Abstract
Management paradigms for newly-diagnosed acute myeloid leukemia (ND-AML) in patients considered unfit to receive intensive chemotherapy have evolved with improved understanding of disease biology. In this setting, management requires clear delineation of goals of therapy that should include preservation of quality-of-life (QoL). Combination of venetoclax (Ven) and a hypomethylating agent (HMA) is the current standard-of-care in most circumstances with flexible options in regard to drug dose and duration of treatment as well as the addition (triplet combinations) or alternative use of targeted therapies, such as inhibitors of FLT3, IDH1, IDH2, or menin for patients with NPM1MUT or KMT2A rearrangements (KMT2Ar). Response rates (CR/CRi:40-90%) and overall survival outcomes (3-year: 0–67%) following Ven-HMA therapy are highly variable and depend primarily on tumor genetics while achievement of complete response with (CR) or without count recovery (CRi) and consolidation with allogeneic stem cell transplant (ASCT) are essential in securing long-term survival. Favorable genomic predictors of response to Ven-HMA include NPM1MUT, IDH2MUT, and DDX41MUT, and unfavorable TP53MUT, FLT3-ITD, and K/NRASMUT. Favorable predictors of overall survival include IDH2MUT, and unfavorable TP53MUT, FLT3-ITD, K/NRASMUT, and KMT2Ar. Whether or not triplet regimens provide significant survival gain over Ven-HMA in genetically targetable subgroups remains to be determined. Particularly frail patients who are considered unfit for Ven-HMA might benefit from monotherapy targeting FLT3MUT, IDH1/2MUT, NPM1MUTor KMT2Ar. Future research projects should focus on incorporating patient-reported outcomes in clinical trials, optimization of Ven-HMA dosing and treatment duration especially in triplet combinations and broadening the use of ASCT and clarification of its timing.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) affects older individuals, with approximately one-third of newly-diagnosed (ND) patients aged 75 years or older [1]. The majority of these patients are unfit to receive intensive chemotherapy and historically had poor outcomes. However, in recent years, treatment with the oral BCL2 inhibitor venetoclax (Ven), in combination with a hypomethylating agent (HMA), has emerged as the standard-of-care for patients with AML who are ineligible to receive intensive chemotherapy, significantly improving survival and quality-of-life (QoL) [2, 3]. In addition to Ven, oral small molecule inhibitors targeting FLT3, IDH1/2 mutations (IDH1/2MUT), and most recently KMT2A rearrangements (KMT2Ar) have also become important additions to the therapeutic armamentarium for AML [4]. With the increase in treatment options, management of AML relies on a shared decision-making process, which not only considers disease features but importantly patient fitness, quality of life metrics and preference. The current review outlines our practical management approach to AML in adults, ineligible to receive intensive therapy, informed by evidence derived from clinical trials as well as real-world experience.
Patient fitness and treatment selection
There is general consensus that chronological age alone is an inadequate measure of patient fitness [5]. In routine clinical care, determination of fitness continues to be a challenge due to lack of validated fitness screening criteria. Over the years, several scores have been developed which rely primarily on co-morbidities and disease characteristics (e.g., Augmented Hematopoietic Cell Transplantation Comorbidity Index [HCT-CI] or AML Composite Model [AML-CM]) [6], or involve consensus from expert panels (e.g., European LeukemiaNet (ELN) criteria, and Italian Society of Hematology, Italian Society of Experimental Hematology (SIES) and Italian Group for Bone Marrow Transplantation (GITMO) Consensus Criteria or Ferrara score for unfitness) [5, 7]. The latter provides a consensus-based definition of unfitness to intensive chemotherapy which includes chronological age > 75 years, Eastern Cooperative Oncology Group performance status (ECOG ≥ 3), medical co-morbidities (cardio-pulmonary, renal, or hepatic disease) and/or psychosocial factors (cognitive status and caregiver support). Application of the aforementioned unfitness criteria to 655 patients with AML treated with intensive regimens including 7 days of standard-dose cytarabine and 3 days of an anthracycline (“7 + 3”), CLAG-M (cladribine, high-dose cytarabine, granulocyte colony-stimulating factor, and mitoxantrone), or reduced-dose CLAG-M, disclosed markedly inferior overall survival in unfit (median 4.8 months) compared to fit patients (36.8 months) [8]. Unsurprisingly, 28 day mortality rates were also significantly higher among unfit vs. fit patients (14% vs. 2%) [8]. Another frailty assessment tool to consider is the HCT-frailty scale developed at Princess Margaret Cancer Center [9], which incorporated eight variables: the clinical frailty scale, instrumental activities of daily living test, grip strength test, timed up and go test, a self-rated health question, a single-item falls question, and serum levels of albumin and C-reactive protein obtained within seven days of frailty assessment. Based on total scores, patients may be categorized as fit (0–1), pre-frail (1.5–5.0), or frail ( ≥ 5.5) [9].
Emerging data suggest that pretreatment geriatric assessment may provide a more sensitive and specific measure of fitness in patients with AML [10]. In a prospective single institutional study involving 74 patients with ND-AML that received intensive chemotherapy (median age: 69 years; 11% ≥ 80 years), geriatric assessment included evaluations of cognition, depression, distress, physical function (PF) (self-reported and objectively measured), and comorbidities. Objective PF was assessed using the Short Physical Performance Battery (SPPB), which comprises a timed 4-meter walk, chair stands, standing balance and grip strength measurement [11]. After adjusting for competing risk factors, overall survival was significantly shorter in participants with cognitive impairment (modified mini-mental state exam (MMSE) < 77) and reduced PF (SPPB < 9) [11].
Building upon these findings, a recent phase 2 trial integrated geriatric assessment (comorbidity burden, physical and cognitive function) along with genetic profile to guide treatment selection in patients with AML aged over 60 years [12]. Seventy-three patients were enrolled and those considered fit by geriatric assessment and with favorable/intermediate risk disease received intensive 7 + 3-like chemotherapy (n = 4), or liposomal daunorubicin-cytarabine (CPX351) (n = 4) as appropriate [12]. Patients unfit for intensive therapy or those with high-risk AML received lower-intensity chemotherapy, including HMA alone (n = 18), Ven-HMA (n = 43) or other therapies (n = 4) [12]. In the particular study, 74% of patients demonstrated impairment in more than one geriatric assessment domain and the median time from enrollment to treatment initiation was 1 day (range: 0-13 days), underlining the feasibility of pre-treatment geriatric assessment and its potential to serve as a more accurate measure of frailty in older adults with AML [12].
