Abstract
The treatment landscape for transplant-eligible patients with newly diagnosed multiple myeloma (TE-NDMM) has evolved with the introduction of daratumumab-based quadruplet regimens. Adding daratumumab to traditional triplet regimens has demonstrated improved response rates and progression-free survival (PFS). However, the impact on long-term outcomes, particularly overall survival (OS), remains uncertain. This systematic review and meta-analysis aimed to compare the survival outcomes of these quadruplet regimens with triplets. Conducted in adherence to Cochrane Collaboration and PRISMA guidelines and registered on PROSPERO (CRD42024571946), the study involved searching PubMed, Embase, and Cochrane databases, from inception to June 2024. We included randomized clinical trials (RCT) and non-randomized controlled studies (NRCS) that compared daratumumab-based quadruplet regimens to triplets, focusing on OS and PFS, with a minimum follow-up of 18 months. The meta-analysis included 3327 TE-NDMM patients from four studies, comprising three RCT and one NRCS. Daratumumab-based regimens were administered to 1328 (40%) patients. The analysis revealed that daratumumab-based quadruplet regimens significantly improved both OS (pooled HR 0.60; 95% CI 0.48–0.75; P < 0.00001; I² = 0%) and PFS (pooled HR 0.49; 95% CI 0.37–0.65; P < 0.00001; I² = 52%). A per-protocol subgroup analysis comparing D-VRD to VRD further confirmed these benefits, with significant improvements in both OS (pooled HR 0.68; 95% CI 0.48–0.97; P = 0.03; I² = 0%) and PFS (pooled HR 0.41; 95% CI 0.31–0.54; P < 0.00001; I² = 0%). This meta-analysis consolidates evidence that daratumumab-based quadruplet regimens significantly improve OS, compared to triplet regimens for TE-NDMM patients.
Similar content being viewed by others
Introduction
Multiple myeloma (MM) is a complex and heterogeneous hematologic malignancy, primarily characterized by the clonal proliferation of malignant plasma cells within the bone marrow [1]. For transplant-eligible newly diagnosed multiple myeloma (TE-NDMM) patients, the choice of induction therapy plays a critical role in determining long-term outcomes. Recent advances in therapeutic strategies have focused on incorporating of novel agents to enhance the depth of response before autologous stem cell transplantation (ASCT) [2]. Among these innovations, daratumumab, a monoclonal antibody targeting CD38, has emerged as a pivotal component in frontline regimens [3].
Traditionally, the standard induction therapy is a triplet regimen combining a proteasome inhibitor (PI), an immunomodulatory agent (IMiD) and corticosteroids. However, the emergence of daratumumab-based quadruplet regimens represents a significant evolution in treatment, offering the potential for deeper responses and improved disease control, with an acceptable safety profile [4,5,6,7,8,9].
Clinical trials have shown promising outcomes with these regimens, particularly in terms of response rates, minimal residual disease (MRD) negativity and progression-free survival (PFS) [4,5,6,7,8,9]. However, the impact on long-term outcomes, particularly overall survival (OS), is still under investigation. This uncertainty underscores the need for a comprehensive evaluation of the available evidence to guide clinical decision-making.
To this end, we conducted a systematic review (SR) and meta-analysis (MA) to compare daratumumab-based quadruplet versus triplet induction regimens in the frontline treatment of TE-NDMM patients. By synthesizing data from relevant studies, we aim to provide a clearer understanding of the impact on overall survival and discuss the implications of these findings for the future landscape of MM therapy.
Methods
This SR and MA were performed and reported following the Cochrane Collaboration Handbook for Systematic Review of Interventions recommendations and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [10, 11]. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42024571946).
Databases search strategy
We systematically searched PubMed, Embase, and Cochrane Library databases from inception to June 2024. The search strategy was as follows: (“myeloma” OR “multiple myeloma”) AND (“daratumumab” OR “darzalex”) AND (“newly diagnosed” OR “first line” OR “front line” OR “eligible” OR “induction” OR “autologous” OR “transplantation”).
Eligibility criteria
Pre-specified Population, Intervention, Comparator, Outcome, Time and Study design (PICOTS) criteria were used to develop the search strategy. Included studies met the following eligibility criteria: (1) randomized clinical trials (RCT) and non-randomized controlled studies (NRCS); (2) that compared daratumumab-based quadruplet induction regimens to triplet induction regimens; (3) in TE-NDMM patients; (4) reported the outcomes of interest: OS and PFS and; (5) with a minimum follow-up of 18 months. We excluded studies that were non-controlled or with overlapping patient populations.
Selection criteria
Citations from all databases were imported into a Zotero reference manager software (Corporation for Digital Scholarship) and duplicates were removed. Two authors (JTDSF and LCO) independently screened search records to identify eligible studies, using the pre-established criteria for search, data extraction and quality assessment. Disagreements were resolved by consensus among the authors.
