquartile rank (IQR): 44–62). Males comprised 52.7% (n = 343) of the patients, and 15.4% (n = 100) had an HCT-CI > 3. Most patients were diagnosed with de novo AML (86.6%, 564), and 13.4% (n = 100) with secondary or therapy-related AML. All patients were treated with at least one line of anthracyclines-induction therapies, and 84.9% (n = 553) of them, underwent their allo-HCT in CR1
Related to the allo-HCT characteristics, 61.3% (n = 399) of the patients received myeloablative conditioning regimens (MAC), while 38.7% (n = 252) underwent allo-HCT using RIC protocols. Allo-HCT was performed from matched sibling donors (MSD) in 32.7% (n = 213) of the patients, from 10/10 HLA-matched unrelated donors (MUD) in 31.2% (n = 203), from 9/10 mismatched unrelated donors (MMUD) in 7.5% (n = 48), and from haploidentical donors in 27.1% (n = 187). GVHD prophylaxis with PTCY platforms were administered to 51.9% (n = 334) of the patients.
Allo-HCT refined ELN 2022 risk classification
According to the Allo-HCT Refined ELN 2022 risk classification, 19.4% (n = 126) were categorized as Fav, 38.1% (n = 248) as Int, 27.2% (n = 177) in the Adv* and 15.4% (n = 100) in the AdvP. As reported in Table 1, median age (58 and 57 vs. 53 and 48 years, p < 0.001), and the proportion of patients with secondary or therapy-related AML (15% and 21.7% vs. 9.3% and 4.2%, p < 0.001) were higher in patients grouped in the AdvP and Adv* groups than in those classified into the Fav and Int groups. In contrast, the proportion of patients undergoing allo-HCT in CR2 or beyond (41.3% vs. 10.9%, 6.2% and 8%, p < 0.001) and with positive MRD status (52.3% vs. 31.9%, 33.9% and 26%, p < 0.001) were superior in the Fav group than in the Int, Adv* and AdvP groups.
Key allo-HCT features, including conditioning intensity, donor type, and GVHD prophylaxis were balanced among the different groups, with no significant differences observed
Allo-HCT refined ELN 2022 and ELN 2022 risk classification outcomes
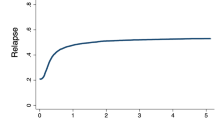
The median follow-up for LFS of the study cohort was 44.2 months (95% CI: 37–49). During follow-up, 28.4% (n = 185) of the patients relapsed and 34.5% (n = 225) died, being relapse the most frequent cause of death (50.7%, n = 109). At 5 years, OS and LFS rates were 63.4% (95%CI: 59.5–67.5) and 55.8% (95%CI: 51.9–60.1) (Fig. 1a). The 5-year CIR of the entire cohort was 30.2% (95%CI: 26.8–33.4), with a 5-year NRM rate of 13.9% (95%CI: 11.9–15.9) (Table 2).
Post-transplant outcomes of patients were analyzed by the ELN 2022 risk classification and Allo-HCT Refined ELN 2022 risk classification. The ELN 2022 risk classification stratified survival accurately among the patients included in the Int and Adv risk (p < 0.001), with 5-year OS rates of 70% (95%CI: 64–76) and 52% (95%CI: 46–59) respectively, while there were no differences between Int and Fav risk groups (74% vs. 70%, p = 0.32). LFS showed the same pattern, discriminating properly between Int and Adv risk (p < 0.001), without significant differences comparing Fav and Int risk groups (p = 0.41) (Supplementary Data, Fig. 1).
According to the Allo-HCT Refined ELN 2022 risk classification, different outcomes were observed in patients classified into the AdvP compared to the other groups. Patients categorized into the AdvP risk group category had lower OS and LFS (5-y OS: 28.4% (95%CI: 20.2–39.9), 5-y LFS: 24.3% (95%CI: 16.8–35.2), both p < 0.001) (Fig. 1b, c) and higher CIR (5-y CIR: 64.3% (95%CI: 54.5–74.1), p < 0.001) (Fig. 2). Patients allocated in the Adv* group had similar OS (5-y OS: 66.7% (95%CI: 59.5–74.7) vs. 70.2% (95%CI: 64.5–76.4), p = 0.69) and LFS (5-y LFS: 55.9% (95%CI: 48.4–64.7) vs. 63.8% (95%CI: 57.9–70.4), p = 0.33) to those classified into the Int risk group. NRM rates were showed no differences among the different risk groups (AdvP 5y-NRM: 11.6% (95%CI: 5.1–18.1), p = 0.523).