Beyond its role in guiding selection of treatment intensity, geriatric assessment is also valuable for predicting treatment outcomes in patients with AML receiving less-intensive therapies; in the CALGB 361101 study which included 96 patients with AML deemed unfit for intensive therapy (median age: 73 years), and randomized to receive decitabine alone or in combination with bortezomib, several baseline factors- including HCT-CI > 3, impaired cognition, and lower global QoL scores were associated with shortened overall survival [13].
Taken together, and pending randomized comparisons, simple geriatric assessments may help guide treatment decisions and reduce the risk of over-treatment in adults with AML. Nonetheless, in routine practice, fitness assessment takes into consideration the treatment regimen as outlined in the recent ELN proposal [5]. In general, unfitness is determined by (i) pre-existing major medical co-morbidities (heart failure with reduced ejection fraction <50%, severe renal or hepatic impairment, concurrent solid tumor), (ii) treating physician’s assessment of performance status (ECOG > 2), and (iii) social circumstances including but not limited to lack of caregiver suppport [5].
Genetic risk factors and treatment selection
Current prognostication in AML is based on the ELN genetic risk models. The ELN-2022 genetic risk model considers three risk categories: favorable (core-binding factor, RUNX1-RUNX1T1 or CBFB-MYH11, NPM1MUT without FLT3-ITD or adverse karyotype, and CEBPA bZIPMUT), intermediate (karyotype not classified as adverse or favorable, NPM1MUT with FLT3-ITD, wild type NPM1 with FLT3-ITD without adverse risk genetic lesions, t(9;11)(p21.3;q23.3)-MLLT3-KMT2A) and adverse (adverse karyotype, BCR/ABL1, TP53MUT, ASXL1MUT, BCORMUT, EZH2MUT, RUNX1MUT, SF3B1MUT, SRSF2MUT, STAG2MUT, U2AF1MUT, and/or ZRSR2MUT) [14]. However, it is now widely recognized that the ELN-2022 model has limited applicability in the context of Ven-HMA therapy [15, 16]. Recently, the ELN-2024 risk stratification was proposed to guide prognostication in patients receiving less intensive therapies including HMA +/-Ven or HMA +ivosidenib (for IDH1MUT AML) and provides the following risk categories; favorable (NPM1MUT, IDH2MUT, IDH1MUT, DDX41MUT in the absence of TP53MUT, KRASMUT, NRASMUT and FLT3-ITD), intermediate (FLT3-ITD, and/or KRASMUT, and/or NRASMUT in the absence of TP53MUT), and adverse (TP53MUT) [17]. IDH1MUT with FLT3-ITD, K/NRASMUT remain favorable when treated with azacitidine + ivosidenib, based on data from the AGILE study reviewed in the section on IDH inhibitor therapy [18].
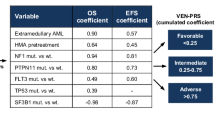
In general, genetic predictors for survival and response tend to overlap. In a pooled analysis of 279 patients with ND-AML treated with Ven-azacitidine in the phase 3 VIALE-A (NCT02993523) and phase 1b study (NCT02203773) [15], a four-gene molecular prognostic risk signature (mPRS) for response was developed based on the mutational status of TP53, KRAS, NRAS, and FLT3-ITD. Complete remission, with (CR) or without (CRi) count recovery rates were 77.2% in TP53WT, K/NRASWT, and FLT3-ITDWT (higher-benefit group), 59.2% in the presence of FLT3-ITD or K/NRASMUT and TP53WT (intermediate-benefit group), and 47.6% in the presence of TP53MUT (lower-benefit group).
In contrast, a Mayo Clinic study including 400 patients treated with Ven-HMA outside of clinical trials identified distinct molecular predictors of treatment response; NPM1MUT, IDH2MUT and DDX41MUT were associated with higher response rates, while TP53MUT, FLT3-ITD and RUNX1MUT were associated with lower responses [19]. Based on these findings, four distinct molecular signatures of treatment response were identified: (i) NPM1MUT, IDH2MUT, or DDX41MUT with TP53WT, RUNX1 WT, and FLT3-ITD WT (CR/CRi, 87%), (ii) NPM1 WT, IDH2 WT, DDX41 WT, TP53 WT, RUNX1 WT, FLT3-ITD WT (CR/CRi, 73%), (iii) NPM1MUT, IDH2MUT, or DDX41MUT with TP53MUT, RUNX1MUT, or FLT3-ITD (CR/CRi, 63%), and (iv) TP53MUT, RUNX1MUT, or FLT3-ITD with NPM1 WT, IDH2 WT, or DDX41 WT (CR/CRi, 44%) [19]. Median overall survival was 13.2 months with 3-year survival rate of 29%. Survival outcomes were better in patients who were bridged to transplant compared to those who were not transplanted (median overall survival: not reached vs. 10.8 months and 3-year survival rates 62% vs. 22%) [19]. On the other hand, presence of adverse karyotype, KMT2Ar, TP53MUT, KRASMUT, and IDH2 WT negatively influenced survival [19]. Additionally and importantly, the particular study demonstrated that achieving CR/CRi outweighed genetic risk factors in predicting survival outcomes across three independent cohorts from the Mayo Clinic, MD Anderson Cancer Center, and University of Chicago [19]. Furthermore, the prognostic importance of achieving measurable residual disease (MRD) negative CR/CRi was underlined in the VIALE-A study, in which patients with MRD-negative status had median overall survival of 34.2 months compared to 19 months in in those with MRD-negative disease [20, 21]. Ultimately, the likelihood of achieving a response significantly influences survival outcomes and remains a crucial factor in guiding treatment decisions.