Data extraction
Data extracted included study characteristics (first author, year of publication, journal, study design, sample size, treatment regimens and duration of follow-up), baseline characteristics of the participants (age, sex, distribution by the International Staging System [ISS] and cytogenetic risk) and outcome data (OS and PFS).
Definition of outcomes
The primary outcome was OS, defined as the time from randomization to the date of death. We also evaluated the PFS, defined as the time from randomization to the date of the first confirmed progression or date of death, whichever occurred earlier. We quantified associations in terms of hazard ratios (HRs) and 95% confidence intervals (95% CI). If multiple publications of the same study were available, the publication with the longest available follow-up results was used to extract the summary effect.
Quality assessment
Two investigators (JTDSF and LCO) independently assessed each study’s quality and risk of bias. Randomized controlled trials were appraised, using Cochrane’s “Risk-of-bias tool for randomized trials Version 2” (RoB-2) [12, 13]. Meanwhile, non-randomized controlled studies were assessed, using Cochrane’s “Risk of bias in non-randomized studies of interventions” (ROBINS-I) [14]. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) assessment was conducted to assess the overall certainty of the evidence for each outcome [15]. The Summary of Findings (SoF) Table was made to facilitate a clear and comprehensive presentation of the evidence [10]. Publication bias was assessed through funnel plot analysis, which examined the relationship between point estimates and study weights [16].
Statistical analysis
We pooled the treatment effects from each study to estimate HRs and their corresponding 95% CIs for time-to-event outcomes. When the reported CI differed from a 95% CI, the provided CI was converted to a 95% CI for consistency, using a calculations spreadsheet [17]. In studies where the HR were not directly reported, they were extracted from the published Kaplan-Meier curves [17]. Heterogeneity across studies was assessed, using Cochrane’s Q test and I2 statistics. A P-value of less than 0.10 was considered significant of significant heterogeneity, with I² values categorized as low (0–40%), moderate (30–60%), substantial (50–90%) and considerable (75–100%) heterogeneity [12]. The DerSimonian and Laird random-effects model was used. Leave-one-out sensitivity analysis were conducted to identify outliers in the MA. All P-values were two-sided, with statistical significance set at P < 0.05. Statistical analyses to pool HRs and generate the corresponding forest plots were conducted, using the Review Manager, version 5.4.1 (The Cochrane Collaboration, Denmark). Additionally, patient-level data of each treatment arm in each trial were reconstructed from Kaplan-Meier curves, using the IPDfromKM method [18]. Kaplan-Meier curves were digitalized with the Engauge Digitizer (version 12.1). Subsequently, the IPDfromKM Shiny app (version: 1.2.3.0, available at https://biostatistics.mdanderson.org/) was used to reconstruct the individual patient data (IPD). Finally, pooled survival curves were generated from the reconstructed IPD, along with the relevant Cox statistics, using the “coxph”, “survfit”, and “ggsurvplot” packages in R Statistical Software (version 4.4.1) [19].
Results
Study selection
The initial search yielded 4789 results. After removing duplicates, 3502 articles were screened by title and abstract, leaving 142 potentially eligible studies for detailed review. Following the exclusion of 135 studies, seven reports from a total of four studies were included in the SR and MA (Supplementary Fig. 1).
Characteristics of studies and study population
Four studies involving 3327 TE-NDMM patients were included, comprising three randomized controlled trials (CASSIOPEIA [4,5,6], GRIFFIN [7, 8], and PERSEUS [9]) and one non-randomized controlled study based on real-world data [20, 21]. The publication years ranged from 2019 to 2024. Across three studies, daratumumab in combination with bortezomib, lenalidomide, and dexamethasone (D-VRD) was compared to bortezomib, lenalidomide, and dexamethasone (VRD) [8, 9, 20], while one study compared daratumumab with bortezomib, thalidomide and dexamethasone (D-VTD) to bortezomib, thalidomide, and dexamethasone (VTD) [6]. Sample sizes ranged from 207 to 1326 patients. The weighted median follow-up duration was 51.7 months, with a range from 19.1 to 88.4 months. The weighted median patient age was 60 years, 57% were male, 46% had ISS stage I, 36% had ISS stage II, 18% had ISS stage III disease and 17.5% of patients had a high-risk cytogenetic profile. The main characteristics of the included studies are reported in Table 1.
Outcome analysis
In the comparative analysis of OS and PFS, data from a total of 3327 patients were included, with 1328 patients (40%) receiving a daratumumab-based quadruplet induction regimen. Specifically, 785 patients (24%) were treated with D-VRD, while 543 patients (16%) received D-VTD. The remaining 1999 patients (60%) were administered a triplet regimen, with 1457 patients (44%) receiving VRD and 542 patients (16%) receiving VTD.