The restricted analysis for patients allografted in CR1 confirmed these poor outcomes for the AdvP risk group (5-y OS: 29.5% (95%CI: 21–41.5), 5-y LFS: 25.1% (95%CI: 17.3–36.3), both p < 0.001) (Supplementary Data, Fig. 2).
The predictive power of the Allo-HCT Refined ELN 2022 risk classification for OS and LFS was further investigated using MVA (Fig. 3). It confirmed the lower OS (HR: 3.05, p < 0.001) and LFS (HR: 2.66, p < 0.001) than the rest of the subgroups. and that patients classified into the Adv* risk group had similar outcomes to those included in the Int risk group (OS (HR: 1.02, p = 0.90), LFS (HR: 1.17, p = 0.37).
In addition, the MVA revealed another two significant values for OS and LFS: adults older than 55 years (OS (HR: 1.72, p = 0.002), LFS (HR: 1.41, p = 0.011)), and those undergoing allo-HCT with positive MRD status (OS (HR: 1.49, p = 0.007), LFS (HR: 1.51, p = 0.002)). Lastly, the use of PTCY-based prophylaxis was associated with an improved LFS (HR: 0.67 (95%CI: 0.5–0.91), p = 0.01). (Supplementary Data, Figs. 3 and 4)
Subanalysis of the Adverse-Plus risk group
Further investigations were conducted in the 100 patients included in the AdvP group based to the genetic abnormalities identified at diagnosis. Among these patients, 22% n = 22) had MECOM(EVI1) rearrangements, and 78% (n = 78) had a CK and/or mutated TP53/del(17p).
During follow-up, 62% (n = 62) of the patients relapsed and 67% (n = 67) died. Two-year OS showed that patients with MECOM(EVI1) rearrangements had better survival compared to those with CK and/or TP53 mutated/del(17p) (2-y OS rate: 51% vs. 34% (p = 0.044), while there was also a trend for longer LFS (2-y LFS: 40% vs. 30% (p = 0.07) (Supplementary Data, Fig. 5). MVA confirmed the UVA results for OS, with patients having CK, TP53 mutations or del(17p) having a higher risk of death compared to those with MECOM(EVI1) rearrangements (HR: 2.39 (95%CI: 1.1–5.2, p = 0.027). There was also a trend towards shorter LFS (HR: 1.91 (95%CI: 0.63–3.9), p = 0.067).
MVA showed worse outcomes in patients older than 55 years (OS (HR: 2.16 (95%CI: 1.15–4.1), p = 0.017), LFS (HR: 1.91 (95%CI: 1.05–3.5), p = 0.033)). Interestingly, a negative pre-transplant MRD status did not show a significant survival benefit in this subgroup, neither for OS (HR: 1.31 (95%CI: 0.95–2.7), p = 0.349) nor for LFS (HR: 1.56, p = 0.11) (Fig. 4).
Discussion
This multicenter retrospective study validates the prognostic value of the Allo-HCT Refined ELN 2022 risk classification for AML patients treated with anthracycline-based induction therapies and undergoing allo-HCT. These findings highlight the enhanced value of this refined classification, particularly in distinguishing outcomes within the broad adverse risk group of the ELN 2022 guidelines.
One of the most important contributions of this study is the reclassification of patients from the ELN 2022 Adv category into two distinct risk groups: Adverse* (Adv*) and Adverse-Plus (AdvP). This refinement is critical, as it highlights the poor prognosis of patients in the AdvP group—those with complex karyotype [20], MECOM(EVI1) rearrangement [21], and/or TP53 mutation/del(17p) [22,23,24,25]. Patients in the AdvP subgroup showed significantly worse outcomes compared to the Adv* group, with a median OS of only 12 months post-transplant and 5-year OS and LFS rates of 32.3% and 24.3%, respectively. These results align with existing literature, which has consistently demonstrated the aggressive nature of these genetic abnormalities and their association with a higher risk of relapse after the procedure [11, 12].
Within the AdvP risk group, patients with MECOM(EVI1) rearrangements had relatively better outcomes compared to those with CK and/or TP53 mutations or del(17p). The 2-year OS rate for MECOM(EVI1) rearranged patients was 51% versus 34% for CK/TP53m patients (p = 0.044). However, despite this apparent difference, the overall prognosis of all patients in the AdvP group remains poor, and there is an urgent need for more personalized and intensive therapeutic strategies in these patients.
Interestingly, patients classified into the Adv* subgroup had similar outcomes to those in the Int group. This suggests that the Allo-HCT Refined ELN 2022 classification more accurately refines risk stratification, revealing that some patients previously classified as high-risk may actually have more favorable outcomes after allo-HCT. These findings align with recent evidence showing that patients with the most common abnormalities within the Adv* group, such as myelodysplasia-related abnormalities [26] and KMT2A rearrangements [27] have better outcomes when transplanted in first complete remission.