Treatment approach
A holistic and individualized approach to the management of ND-AML is strongly advised, taking into consideration both disease-related factors (genetic risk) and patient characteristics (including fitness and treatment goals/preference). It is recommended to await genetic test results before initiating treatment [22], especially in patients who present with low or normal leukocyte count. For cases with hyperleukocytosis, temporizing measures such as leukapheresis, hydroxyurea +/- cytarabine should be promptly implemented and can stabilize most patients until genomic results are available. In general, AML in adults is associated with inferior survival but outcomes are improving. Age-specific survival analysis of patients with AML from the Nordic countries over five decades (1972–2021) revealed a steady improvement in survival across all age groups except those aged 80–89-year-old [23]. Notably, minimal survival gains were noted in the 70–79 year group, whereas survival was substantially improved in younger patients (<50 years), mainly due to the use of more intensive therapies and allogeneic stem cell transplant (ASCT) [23]. It is important to note that this study predated Ven approval in Europe which has shown survival and QoL benefits in patients otherwise ineligible for intensive therapy [23].
Treatment for patients unfit/ineligible for intensive therapies
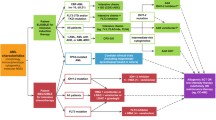
Figure 1 provides an algorithmic approach to treatment of patients with ND-AML who are ineligible to receive intensive therapies based on advanced age, presence of major medical co-morbidities and marginal performance status. Foremost, investigational therapies should be offered and if not available or feasible, Ven-HMA is our preferred first-line treatment for most patients including those with actionable mutations due to its proven efficacy and favorable safety profile in unfit patients, including octogenarians and nonagenarians [2, 24]. CDD and NG are generally aligned in their treatment approach. The exceptions include IDH1MUT patients, where azacitidine + ivosidenib is considered the preferred approach by CDD [18]. In TP53MUT patients, the addition of Ven does not improve remission or survival, and HMA monotherapy may be considered [25, 26]. The Ven-HMA regimen yields high CR/CRi rates, especially in patients with favorable genetic profiles (CR/CRi in ~90%), with responses occurring within one to two months of treatment initiation [2]. Treatment can be administered in the outpatient setting, provided patients are closely monitored for tumor lysis syndrome during the initial venetoclax ramp-up phase of the first cycle. Early treatment-related mortality remains low ( <5%), and therapy often leads to improvements in performance status which may enable ASCT in a subset of patients [3, 27]. However, the financial and psychosocial burdens associated with ongoing treatment should be considered, as Ven-HMA is not a time-limited therapy and is frequently accompanied by significant myelosupression [21, 28]. In addition, several studies including clinical trials and real-world series have shown that roughly one-third of ND patients with AML patients receiving Ven-HMA fail to respond, and nearly all responders will inevitably relapse, underlining the non-curative nature of this therapy [16, 19, 21, 29]. It should be noted that a subset of especially frail patients with AML are considered unfit for Ven-HMA based on concern for myelosuppression and might benefit from monotherapy with agents targeting FLT3MUT, IDH1/2MUT, NPM1MUT or KMT2Ar (Fig. 2).
Venetoclax-hypomethylating agent therapy in the front-line setting
Ven in combination with HMA received FDA approval in November 2018 for the treatment of ND-AML in patients ineligible for intensive chemotherapy [30, 31]. The pivotal phase-III VIALE-A trial evaluated Ven (400 mg orally for 28 days) and azacitidine (75 mg/m2 S/C or I/V days 1-7), vs. placebo plus azacitidine (n = 145) in patients with ND-AML [2]. Key eligibility criteria included age ≥75 years (median: 76 years; range 49–91), or presence of significant medical comorbidities including treatment-requiring congestive heart failure or ejection fraction <50%, chronic stable angina, impaired pulmonary function (diffusing capacity of the lung for carbon monoxide of <65% or a forced expiratory volume in 1 second of <65%), or an ECOG performance status of 2-3 [2]. Ven-azacitidine demonstrated significantly superior outcomes compared to azacitidine alone with higher CR/CRi rates (66 vs. 28%) and improved median overall survival (14.7 vs. 9.6 months) [2]. Long-term follow-up (median: 43.2 months), identified a subgroup of patients with durable response to Ven-azacitidine [21]. Among patients achieving CR/CRi, the median duration of response was 18.2 months and MRD < 10-3 by flow cytometry was achieved in 42% of evaluable patients. Notably, two-thirds of MRD responders harbored either IDH1/2MUT (33%) or NPM1MUT (33%) [21, 32].
CR/CRi was found to be indispensable for long-term survival and 51% and 29% of patients who achieved CR/CRi were alive beyond 2 and 3 years, respectively [21]. Moreover, survival was superior among responders with negative MRD (median: 34.2 months vs. 19 months in MRD-positive). More than half of responders and long-term survivors (>2 years) harbored either IDH1/2MUT (35%) or NPM1MUT(20%), while, FLT3MUT (11%) and TP53MUT (8%) were less frequent [21].
In long-term follow-up from the VIALE-A study, 52% of patients in CR/CRi experienced disease progression/relapse and had a median survival of 6.8 months, despite the use of salvage therapy in 42% of patients [2, 21]. Also, in the real-world setting, patients who were relapsed/refractory to front-line Ven-HMA had median overall survival of ~ 3 months, moreover survival was exceedingly poor in patients with either TP53MUT, K/NRASMUT or ASXL1MUT (1-year survival <1% vs. 42% in the presence vs. absence of either of these mutations) [33].
Several real-world series have recapitulated the findings from the VIALE-A trial with some differences [16, 19, 34,35,36] (Table 1). In a Mayo Clinic study of 400 patients with ND-AML treated with Ven (median dose: 200 mg daily) in combination with either decitabine (n = 265) or azacitidine (n = 148), 38% patients achieved CR, and 24% achieved CRi, resulting in an overall CR/CRi rate of 62% [19]. In the Mayo analysis, the highest CR/CRi rate (87%) was observed in patients harboring one or more favorable mutations (NPM1, IDH2, DDX41) without unfavorable mutations (TP53, FLT3-ITD, RUNX1) and lowest response (CR/CRi; 44%) in patients with at least one unfavorable mutation and no favorable mutation [19]. Similarly, survival was inferior in patients harboring adverse karyotype, KMT2Ar, TP53MUT, KRASMUT, and IDH2 WT [19]. The above risk factors delineated low, intermediate, and high-risk groups with corresponding median transplant-censored survival of: not reached (3-year survival: 67%), 19.1 (33%), and 7.1 months (0%), respectively [19]. In a separate multicenter retrospective study including 154 Ven-HMA treated patients (77% with ND-AML) that were older than 80 years (range; 80–92), 64% patients received Ven 400 mg, with treatment duration ranging from ≤ 7 days (n = 6, 3.9%), 8-14 (n = 11, 7.1%), 15–21 (n = 8, 5.2%) and > 21 days (n = 116, 75.3%) [24]. CR/CRi was achieved in 73%, with median overall survival of 13.2 months in treatment responders [24]. 30-day and 60-day mortality rates were 8.5% and 17%, respectively [24].