Among the four studies evaluating the addition of daratumumab to backbone regimens, the HRs for PFS, using the most recent follow-up data, were 0.61 (95% CI, 0.52–0.72) in the CASSIOPEIA study [6], 0.45 (95% CI, 0.21–0.95) in the GRIFFIN study [8], 0.42 (95% CI, 0.30–0.59) in the PERSEUS study [9], and 0.34 (91% CI, 0.2–0.67) in the study by Joseph et al. [20]. Since the Joseph et al. study reported its results with a 91% CI, the interval was converted to a 95% CI (0.17–0.68) for consistency [17]. All these findings were statistically significant.
Regarding OS, the HR for quadruplet therapy was 0.55 (95% CI, 0.42–0.73) in the CASSIOPEIA study [6], 0.90 (95% CI, 0.31–2.56) in the GRIFFIN study [8], and 0.53 (91% CI, 0.3–0.96) in the Joseph et al. study, which was again converted to a 95% CI (0.27–1.04) [20]. In the PERSEUS study, the HR for D-VRD was reported as 0.73; however, the confidence intervals (CIs) were not directly provided. In this manner, the 95% CI was extracted from the published Kaplan-Meier curves (95% CI, 0.47–1.14) [9, 22]. Among these studies, only the CASSIOPEIA trial demonstrated a statistically significant improvement in OS with a 95% CI. Regarding postprotocol therapies, only the CASSIOPEIA trial presented these data, documenting that 68% of patients in the observation group (VTd plus observation) received an anti-CD38–based regimen as their first subsequent therapy [6].
Pooled analyses of OS and PFS
In this MA, we evaluated the impact of daratumumab-based quadruplet regimens, compared to triplet regimens, on OS and PFS.
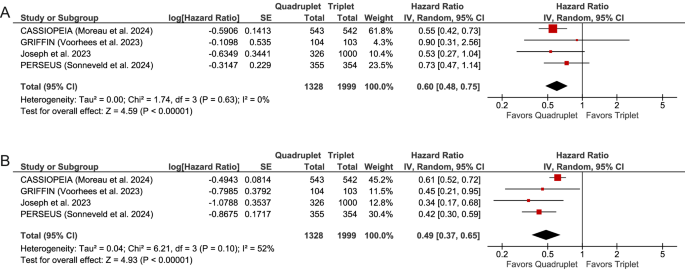
For OS, our analysis demonstrated that quadruplet regimens incorporating daratumumab resulted in a significantly improved survival compared to triplet regimens, with a pooled HR of 0.60 (95% CI, 0.48–0.75; P < 0.00001). The heterogeneity among studies was low, as indicated by Cochran’s Q test (P = 0.63) and an I² statistic of 0%. For PFS, the pooled HR was 0.49 (95% CI, 0.37–0.65; P < 0.00001), with a moderate level of heterogeneity (Cochran’s Q, P = 0.10; I² = 52%). These findings are illustrated in the forest plot (Fig. 1).
Sensitivity analyses using the leave-one-out method, which involved omitting one study at a time, yielded HRs ranging from 0.57 to 0.68 for OS and from 0.41 to 0.52 for PFS (Supplementary Figs. 2 and 3). Notably, excluding the NRCS from the analysis had minimal impact, yielding HRs of 0.61 (95% CI, 0.48–0.77; P < 0.0001) for OS and 0.52 (95% CI, 0.39–0.69; P < 0.00001) for PFS (Supplementary Figs. 2C and 3C). These findings underscore the robustness of the pooled estimates, confirming that no single study significantly influenced the results.