Furthermore, the refined Allo-HCT Refined ELN 2022 classification identifies a subgroup of patients (AdvP) with particularly dismal outcomes after allo-HCT. These findings indicate a potential but uncertain advantage of the role of allo-HCT consolidation in AdvP patients and highlight the urgent need for more effective strategies both before and after transplantation. Despite the poor outcomes for AdvP patients, the allo-HCT remains the only potentially curative option, particularly for younger individuals. Improving the response prior to allo-HCT with new frontline treatments, promoting a stronger graft-versus-leukemia effect through maintenance therapies or implementing preemptive treatment after molecular relapse could contribute to increase survival rates in this challenging subgroup.
Patients over 55 years and those with positive MRD status prior to allo-HCT had lower OS and LFS. The higher proportion of older patients in the Adv and Int groups may partially explain their worse prognosis, as well as the fact that older adults are often treated with RIC regimens, which, while improving safety, may be associated with higher relapse rates.
MRD positive status, measured according to the ELN-DAVID MRD 2021 recommendations, was found to be a predictor for disease relapse in the global MVA [28, 29]. However, MRD-positive status prior to allo-HCT was not associated with worse outcomes in the subgroup of patients classified into AdvP subgroup. This result may suggest that the genetic features of the AdvP subgroup confer such a poor prognosis that MRD status becomes less relevant. However, no molecular markers were used (e.g., NPM1 [30] and CBF mutation) to assess MRD, limiting the whole analysis to MFC. Recent evidence highlights that using more sensible MRD techniques in this context, such as NGS-MRD [31, 32], DNA sequencing [33,34,35] or ddPCR analysis of clonal hematopoiesis linked mutations [36] may be more sensitive for defining the AML response after treatments, guide therapeutic options, and help on decision making.
Finally, using PTCY-based prophylaxis was associated with improved LFS, supporting its ongoing use in clinical practice. The potential benefit of PTCY may stem from an inferior risk of severe chronic GVHD or shorter immunosuppressive therapy and potentially reducing relapse risk. This observation suggests that PTCY may be particularly advantageous for high-risk patients, as it could enable quicker immune reconstitution and a stronger graft-versus-leukemia effect, thereby improving outcomes [37].
There are limitations in the study that should be mentioned. First, the retrospective nature of the study and the limited number of patients in some subgroups may have impacted the robustness of the findings. Second, the MRD measurement heterogeneity across institutions limited the ability to thoroughly assess its impact on outcomes in the AdvP group. Lastly, there is a lack of information regarding the impact of new findings on disease biology, such as DDX41 mutations [38,39,40], or the effects of lower-intensity induction treatments such as venetoclax and azacitidine [41], which have been increasingly used in clinical practice and may affect outcomes in the future.
To conclude, this study validates the Allo-HCT Refined ELN 2022 classification for prognostic stratification of AML patients undergoing allo-HCT, particularly by identifying the AdvP subgroup, which requires special attention due to its significantly poorer outcomes [12]. This refined classification provides clinicians with a more precise tool for acknowledging allo-HCT outcomes and may help physicians to improve personalized treatment strategies for patients allocated in the AdvP risk group in order to prevent post-transplant relapse and improve survival.
Data availability
Due to privacy and ethical concerns, the data that support the findings of this study are available upon request from the corresponding author.
References
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Salas MQ, Atenafu EG, Pasic I, Al-Shaibani E, Bascom O, Wilson L, et al. Impact of hematopoietic cell transplant frailty scale on transplant outcome in adults. Bone Marrow Transpl. 2023;58:317–24.
Salas MQ, Atenafu EG, Pasic I, Bascom O, Wilson L, Lam W, et al. HCT frailty scale for younger and older adults undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2023;58:1237–46.
DeFilipp Z, Alousi AM, Pidala JA, Carpenter PA, Onstad LE, Arai S, et al. Nonrelapse mortality among patients diagnosed with chronic GVHD: an updated analysis from the Chronic GVHD Consortium. Blood Adv. 2021;5:4278–84.
Yanada M, Konuma T, Mizuno S, Saburi M, Shinohara A, Tanaka M, et al. Predicting non-relapse mortality following allogeneic hematopoietic cell transplantation during first remission of acute myeloid leukemia. Bone Marrow Transpl. 2021;56:387–94.
Braamse AMJ, Gerrits MMJG, van Meijel B, Visser O, van Oppen P, Boenink AD, et al. Predictors of health-related quality of life in patients treated with auto- and allo-SCT for hematological malignancies. Bone Marrow Transpl. 2012;47:757–69.