From a practical standpoint, it is critical to optimize Ven dosing schedule during cycle 1, since the majority of patients treated with standard Ven dosing of 28 days experience clinically significant myelosuppression. In the VIALE-A trial, hematologic adverse events including grade ≥3 thrombocytopenia (46%), neutropenia (43%), and febrile neutropenia (43%) were frequently observed [2, 21, 37]. Furthermore, retrospective studies have consistently shown similar efficacy and less toxicity with shorter Ven duration. Accordingly, implementation of an abbreviated Ven dosing schedule during the first cycle (14–21 days) is highly recommended [38]. In a Mayo Clinic study, ND patients with AML received Ven 14 days (n = 40, 15%), 21 days (n = 41, 15%), or 28 days (n = 189, 70%) during cycle 1. CR/CRi rates were similar (68% vs. 66% vs. 62%) and survival was also similar in patients receiving Ven for 14, 21 vs. 28 days, with respective median survival of 18.6, 21.3, and 13.2 months [39]. Furthermore, Ven treatment duration did not appear to influence response rates in patients harboring one or more unfavorable mutations (TP53, FLT-ITD, RUNX1) without favorable mutations (NPM1, IDH2, DDX41) [39]. On the other hand, a non-significantly higher rate of grade 3 or higher infections was documented in patients receiving Ven for 28 vs. 21 vs. 14 days (28% vs. 18% vs. 20%) [39]. Similar findings were recently reported in a comparative analysis of 82 patients with ND-AML receiving azacitidine x 7 days plus Ven x 7 days (7 + 7 regimen) at seven French centers vs. standard dose Ven-HMA in cycle 1 (n = 111 receiving ≥21 days Ven) at MD Anderson Cancer Center [40]. CR/CRi (72% each) and overall survival (11.2 vs. 10.3 months) were comparable, however, early mortality at 8 weeks (6% vs. 16%) and platelet transfusion requirements were lower with abbreviated Ven dosing (62% vs. 77%) [40]. Moreover, Ven duration did not appear to influence overall survival in patients with the “less Ven sensitive” genetics of TP53MUT, FLT3-ITD, KRASMUT, or NRASMUT [40].
Another key question is whether treatment-free remission (TFR) can be achieved with Ven-HMA therapy. A multicentric study from 12 centers of the French Innovative Leukemia Organization (FILO) group and Moffitt Cancer Center, analyzed the outcome of patients in remission who discontinued therapy due to poor tolerance [41]. Among 62 patients with ND-AML, 28 patients stopped Ven-azacitidine and 34 patients continued azacitidine monotherapy and both groups had similar outcomes [42]. At a median follow-up of 23 months, treatment-free survival was 16 months (MRD-negative: 2-year treatment-free survival 80%), and was significantly influenced by number of cycles of treatment [41]. Patients who received more than 10 prior cycles had the longest treatment-free survival, however, a minimum of five cycles was found to be associated with an acceptable TFR (median TFR, not-reached vs. 10 months) [41]. In a separate retrospective comparative analysis of patients in remission for ≥12 months on Ven-based therapy, 55% continued therapy until disease progression, while 45% discontinued treatment (STOP) [43]. Long-term follow-up ( > 5 years) showed median TFR of 45.8 months among the STOP cohort, with >50% of patients still in sustained remission; successful TFR was more likely in patients with NPM1MUT and/or IDH2MUT, in MRD-negative CR, and patients who received ≥12 months of Ven-based therapy, suggesting that TFR is achievable in a subset of patients [43]. Based on the available evidence, we recommend treatment discontinuation be considered in patients who received ≥12 cycles of Ven-based therapy and have achieved MRD-negative CR.
Allogeneic stem cell transplantation (ASCT)
ASCT remains essential for securing long-term survival in AML, and Ven-HMA therapy provides an opportunity to improve performance status and fitness, thereby enabling eligibility for transplant. In a real-world study from the Mayo Clinic, 55 of 400 (13.8%) patients (median age: 69 years) were bridged to ASCT following a median of 3 cycles of Ven-HMA therapy [44]. At the time of transplant, 98% were in CR/CRi or morphologic leukemia-free state. Median post-transplant survival was not reached with 3-year survival of 62%. Thirteen patients (24%) experienced post-transplant relapse and the 3-year cumulative incidence of relapse was 25%, significantly higher in patients with adverse karyotype (80% vs. 17% for non-adverse) [44]. Similarly, in a study of 29 patients receiving ASCT following Ven-HMA, median post-transplant survival was 14.3 months with outcomes comparable to those who were transplanted following intensive chemotherapy [45]. In another study, 21 of 119 (18%) ND non-core binding factor patients with AML ≥ 60 years old, received Ven-azacitidine as initial therapy and underwent ASCT with significantly superior overall survival compared to 31 patients who deferred ASCT (median survival not reached vs. 518 days) [46]. Additionally, among 33 patients treated on Ven-azacitidine clinical trials and bridged to transplant, the median post-transplant survival was 29.9 months and 1-year survival was 76% in MRD-negative patients [47]. The above studies suggest that Ven-HMA can serve as an effective bridging therapy to ASCT and offer long-term survival benefits for patients deemed ineligible for intensive therapies.
FLT3 inhibitors in the front-line setting
Mutations in FMS-like tyrosine kinase 3 receptor (FLT3) involving internal tandem duplication and tyrosine kinase domain occur in ~20% of ND patients with AML ≥ 70 years old, and have differential prognostic impact [48]. FDA-approved oral small molecule FLT3 inhibitors include midostaurin and quizartinib, approved for front-line use in combination with 7 + 3 chemotherapy [49, 50], and gilteritinib which is approved as monotherapy in the relapsed/refractory setting [51]. Additionally, crenolanib remains under clinical investigation [51], and sorafenib (approved for renal and hepatocellular carcinoma) is available for off-label use [52, 53].