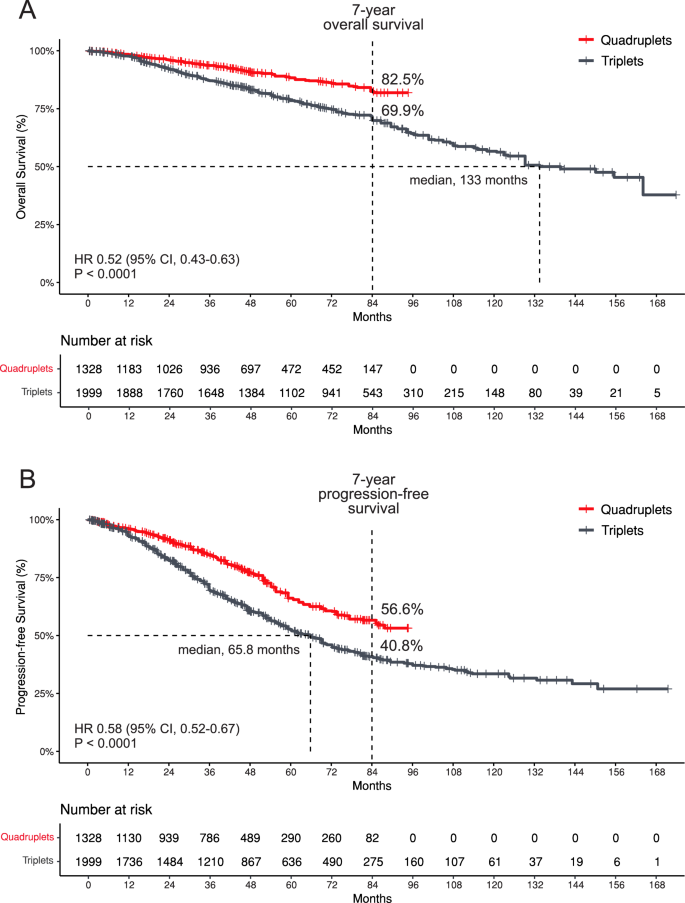
Pooled Kaplan-Meier survival curves (Fig. 2) indicate that, at 84 months, the OS rate for patients receiving quadruplet therapy was 82.5%, compared to 69.9% for those receiving triplet therapy. The median OS for the quadruplet group was not reached, while the median OS for the triplet group was 133 months (HR 0.52; 95% CI, 0.43–0.63; P < 0.0001). For PFS, the 84-month rate was 56.6% for quadruplet therapy, versus 40.8% for triplet therapy, with the median PFS not reached for the quadruplet group and 65.8 months for the triplet group (HR 0.58; 95% CI, 0.52–0.67; P < 0.0001).
Pooled Kaplan-Meier survival curves, based exclusively on RCT and excluding the NRCS, were generated to assess the NRCS’s contribution to the overall estimate. At 84 months, the OS rate was 82.6% for patients receiving quadruplet therapy, compared to 73.2% for those receiving triplet therapy (HR 0.62; 95% CI, 0.49–0.77; P < 0.0001). For PFS, the 84-month rates were 55.3% for quadruplet therapy and 35.7% for triplet therapy (HR 0.56; 95% CI, 0.48–0.65; P < 0.0001). Median OS and PFS were not reached in either group (Supplementary Fig. 4).
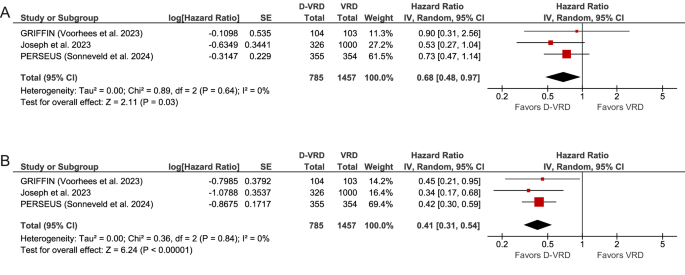
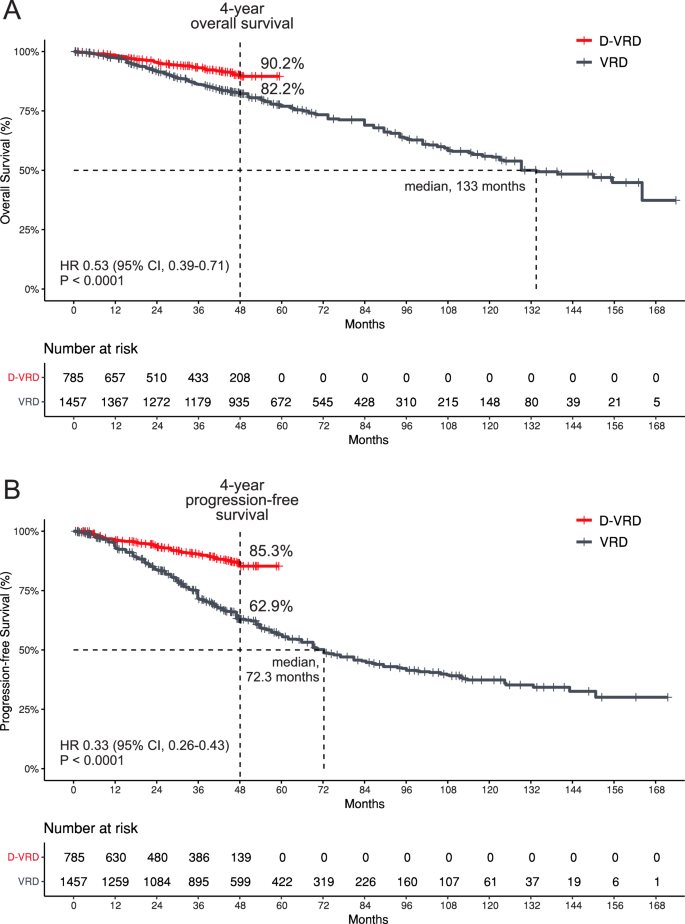
A per-protocol subgroup analysis comparing the D-VRD regimen to VRD further confirmed the benefit of adding daratumumab. This analysis showed a significant improvement in both OS (pooled HR 0.68; 95% CI, 0.48–0.97; P = 0.03), with low heterogeneity (Cochran’s Q, P = 0.64; I² = 0%), and PFS (pooled HR 0.41; 95% CI, 0.31–0.54; P < 0.00001), also with low heterogeneity (Cochran’s Q, P = 0.84; I² = 0%). These results are depicted in the forest plot (Fig. 3).