Blaise D, Devillier R, Lecoroller-Sorriano AG, Boher JM, Boyer-Chammard A, Tabrizi R, et al. Low non-relapse mortality and long-term preserved quality of life in older patients undergoing matched related donor allogeneic stem cell transplantation: a prospective multicenter phase II trial. Haematologica. 2015;100:269–74.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377.
Rausch C, Rothenberg-Thurley M, Dufour A, Schneider S, Gittinger H, Sauerland C, et al. Validation and refinement of the 2022 European LeukemiaNet genetic risk strati fi cation of acute myeloid leukemia. Leukemia. 2023;37:1234–44.
Lachowiez CA, Long N, Saultz J, Gandhi A, Newell LF, Hayes-lattin B, et al. Comparison and validation of the 2022 European LeukemiaNet guidelines in acute myeloid leukemia. Blood Adv. 2023;7:1899–909.
Jentzsch M, Bischof L, Ussmann J, Backhaus D, Brauer D, Metzeler KH, et al. Prognostic impact of the AML ELN2022 risk classification in patients undergoing allogeneic stem cell transplantation. Blood Cancer J. 2022;12:170.
Jiménez-Vicente C, Charry P, Castaño-Diez S, Guijarro F, López-Guerra M, Pérez-Valencia AI, et al. Evaluation of European LeukemiaNet 2022 risk classification in patients undergoing allogeneic haematopoietic stem cell transplantation for acute myeloid leukaemia: identification of a very poor prognosis genetic group. Br J Haematol. 2024;205:256–67.
Arber D, Orazi A, Hasserijan RP, Borowitz MJ, Calvo KR, Kvasnica H-M, et al. International consensus classification of myeloid neoplasms and acute leukemia: integrating morphological, clinical, and genomic data. Blood. 2022;140:1200–28.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138:2753–67.
Przepiorka D, Weisdorf D, Martin P, Al E. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from Hl-A-matched sibling donors. Transplantation. 1974;18:295–304.
Robert Z, BB R. Acute graft-versus-host disease—biologic process, prevention, and therapy. N Engl J Med. 2024;377:2167–79.
Robert Z, BB R. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2024;377:2565–79.
Mrózek K, Eisfeld AK, Kohlschmidt J, Carroll AJ, Walker CJ, Nicolet D, et al. Complex karyotype in de novo acute myeloid leukemia: typical and atypical subtypes differ molecularly and clinically. Leukemia. 2019;33:1620–34.
Richard-Carpentier G, Rausch CR, Sasaki K, Hammond D, Morita K, Takahashi K, et al. Characteristics and clinical outcomes of patients with acute myeloid leukemia with inv(3)(q21q26.2) or t(3;3)(q21;q26.2). Haematologica. 2023;108:2331–42.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Görlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98.
Grob T, Al Hinai ASA, Sanders Mathijs A, Kavelaars FG, Rijken M, Gradowska PL, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2022;139:2347–54.
Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH, et al. TP53-mutated myelodysplastic syndrome and acute myeloid leukemia: biology, current therapy, and future directions. Cancer Discov. 2022;12:2516–29.
Pollyea DA, Pratz KW, Wei AH, Pullarkat V, Jonas BA, Recher C, et al. Outcomes in patients with poor-risk cytogenetics with or without TP53 mutations treated with venetoclax and azacitidine. Clin Cancer Res: Off J Am Assoc Cancer Res. 2022;28:5272–9.
Song GY, Kim T, Ahn SY, Jung SH, Kim M, Yang DH, et al. Allogeneic hematopoietic cell transplantation can overcome the adverse prognosis indicated by secondary-type mutations in de novo acute myeloid leukemia. Bone Marrow Transplant. 2022;57:1810–9.
Hernández-Sánchez A, González T, Sobas M, Sträng E, Castellani G, Abáigar M, et al. Rearrangements involving 11q23.3/KMT2A in adult AML: mutational landscape and prognostic implications – a HARMONY study. Leukemia. 2024;38:1929–37.
Short NJ, Zhou S, Fu C, Berry DA, Walter RB, Freeman SD, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: a systematic review and meta-analysis. JAMA Oncol. 2020;6:1890–9.
Paras G, Morsink LM, Othus M, Milano F, Sandmaier BM, Zarling LC, et al. Conditioning intensity and peritransplant flow cytometric MRD dynamics in adult AML. Blood. 2022;139:1694–1706.
Dillon R, Hills R, Freeman S, Potter N, Jovanovic J, Ivey A, et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood. 2020;135:680–8.
Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132:1703–13.
Heuser M, Heida B, Büttner K, Wienecke CP, Teich K, Funke C, et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv. 2021;5:2294–304.
Nakamura S, Yokoyama K, Shimizu E, Yusa N, Kondoh K, Ogawa M, et al. Prognostic impact of circulating tumor DNA status post–allogeneic hematopoietic stem cell transplantation in AML and MDS. Blood. 2019;133:2682–95.
Dillon LW, Gui G, Page KM, Ravindra N, Wong ZC, Andrew G, et al. DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA. 2023;329:745–55.
Dillon LW, Gui G, Ravindra N, Andrew G, Mukherjee D, Wong ZC, et al. Measurable residual FLT3 internal tandem duplication before allogeneic transplant for acute myeloid leukemia. JAMA Oncol. 2024;10:1104–10.
Pasca S, Guo MZ, Wang S, Stokvis K, Shedeck A, Pallavajjala A, et al. Cell-free DNA measurable residual disease as a predictor of postallogeneic hematopoietic cell transplant outcomes. Blood Adv. 2023;7:4660–70.
Salas MQ, Charry P, Pedraza A, Martínez-Cibrian N, Solano MT, Domènech A, et al. PTCY and tacrolimus for GVHD prevention for older adults undergoing HLA-matched sibling and unrelated donor AlloHCT. Transplant Cell Ther. 2022;28:489.e1–489.e9.
Cheloor Kovilakam S, Gu M, Dunn WG, Marando L, Barcena C, Nik-Zainal S, et al. Prevalence and significance of DDX41 gene variants in the general population. Blood. 2023;142:1185–92.
Duployez N, Largeaud L, Duchmann M, Kim R, Rieunier J, Lambert J, et al. Prognostic impact of DDX41 germline mutations in intensively treated acute myeloid leukemia patients: an ALFA-FILO study. Blood. 2022;140:756–68.
Bataller A, Loghavi S, Gerstein Y, Bazinet A, Sasaki K, Chien KS, et al. Characteristics and clinical outcomes of patients with myeloid malignancies and variants. Am J Hematol. 2023;98:1780–90.
Pollyea DA, Winters A, McMahon C, Schwartz M, Jordan CT, Rabinovitch R, et al. Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transplant. 2022;57:160–6.
Acknowledgements
We thank our patients and the Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH-TC) for the support provided during the study development. In addition, Dr. Jordi Esteve and María Jesús Pascual Cascón for their mentorship and support during the study period. We additionally thank REDCap (Research Electronic Data Capture) service for permitting the use of their service without costs. REDCap is a secure, web-based software platform designed to support data capture for research studies.
Author information
Authors and Affiliations
Contributions
CJV, JE, and MQS designed the study. CJV performed the statistical analysis and wrote the manuscript; MQS and JE supervised the study and contributed to manuscript writing; MJPC provided orientation and support for the study conduction on behalf of GETH-TC; CJV, MBG, EPL, CMC, CA, IOO, AE, FPM, SFL, IHF, APGR, JLL-L, ALG, TT, AJSM, CAF, LG, SV, SF and PB collected data. All authors contributed valuable revisions and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
CJV serves as an educational speaker for AbbVie and received travel grants from AbbVie, Jazz Pharmaceuticals, and Pfizer. JE declares consultancy honoraria from AbbVie, Novartis, Astellas, Jazz Pharmaceuticals, BMS-Celgene, Pfizer, and Daichii-Sankyo, and received research grants from Novartis, Jazz Pharmaceuticals, and Pfizer. The remaining authors declare no competing financial interests. The founders had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Ethics approval statement
The study was approved by the Ethics Committee of the Hospital Clínic de Barcelona and conducted following standards set forth by the Declaration of Helsinki.
Informed consent
All patients provided their informed consent for the use of laboratory results and genetic analyses.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiménez-Vicente, C., Esteve, J., Baile-González, M. et al. Allo-HCT refined ELN 2022 risk classification: validation of the Adverse-Plus risk group in AML patients undergoing allogeneic hematopoietic cell transplantation within the Spanish Group for Hematopoietic Cell Transplantation (GETH-TC). Blood Cancer J. 15, 42 (2025). https://doi.org/10.1038/s41408-025-01223-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41408-025-01223-x
This article is cited by
-
Emerging strategies and novel therapeutic targets in acute myeloid leukemia: current advances and future directions
Biomarker Research (2025)
-
Comprehensive analysis of FLT3-mutated patients with acute myeloid leukemia with updated 2022 European LeukemiaNet recommendations: insights from the Turkish AML registry project
BMC Cancer (2025)