The potential benefit of FLT3 inhibitors as part of lower-intensity therapy for unfit patients with AML (reviewed in Table 2) has not been confirmed in randomized studies. In a phase 3 trial of gilteritinib plus azacitidine vs. azacitidine in patients with FLT3MUT AML ineligible for intensive therapy, although CR/CRi rates were higher (58% vs. 27%) in the gilteritinib-azacitidine arm, CR rates (16% vs. 14%) and overall survival were similar (9.8 vs. 8.9 months) [54]. Limited data on the efficacy of quizartinib or sorafenib in combination with HMA exists. In a phase 1/2 trial in patients with FLT3-ITD AML or myelodysplastic syndrome (MDS) unfit for intensive therapy, quizartinib was administered with either azacitidine or low-dose cytarabine (LDAC) based on physician discretion. Among 34 patients that were previously untreated, CR/CRi was documented in 87% (CR in 53%) and 74% (CR in 5%) patients receiving quizartinib-azacitidine, and quizartinib-LDAC, respectively, while grade 3 QTc prolongation occurred in two patients. Median overall survival was numerically better in patients receiving quizartinib with azacitidine vs. LDAC (19.2 vs. 8.5 months). Like other FLT3 inhibitors, sorafenib may be used off-label based on data from two phase 2 studies (NCT01254890 and NCT02196857) which evaluated sorafenib 400 mg twice daily plus azacitidine in unfit patients with FLT3-ITD AML [53]. CR/CRi was achieved in 19 of 27 (70%) patients with median response duration of 14.5 months. Notable toxicities included grade 1/2 hyperbilirubinemia (22%), diarrhea (22%), fatigue (22%), and nausea (19%), and grade 3/4 infections (26%) and neutropenic fever (26%) [53].
A key unanswered question remains: how does the efficacy and tolerability of FLT3 inhibitor-HMA combinations compare to those of Ven-HMA in FLT3MUT AML? In general, presence of FLT3-ITD is associated with lower response to Ven-HMA therapy; in a pooled analysis of FLT3MUT patients treated on Ven-azacitidine clinical trials, CR/CRi rate in patients with FLT3-ITD and FLT3-TKD was 63% and 77% and median overall survival was 9.9 and 19.2 months, respectively [55]. Similarly, in a Mayo Clinic study of ND-AML treated with Ven-HMA, CR/CRi rates were found to be significantly lower (41%) among 39 patients who harbored FLT3-ITD compared to 64% in those without FLT3-ITD [19].
Current efforts to improve outcomes in FLT3MUT AML, leverage the preclinical synergy between FLT3 inhibitors, as well as the promising clinical activity observed with these combinations in the relapsed/refractory setting [56, 57]. The triplet combination of azacitidine, Ven (cycle 1 28 days, cycle 2 days 1–14), and gilteritinib (days 1-28) was investigated in ND unfit patients with AML (median age; 71 years, 73% FLT3-ITD) [58]. Among 30 patients treated in the front-line setting, CR (90%), and CRi (6%) was achieved in 96%, with 93% of patients MRD- negative by flow cytometry [58]. Moreover, responses were durable with only five relapses and 18-month overall survival was 72% [58]. Significant toxicities included grade 3 febrile neutropenia (in 33%), and infection (in 50%) [58]. Similar high rates of efficacy have also been reported with the triplet regimen of decitabine, quizartinib and Ven [59]. Ultimately, results from the phase II MyeloMATCH trial which compares treatment Ven-azacitidine to the combination of Ven-azacitidine and gilteritinib in older and unfit patients with AML and FLT3MUT (NCT06317649), will inform optimal management.
IDH1/2 inhibitors in the front-line setting
Twenty percent of older patients with AML harbor mutations in isocitrate dehydrogenase 1 (IDH1) (6%), or IDH2 (12–15%), typically seen in association with normal karyotype and NPM1MUT [60]. Ivosidenib is an oral targeted FDA-approved agent for ND-IDH1MUT AML as monotherapy or in combination with azacitidine. Results from pivotal clinical trials with IDH1/2 inhibitors in ND-AML are summarized in Table 3.
The clinical activity of ivosidenib monotherapy in ND-AML ineligible for intensive therapy was established through a phase 1 sub-study conducted in 34 patients with IDH1MUT ND-AML (median age: 76.5 years) who received ivosidenib 500 mg daily. CR/CRi was achieved in 42% (CR in 30%), median duration was not reached and 62% remained in remission at 1 year [61]. Major toxicities included differentiation syndrome and QTc prolongation in 9% of patients each [61]. Subsequent preclinical work suggested addition of azacitidine to ivosidenib enhances differentiation and apoptosis, and led to the clinical study of azacitidine-ivosidenib in ND-AML unfit for intensive therapy [62]. A total of 23 patients were treated with ivosidenib-azacitidine combination therapy (median age: 76 years), CR was achieved in 61% (median time to CR: 3.7 months), with IDH1MUT clearance in 71%, and 1-year survival rate of 82% [62]. In terms of treatment-related toxicities, differentiation syndrome and Qtc prolongation were documented in 17% and 26%, respectively [62]. These findings paved the way for a phase 3 trial of ivosidenib-azacitidine (n = 72) vs. placebo-azacitidine (n = 74) in which both CR/CRi rates (54% vs. 16%) and median overall survival (24 vs. 7.9 months) were superior in the ivosidenib-azacitidine arm [18].
Unlike the case with ivosidenib, enasidenib, an inhibitor of mutant IDH2 is not approved for front-line use due to lack of survival advantage over azacitidine monotherapy. In a phase 1/2 investigation in ND-AML harboring IDH2MUT, 39 patients (median age: 77 years) received enasidenib 100 mg daily, CR rate was only 18%; common treatment-related adverse events included indirect hyperbilirubinemia (in 31%), nausea (in 23%), and fatigue, anorexia, and rash (in 18% each) [63]. This was followed by a randomized non-blinded phase 2 study which compared enasidenib-azacitidine (n = 68) with azacitidine alone (n = 33); 57% patients in the enasidenib-azacitidine group compared to 18% patients in the azacitidine monotherapy group achieved CR/CRi, however, overall survival remained similar in both treatment groups (median: 22 vs. 22.3 months) [64]. Treatment-related grade 3 or 4 adverse events were more likely with enasidenib-azacitidine - thrombocytopenia (37% vs. 19% in the azacitidine-only group), neutropenia (37% vs. 25%), and febrile neutropenia (16% vs. 16%) [64]. A separate phase 2/1b study applied a risk-adapted treatment strategy with enasidenib monotherapy for up 5 cycles and in patients who did not achieve CR/CRi by cycle 5 or who progressed, azacitidine was added to enasidenib [65]. CR/CRi was achieved in 40% and 30%, with enasidenib monotherapy (n = 60), and enasidenib + azacitidine (n = 17), respectively [65].