Survival data from pooled Kaplan-Meier curves (Fig. 4) reveal that, at 48 months, the OS rate for patients treated with D-VRD was 90.2%, compared to 82.2% for those receiving VRD. The median OS for the D-VRD group was not reached, whereas the median OS for the VRD group was 133 months (HR 0.53; 95% CI, 0.39–0.71; P < 0.0001). For the PFS, the 48-month rate was 85.3% for D-VRD versus 62.9% for VRD, with the median PFS not reached for the D-VRD group and 72.3 months for the VRD group (HR 0.33; 95% CI, 0.26–0.43; P < 0.0001).
Quality assessment
Three of the included studies showed a low risk of bias, according to the RoB 2 tool (Supplementary Fig. 5), whereas one showed a moderate risk, according to the ROBINS-I (Supplementary Fig. 6). The funnel plot exhibited a symmetrical distribution of studies around the combined effect size, suggesting that there is no substantial publication bias (Supplementary Fig. 7). The certainty of the evidence, assessed via the GRADE approach, was rated as moderate for both OS and PFS (Supplementary Fig. 8).
Discussion
This SR and MA provide a robust and unique evaluation of daratumumab-based quadruplet regimens, compared to traditional triplet regimens in the frontline treatment of TE-NDMM patients. By pooling data from 3327 patients across multiple studies, our analysis highlights significant improvements in OS and PFS when daratumumab is incorporated into standard therapies.
The findings align with the evolving treatment paradigm for TE-NDMM, where the inclusion of novel agents has shifted the standard of care. Historically, the VRD has been widely accepted as the standard induction regimen [23,24,25]. However, recent trials, including CASSIOPEIA, GRIFFIN and PERSEUS, have demonstrated the superiority of adding daratumumab to triplet regimens, establishing quadruplet regimens as the new standard [4,5,6,7,8,9].
The pivotal phase III CASSIOPEIA trial established the D-VTD regimen, which significantly improved PFS, compared to VTD alone [6]. This led to the European Medicines Agency approval of D-VTD as the new standard for induction regimen prior to ASCT and it has been incorporated into clinical guidelines [26]. Subsequently, the phase II GRIFFIN trial demonstrated the clear benefits of D-VRD for induction and consolidation, followed by daratumumab and lenalidomide maintenance, showing significantly longer PFS compared to VRD with lenalidomide maintenance [8]. The phase III PERSEUS trial further supported these findings, confirming the superiority of D-VRD over VRD regarding response rates, MRD negative rates, and PFS [9].
Emerging data suggest that incorporating carfilzomib into quadruplet regimens may offer additional benefits. The phase II MASTER trial showed promising results with the D-KRD regimen (daratumumab, carfilzomib, lenalidomide, and dexamethasone), achieving an 81% MRD negativity rate [27, 28]. Similarly, the phase II IFM 2018-04 study reported high MRD negativity (94%) and a 30-month PFS of 80% in high-risk TE-NDMM patients treated with D-KRD and tandem transplantation [29]. However, no direct comparisons exist between carfilzomib- and bortezomib-based quadruplets, leaving unanswered questions regarding their relative efficacy.
Isatuximab, another anti-CD38 monoclonal antibody, has also been incorporated into quadruplet regimens. The phase III GMMG-HD7 trial demonstrated higher MRD negativity rates with I-VRD (isatuximab, VRD) compared to VRD alone (50% vs 36%) [30]. Additionally, the phase II GMMG-CONCEPT and phase III ISKIA trials evaluated isatuximab, carfilzomib, lenalidomide, and dexamethasone (I-KRD) [31, 32]. The ISKIA trial reported MRD negativity rates of 77% with I-KRD versus 67% with KRD (OR 1.67; P = 0.049) [32]. However, these studies have yet to provide long-term OS or PFS data due to limited follow-up durations [30,31,32].
Lenalidomide maintenance remains the standard of care due to its OS benefit compared to observation [33]. Except for the CASSIOPEIA trial, which compared daratumumab maintenance with observation, all other quadruplet trials—GRIFFIN [8], PERSEUS [9], ISKIA [32], GMMC-HD7 [30], GMMC-CONCEPT [31] and MASTER [28]—incorporated either a prolonged consolidation with three drugs or a two-drug maintenance regimen involving an anti-CD38 antibody plus lenalidomide, whereas the control arms received lenalidomide monotherapy. Consequently, the long-term benefits reported in these studies likely reflect the cumulative effects of anti-CD38 antibody therapy across the induction, consolidation and maintenance phases. However, it remains unclear whether the PFS advantage observed in these trials is predominantly attributable to daratumumab use during induction phase, maintenance phase, or a synergistic effect across both phases.