Overall, presence of IDH1/2MUT, particularly IDH2MUT have demonstrated favorable response and survival following Ven-HMA therapy in clinical trial as well as real-word analyses [19, 21]. In a pooled analysis of IDH1/2MUT patients treated on Ven-azacitidine trials, CR/CRi rates were 67% and 86% among IDH1MUT and IDH2MUT cases, respectively [66]. Median overall survival was not reached vs. 15.2 months among IDH2MUT and IDH1MUT mutated cases, respectively [66]. Notably, CR/CRi rates were higher in IDH2R172 (93%) compared to 1DH2R140 (83%).
Preclinical and clinical studies have demonstrated synergy between IDH inhibitors and Ven [67]. Accordingly, Ven-HMA + IDH inhibitor triplet combinations are currently under active investigation [68]. A phase 1b/II study evaluated the safety and efficacy of ivosidenib + Ven, with or without the addition of azacitidine in patients with ND-IDH1MUT AML; CR/CR rate was 83% vs. 90% with doublet vs. triplet combination [69]. Furthermore, responses were deeper and more durable in patients receiving the triplet compared to the ivosidenib-Ven doublet, with flow MRD–negative CR/CRi rates of 75% vs. 50% and a 24-month overall survival of 75% vs. 58% [69]. Also, in a recently published pooled analysis of 60 patients with IDH1/2MUT ND-AML, unfit to receive intensive therapy, treated with IDH inhibitor triplet either ivosidenib-Ven-azacitidine or ivosidenib/enasidenib-oral decitabine-Ven, CR/CRi was achieved in 92%; 2 year overall survival was 69% with cumulative incidence of relapse of 24% [70]. In ND patients with AML who had not received prior HMA or Ven for prior myelodysplastic syndrome, the 2-yr overall-survival was 84% and cumulative incidence of relapse was 20% [70]. A Phase 3 randomized trial (EVOLVE-1) is now enrolling to confirm the benefit of a front-line triplet regimen for IDH1MUTAML.
Menin inhibitors in the front-line setting
KMT2Ar occur infrequently in adult AML (~5%,), and are associated with exceedingly poor survival witth Ven-HMA therapy [71]. In a Mayo clinic study of Ven-HMA treated ND-AML, among 7 of 400 (2%) patients with KMT2Ar, CR/CRi rate was 43% and median overall survival only 2.5 months [19], underlining an unmet need for new therapeutic approaches. Revumenib is a first-in-class menin inhibitor approved for relapsed/refractory KMT2Ar with CR rate OF 17.5% and median OS of 8 months [72]. Recently published results of the combination of revuminib with Ven-azacitidine in the front-line setting, showed CR/CRi in 89% (CR in 78%) patients with KMT2Ar [73]. Moreover, all patients were MRD-negative by flow cytometry [73]. Beyond KMT2Ar, menin inhibitors have also displayed activity in NPM1MUT AML [74], and in the aforementioned study, 79% of patients achieved CR/CRi with CR in 65% [73]. Overall, the triplet combination was deemed safe with differentiation syndrome (in 19%) and Qtc prolongation (in 44%) patients, and a protocol amendment has now reduced the Ven duration per cycle to improve myelosuppression related complications [73]. Other menin inhibitors under development include ziftomenib [75, 76] and bleximenib, being studied in combination with Ven and azacitidine [77]. An all oral combination regimen of revumenib with decitabine/cedazuridine and Ven is also under investigation [78]. Randomized studies of the triplet combination vs. Ven-HMA are planned to evaluate the added benefit of menin inhibition on response rates and survival outcomes.
Concluding remarks
In recent years, there is an acute awareness that treatment response and survival outcomes among unfit patients with ND-AML receiving Ven-HMA are highly heterogeneous and strongly associated with underlying genomic profile. Given that Ven-HMA therapy is not curative in the vast majority of patients, an individualized approach to treatment selection is recommended and ASCT in remission should be strongly considered. We recommend comprehensive NGS testing, or at minimum testing for actionable mutations, with consideration of rapid turnaround times to facilitate timely selection of appropriate therapies. Importantly, overtreatment should be avoided. Recent data suggest that reduced Ven exposure during cycle 1, induces response rates comparable to the standard 28-day dosing, while mitigating toxicity, and without compromising overall survival. A randomized study of 14 vs. 28 days of Ven with azacitidine is ongoing to address this question. Current clinical trials are investigating Ven-HMA in combination with targeted therapies (FLT3, IDH1/2, or menin inhibitors) in patients with actionable abnormalities in order to improve depth and durability of response [58, 69, 70, 73]. These combinatorial strategies may also mitigate on-target, off-tumor toxicity while optimizing the overall benefit-risk ratio [79]. Several randomized trials are ongoing to confirm whether triplet regimens add incremental value (i.e. improvements in response rates and overall survival without incurring additional toxicity) for these various genomic AML subgroups.
References
https://seer.cancer.gov/statfacts/html/amyl.html [Internet].
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid Leukemia. N. Engl J Med. 2020;383:617–29.
Pratz KW, Panayiotidis P, Recher C, Wei X, Jonas BA, Montesinos P, et al. Venetoclax combinations delay the time to deterioration of HRQoL in unfit patients with acute myeloid leukemia. Blood Cancer J. 2022;12:71.
Shimony S, Stahl M, Stone RM. Acute Myeloid Leukemia: 2025 Update on diagnosis, risk-stratification, and management. Am J Hematol. 2025;100:860–91.
Venditti A, Palmieri R, Maurillo L, Röllig C, Wierzbowska A, de Leeuw D, et al. Fitness assessment in acute myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood Adv. 2025;9:2207–20.
Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC, Shami P, et al. Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol. 2017;3:1675–82.
Ferrara F, Barosi G, Venditti A, Angelucci E, Gobbi M, Pane F, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27:997–9.