Traditionally, PFS has served as the primary endpoint in MM clinical trials to assess treatment efficacy [34]. However, MRD negativity has emerged as a more sensitive and timely surrogate endpoint for long-term outcomes [35, 36]. Despite its utility, many trials lack sufficient follow-up to demonstrate OS benefits conclusively. In this context, the MA has become a valuable and important tool. By aggregating data from various trials, the MA can provide more robust and comprehensive insights into the survival benefits. Therefore, we present a MA focused on survival outcomes.
Our MA demonstrates a significant survival benefit with daratumumab-based quadruplet regimens. The pooled HR for OS was 0.60 (95% CI: 0.48–0.75), corresponding to a 40% reduction in the risk of death compared to triplet regimens. Except for the CASSIOPEIA trial, no other individual study has shown a similar OS benefit [4,5,6]. This pooled analysis underscores the efficacy of daratumumab-based regimens, given that the OS is the most reliable endpoint in hematology-oncology clinical trials [37].
Additionally, the marked improvement in PFS with a pooled HR of 0.49 (95% CI: 0.37–0.65) further supports the enhanced disease control provided by these regimens. Daratumumab addition reduces the risk of disease progression or death by 51%, thereby enhancing both the depth and durability of the response. These findings are consistent with the results from the individual trials CASSIOPEIA, GRIFFIN, and PERSEUS [4,5,6,7,8,9].
A per-protocol subgroup analysis comparing D-VRD to VRD provides further insights into the benefits of adding daratumumab to the VRD regimen. The inclusion of daratumumab resulted in a remarkable improvement in the OS, with a 32% reduction in the risk of death, and the PFS, showing a 59% reduction in the risk of disease progression or death, for patients receiving D-VRD, compared to those on VRD alone. While individual clinical trials, such as GRIFFIN and PERSEUS did not demonstrate a significant impact of daratumumab on the OS—likely due to their shorter follow-up periods of 49.6 and 47.5 months, respectively—this MA highlights that D-VRD significantly outperforms VRD in terms of the OS [7,8,9]. These results are particularly important, given that VRD has long been established as the standard of care for TE-NDMM.
When calculating the pooled HR for the OS, we observed low heterogeneity in both comparisons: daratumumab quadruplet regimens versus triplet regimens (Cochran’s Q, P = 0.63; I² = 0%) and D-VRD versus VRD (Cochran’s Q, P = 0.64; I² = 0%). This minimal heterogeneity indicates that the studies included in this analysis are highly consistent in their results, thereby enhancing the reliability and generalizability of the meta-analytic findings.
In this MA, we employed two distinct methodological approaches: a standard MA of aggregate data and a reconstructed patient data analysis to generate survival curves. Both methods demonstrated strong agreement in estimating the effectiveness of the daratumumab-based quadruplet regimen on the OS and PFS (Figs. 1–4). The simultaneous application of these approaches enabled a more robust and comprehensive dataset interpretation [38].
Both RCT and NRCS were included in this MA to address gaps in the evidence. While RCTs provide high-quality evidence on efficacy, NRCS were included to capture real-world effectiveness not adequately addressed by RCTs [10]. A sensitivity analyses using the leave-one-out method were performed to evaluate the impact of their inclusion to the overall estimate. The results remained consistent even after repeating the MA omitting the NRCS from the analysis (Supplementary Figs. 2C, 3C, and 4).
Prior meta-analyses have established that daratumumab-based regimens demonstrate superior efficacy outcomes relative to control groups, as evidenced by enhanced overall response rates, complete responses, stringent complete responses, and increased MRD negativity. Conversely, these regimens are associated with a significantly higher incidence of adverse events, notably grade ≥3 neutropenia, grade ≥3 infections, pneumonia, and grade ≥3 thrombocytopenia [39,40,41,42,43,44,45,46]. Unlike most previous analyses that aggregated patients with both transplant-eligible and transplant-ineligible MM, our study focuses exclusively on TE-NDMM patients, thereby offering a more precise and tailored comparison of treatment regimens within this specific population. Furthermore, we provide a comprehensive analysis of OS in TE-NDMM patients, a critical endpoint that has not been thoroughly addressed in earlier meta-analyses.
Despite the compelling evidence, several limitations must be acknowledged. First, all data were derived or calculated from published studies rather than individual patient data, introducing potential heterogeneity due to variability in study designs, patient characteristics, and maintenance therapies, which may affect the generalizability of our findings. Second, in the PERSEUS and GRIFFIN trials, the observed benefits of quadruplet regimens may reflect the cumulative effects of daratumumab across induction, consolidation, and maintenance phases. Third, in the CASSIOPEIA trial, the randomization of patients to daratumumab maintenance or observation in the second phase may have influenced outcomes. Fourth, the limited reporting of postprotocol therapies raises concerns about the potential impact of suboptimal treatment, particularly the absence of anti-CD38 antibodies upon relapse in the control arms, which may undermine the observed OS benefit. Additionally, the relatively short follow-up duration in some studies limits the assessment of long-term outcomes, which may evolve with extended follow-up. Finally, the inclusion of one non-randomized controlled study, despite its rigorous and favorable quality assessment, introduces a potential source of bias.
In conclusion, this MA consolidates evidence that daratumumab-based quadruplet regimens significantly improve the overall survival in the frontline treatment of TE-NDMM, compared to traditional triplet regimens. These results provide valuable guidance for selecting optimal first-line treatments, especially in the absence of long-term follow-up data from ongoing randomized trials.
Data availability
This meta-analysis was conducted using data sourced from previously published studies, all of which are publicly accessible. The authors did not have access to individual patient-level data from the included studies. All pertinent datasets generated and analyzed during this study are provided within the article and its supplementary materials. For further inquiries or requests for additional data, please contact the corresponding author.
References
Malard F, Neri P, Bahlis NJ, Terpos E, Moukalled N, Hungria VTM, et al. Multiple myeloma. Nat Rev Dis Primers. 2024;10:45.
Perrot A. How I treat frontline transplantation-eligible multiple myeloma. Blood. 2022;139:2882–8.
Rajkumar SV. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2024;99:1802–24.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38.
Moreau P, Hulin C, Perrot A, Arnulf B, Belhadj K, Benboubker L, et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:1378–90.
Moreau P, Hulin C, Perrot A, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab and followed by daratumumab maintenance or observation in transplant-eligible newly diagnosed multiple myeloma: long-term follow-up of the CASSIOPEIA randomised controlled phase 3 trial. Lancet Oncol. 2024;25:1003–14.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936–45.
Voorhees PM, Sborov DW, Laubach J, Kaufman JL, Reeves B, Rodriguez C, et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023;10:e825–37.
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl J Med. 2024;390:301–13.
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. Cochrane; 2023. http://www.training.cochrane.org/handbook
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Schünemann HJ, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. https://guidelinedevelopment.org/handbook
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21:111.
Messori A. Synthetizing published evidence on survival by reconstruction of patient-level data and generation of a multi-trial Kaplan-Meier curve. Cureus. 2021;13:e19422.
Joseph NS, Kaufman JL, Dicamillo S, Roberts D, Gupta VA, Hofmeister CC, et al. Comparison of response and survival outcomes in standard- and high-risk newly diagnosed transplant-eligible multiple myeloma (NDMM) patients treated with lenalidomide, bortezomib and dexamethasone (RVD) versus daratumumab, lenalidomide, bortezomib and dexamethasone (D-RVD). Blood. 2023;142:647–647.
Joseph NS, Kaufman JL, Gupta VA, Hofmeister CC, Dhodapkar MV, Boise LH, et al. Quadruplet therapy for newly diagnosed myeloma: comparative analysis of sequential cohorts with triplet therapy lenalidomide, bortezomib and dexamethasone (RVd) versus daratumamab with RVD (DRVd) in transplant-eligible patients. Blood Cancer J. 2024;14:159.
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Phase 3 Randomized Study of Daratumumab (DARA) + Bortezomib, Lenalidomide, and Dexamethasone (VRd) Versus Vrd Alone in Patients (Pts) with Newly Diagnosed Multiple Myeloma (NDMM) Who Are Eligible for Autologous Stem Cell Transplantation (ASCT): Primary Results of the Perseus Trial. Blood. 2023;142:LBA-1.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387:132–47.
Rosiñol L, Oriol A, Rios R, Sureda A, Blanchard MJ, Hernández MT, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134:1337–45.
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:309–22.
Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol. 2022;40:2901–12.
Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma (MASTER): final report of the multicentre, single-arm, phase 2 trial. Lancet Haematol. 2023;10:e890–901.
Touzeau C, Perrot A, Hulin C, Manier S, Macro M, Chretien M-L, et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with tandem transplant for high-risk newly diagnosed myeloma. Blood. 2024;143:2029–36.
Goldschmidt H, Mai EK, Bertsch U, Fenk R, Nievergall E, Tichy D, et al. Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol. 2022;9:e810–21
Leypoldt LB, Tichy D, Besemer B, Hänel M, Raab MS, Mann C, et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone for the treatment of high-risk newly diagnosed multiple myeloma. J Clin Oncol. 2024;42:26–37.