Palmieri R, Othus M, Halpern AB, Percival MM, Godwin CD, Becker PS, et al. Accuracy of SIE/SIES/GITMO Consensus Criteria for Unfitness to Predict Early Mortality After Intensive Chemotherapy in Adults With AML or Other High-Grade Myeloid Neoplasm. J Clin Oncol. 2020;38:4163–74.
Salas MQ, Salinas-González R, Guardia L, Solano MT, Padilla C, Moreno C, et al. Frailty assessment and outpatient pre-habilitation for adults undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2025;60:841–50.
Bhatt VR, Uy GL, Klepin HD. Determining treatment tolerance and fitness for intensive chemotherapy in older adults with AML: a call to action. Blood. 2024;143:483–7.
Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–94.
Bhatt VR, Wichman CS, Koll TT, Fisher AL, Wildes TM, Haddadin M, et al. A Phase II trial of geriatric assessment-guided selection of treatment intensity in older adults with AML. Am J Hematol. 2025;100:1163–72.
Ritchie EK, Klepin HD, Storrick E, Major B, Le-Rademacher J, Wadleigh M, et al. Geriatric assessment for older adults receiving less-intensive therapy for acute myeloid leukemia: report of CALGB 361101. Blood Adv. 2022;6:3812–20.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Döhner H, Pratz KW, DiNardo CD, Wei AH, Jonas BA, Pullarkat V, et al. Genetic Risk Stratification and Outcomes Among Treatment-Naive Patients With AML Treated With Venetoclax and Azacitidine. Blood. 2024;144:2211–22.
Gangat N, Karrar O, Iftikhar M, McCullough K, Johnson IM, Abdelmagid M, et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: Genotype signatures for response and survival among 301 consecutive patients. Am J Hematol. 2024;99:193–202.
Döhner H, DiNardo CD, Appelbaum F, Craddock C, Dombret H, Ebert BL, et al. Genetic risk classification for adults with AML receiving less-intensive therapies: the 2024 ELN recommendations. Blood. 2024;144:2169–73.
Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and Azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386:1519–31.
Gangat N, Elbeih A, Ghosoun N, McCullough K, Aperna F, Johnson IM, et al. Mayo genetic risk models for newly diagnosed acute myeloid leukemia treated with venetoclax + hypomethylating agent. Am J Hematol. 2025;100:260–71.
Pratz KW, Jonas BA, Pullarkat V, Recher C, Schuh AC, Thirman MJ, et al. Measurable residual disease response and prognosis in treatment-naïve acute myeloid leukemia with venetoclax and azacitidine. J Clin Oncol. 2022;40:855–65.
Pratz KW, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Döhner H, et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am J Hematol. 2024;99:615–24.
Röllig C, Kramer M, Schliemann C, Mikesch J-H, Steffen B, Krämer A, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136:823–30.
Hemminki K, Zitricky F, Försti A, Kontro M, Gjertsen BT, Severinsen MT, et al. Age-specific survival in acute myeloid leukemia in the Nordic countries through a half century. Blood Cancer J. 2024;14:44.
Madarang E, Lykon J, Zhao W, Sekeres MA, Bradley T, Chandhok NS, et al. Venetoclax and hypomethylating agents in octogenarians and nonagenarians with acute myeloid leukemia. Blood Neoplasia. 2024;1:100016.
Badar T, Nanaa A, Atallah E, Shallis RM, Guilherme SdCC, Goldberg AD, et al. Comparing venetoclax in combination with hypomethylating agents to hypomethylating agent-based therapies for treatment naive TP53-mutated acute myeloid leukemia: results from the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND). Blood Cancer J. 2024;14:32.
Patel SA. Managing the unmanageable: evidence-driven approaches to real-world patient prototypes of TP53-mutant myelodysplastic neoplasms and acute myeloid leukemia. Leukemia. 2024;38:2544–51.
Gangat N, Tefferi A. To live is well but to live well is better: Venetoclax combination therapy and quality-of-life in acute myeloid leukemia. Blood Cancer J. 2022;12:75.
DiNardo CD, Pratz KW, Panayiotidis P, Wei X, Vorobyev V, Illés Á, et al. The impact of post-remission granulocyte colony-stimulating factor use in the phase 3 studies of venetoclax combination treatments in patients with newly diagnosed acute myeloid leukemia. Am J Hematol. 2025;100:185–8.
Gangat N, Johnson I, McCullough K, Farrukh F, Al-Kali A, Alkhateeb H, et al. Molecular predictors of response to venetoclax plus hypomethylating agent in treatment-naive acute myeloid leukemia. Haematologica. 2022;107:2501–5.
DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–28.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791–803.
Gangat N, Ilyas R, Johnson IM, McCullough K, Al-Kali A, Alkhateeb HB, et al. Outcome of patients with acute myeloid leukemia following failure of frontline venetoclax plus hypomethylating agent therapy. Haematologica. 2023;108:3170–4.
Bataller A, Bazinet A, DiNardo CD, Maiti A, Borthakur G, Daver NG, et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 2024;8:927–35.
Morsia E, McCullough K, Joshi M, Cook J, Alkhateeb HB, Al-Kali A, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am J Hematol. 2020;95:1511–21.
Gangat N, Johnson I, McCullough K, Farrukh F, Al-Kali A, Alkhateeb H, et al. Molecular predictors of response to venetoclax plus hypomethylating agent in treatment-naïve acute myeloid leukemia. Haematologica. 2022;107:2501–5.
Pratz KW, DiNardo CD, Selleslag D, Li J, Yamamoto K, Konopleva M, et al. Postremission cytopenia management in patients with acute myeloid leukemia treated with venetoclax and azacitidine in VIALE-A. Am J Hematol. 2022;97:E416–e9.
Gangat N, Tefferi A. Venetoclax schedule in AML: 7 vs 14 vs 21 vs 28 days. Blood Cancer J. 2025;15:56.
Karrar O, Abdelmagid M, Rana M, Iftikhar M, McCullough K, Al-Kali A, et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: Comparative analysis of response, toxicity, and survival. Am J Hematol. 2024;99:E63–E6.
Willekens C, Bazinet A, Chraibi S, Bataller A, Decroocq J, Arani N, et al. Reduced venetoclax exposure to 7 days vs standard exposure with hypomethylating agents in newly diagnosed AML patients. Blood Cancer J. 2025;15:68.