Gay F, Roeloffzen W, Dimopoulos MA, Rosiñol L, van der Klift M, Mina R, et al. Results of the phase III randomized iskia trial: isatuximab-carfilzomib-lenalidomide-dexamethasone vs carfilzomib-lenalidomide-dexamethasone as pre-transplant induction and post-transplant consolidation in newly diagnosed multiple myeloma patients. Blood. 2023;142:4–4.
McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Pawlyn C, Schjesvold FH, Cairns DA, Wei LJ, Davies F, Nadeem O, et al. Progression-free survival as a surrogate endpoint in myeloma clinical trials: an evolving paradigm. Blood Cancer J. 2024;14:134.
Landgren O, Prior TJ, Masterson T, Heuck C, Bueno OF, Dash AB, et al. EVIDENCE meta-analysis: evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma. Blood. 2024;144:359–67.
Costa LJ, Derman BA, Bal S, Sidana S, Chhabra S, Silbermann R, et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia. 2021;35:18–30.
Bommier C, Maurer MJ, Lambert J. What clinicians should know about surrogate end points in hematologic malignancies. Blood. 2024;144:11–20.
Messori A, Fadda V, Romeo MR, Veneziano S, Trippoli S. A comparison of statistical analysis between “Real” patients reported in Kaplan-Meier curves and “Reconstructed” patients estimated through the IPDfromKM method: analysis of eight trials evaluating catheter ablation of ventricular tachycardia. Cureus. 2023;15:e47891.
Wang Y, Li Y, Chai Y. Efficacy and safety of daratumumab in the treatment of multiple myeloma: a systematic review and meta-analysis. J Int Med Res. 2021;49:3000605211038135.
Kiss S, Gede N, Hegyi P, Nagy B, Deák R, Dembrovszky F, et al. Addition of daratumumab to multiple myeloma backbone regimens significantly improves clinical outcomes: a systematic review and meta-analysis of randomised controlled trials. Sci Rep. 2021;11:21916.
Menon T, Kataria S, Adhikari R, Khan H, Khalid MZ, Saeeduddin MO, et al. Efficacy of daratumumab-based regimens compared to standard of care in transplant-eligible multiple myeloma: a meta-analysis. Cureus. 2021;13:e15098.
Ficek J, Kalaitzaki E, Yuan SS, Tosolini A, Du L, Kremer BE, et al. Association of minimal residual disease negativity rates with progression free survival in frontline therapy trials for newly diagnosed multiple myeloma: a meta-analysis. Clin Lymphoma Myeloma Leuk. 2023;23:e213–21.
Chong LL, Soon YY, Soekojo CY, Ooi M, Chng WJ, de Mel S. Daratumumab-based induction therapy for multiple myeloma: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;159:103211.
Htut TW, Thein KZ, Lawrie A, Tighe J, Preston G. Efficacy of daratumumab combination regimen in patients with multiple myeloma: a combined analysis of phase III randomized controlled trials. EJHaem. 2020;1:262–6.
Ebraheem MS, Chakraborty R, Rochwerg B, Visram A, Mohyuddin GR, Venner CP, et al. Quadruplet regimens for patients with newly diagnosed multiple myeloma: a systematic review and meta-analysis. Blood Adv. 2024;8:5993–6002.
Paul B, Anwer F, Raza S, Mammadzadeh A, Khasawneh B, Shatnawi S, et al. Comparative meta-analysis of triplet vs. quadruplet induction regimens in newly diagnosed, treatment naïve, multiple myeloma. Cancers. 2024;16:2938.
Acknowledgements
This study was presented as an oral presentation at the 66th ASH Annual Meeting and Exposition, December 7–10, 2024, San Diego, California, USA.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
JTDSF designed the research and analyzed the data. JTDSF and LOC were responsible for data acquisition and interpretation. JTDSF drafted the manuscript, while LOC, EQC, VH, and AM contributed to its revision. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JTDSF reported receiving honoraria from AbbVie, AstraZeneca, BeiGene, BMS, Johnson & Johnson, Novartis, and Sanofi. LOC reported receiving honoraria from Johnson & Johnson. EQC reported receiving research funding from Johnson & Johnson and honoraria from Amgen, BMS, Johnson & Johnson, Sanofi, GSK, and Takeda. VH reported receiving honoraria from BMS, GSK, Johnson & Johnson, Regeneron, Sanofi, Takeda, Amgen, and AbbVie. AM reported receiving honoraria from Johnson & Johnson, BMS, Sanofi, Takeda, Pfizer, and Amgen. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Souto Filho, J.T.D., Cantadori, L.O., Crusoe, E.d.Q. et al. Daratumumab-based quadruplet versus triplet induction regimens in transplant-eligible newly diagnosed multiple myeloma: a systematic review and meta-analysis. Blood Cancer J. 15, 37 (2025). https://doi.org/10.1038/s41408-025-01253-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-025-01253-5