Garciaz S, Dumas P-Y, Bertoli S, Sallman DA, Decroocq J, Belhabri A, et al. Outcomes of acute myeloid leukemia patients who responded to venetoclax and azacitidine and stopped treatment. Am J Hematol. 2024;99:1870–6.
Garciaz S, Hospital MA, Alary AS, Saillard C, Hicheri Y, Mohty B, et al. Azacitidine plus venetoclax for the treatment of relapsed and newly diagnosed acute myeloid leukemia patients. Cancers. 2022;14:2025.
Chua CC, Hammond D, Kent A, Tiong IS, Konopleva MY, Pollyea DA, et al. Treatment-free remission after ceasing venetoclax-based therapy in patients with acute myeloid leukemia. Blood Adv. 2022;6:3879–83.
Johnson I, Elbeih A, Ghosoun N, McCullough K, Al-Kali A, Alkhateeb HB, et al. Predictors of post-transplant survival in patients with newly diagnosed acute myeloid leukemia receiving frontline venetoclax and hypomethylating agents. J Clin Oncol. 2025;43:e18523–e.
Winters AC, Bosma G, Abbott D, Minhajuddin M, Jordan C, Pollyea DA, et al. Outcomes are similar after allogeneic hematopoietic stem cell transplant for newly diagnosed acute myeloid leukemia patients who received venetoclax + azacitidine versus intensive chemotherapy. Transpl Cell Ther. 2022;28:694.e1–.e9.
Pollyea DA, Winters A, McMahon C, Schwartz M, Jordan CT, Rabinovitch R, et al. Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transpl. 2022;57:160–6.
Pratz K, Dinardo C, L Arellano M, Thirman M, Pullarkat V, S. Becker P, et al. P525: Long-term outcomes of stem cell transplant in older patients with acute myeloid leukemia treated with venetoclax + HMA therapies. HemaSphere. 2023;7:e68978fe.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl J Med. 2017;377:454–64.
Erba HP, Montesinos P, Kim HJ, Patkowska E, Vrhovac R, Žák P, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1571–83.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-Mutated AML. N Engl J Med. 2019;381:1728–40.
Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121:4655–62.
Ohanian M, Garcia-Manero G, Levis M, Jabbour E, Daver N, Borthakur G, et al. Sorafenib combined with 5-azacytidine in older patients with untreated FLT3-ITD mutated acute myeloid leukemia. Am J Hematol. 2018;93:1136–41.
Wang ES, Montesinos P, Minden MD, Lee J-H, Heuser M, Naoe T, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140:1845–57.
Konopleva M, Thirman MJ, Pratz KW, Garcia JS, Recher C, Pullarkat V, et al. Impact of FLT3 Mutation on Outcomes after Venetoclax and Azacitidine for Patients with Treatment-Naïve Acute Myeloid Leukemia. Clin Cancer Res. 2022;28:2744–52.
Singh Mali R, Zhang Q, DeFilippis RA, Cavazos A, Kuruvilla VM, Raman J, et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica. 2021;106:1034–46.
Daver N, Perl AE, Maly J, Levis M, Ritchie E, Litzow M, et al. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2022;40:4048–59.
Short NJ, Daver N, Dinardo CD, Kadia T, Nasr LF, Macaron W, et al. Azacitidine, venetoclax, and gilteritinib in newly diagnosed and relapsed or refractory FLT3-Mutated AML. J Clin Oncol. 2024;42:1499–1508.
Yilmaz M MM, Short N, Loghavi S, Kadia T, DiNardo C, et al. PHASE I/II Study of decitabine, venetoclax, and quizartinib triplet combination in FLT3-ITD mutated AML. HemaSphere. 2025;9.
Issa GC, DiNardo CD. Acute myeloid leukemia with IDH1 and IDH2 mutations: 2021 treatment algorithm. Blood Cancer J. 2021;11:107.
Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135:463–71.
DiNardo CD, Stein AS, Stein EM, Fathi AT, Frankfurt O, Schuh AC, et al. Mutant Isocitrate Dehydrogenase 1 Inhibitor Ivosidenib in Combination With Azacitidine for Newly Diagnosed Acute Myeloid Leukemia. J Clin Oncol. 2021;39:57–65.
Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33:2575–84.
DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei AH, de Botton S, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22:1597–608.
Cai SF, Huang Y, Lance JR, Mao HC, Dunbar AJ, McNulty SN, et al. A study to assess the efficacy of enasidenib and risk-adapted addition of azacitidine in newly diagnosed IDH2-mutant AML. Blood Adv. 2024;8:429–40.
Pollyea DA, DiNardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, et al. Impact of Venetoclax and Azacitidine in Treatment-Naïve Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin Cancer Res. 2022;28:2753–61.
Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–84.
Cathelin S, Sharon D, Subedi A, Cojocari D, Phillips DC, Leverson JD, et al. Enasidenib-induced differentiation promotes sensitivity to venetoclax in IDH2-mutated acute myeloid leukemia. Leukemia. 2022;36:869–72.
Lachowiez CA, Loghavi S, Zeng Z, Tanaka T, Kim YJ, Uryu H, et al. A Phase Ib/II Study of Ivosidenib with Venetoclax ± Azacitidine in IDH1-Mutated Myeloid Malignancies. Blood Cancer Discov. 2023;4:276–93.
DiNardo CD, Marvin-Peek J, Loghavi S, Takahashi K, Issa GC, Jen WY, et al. Outcomes of Frontline Triplet Regimens With a Hypomethylating Agent, Venetoclax, and Isocitrate Dehydrogenase Inhibitor for Intensive Chemotherapy-Ineligible Patients With Isocitrate Dehydrogenase-Mutated AML. J Clin Oncol. 2025:Jco2500640. https://doi.org/10.1200/JCO-25-00640.
Issa GC, Zarka J, Sasaki K, Qiao W, Pak D, Ning J, et al. Predictors of outcomes in adults with acute myeloid leukemia and KMT2A rearrangements. Blood Cancer J. 2021;11:162.
Issa GC, Aldoss I, Thirman MJ, DiPersio J, Arellano M, Blachly JS, et al. Menin inhibition with revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101). J Clin Oncol. 2025;43:75–84.
Zeidner JF, Lin TL, Welkie RL, Curran E, Koenig K, Stock W, et al. Azacitidine, venetoclax, and revumenib for newly diagnosed NPM1-Mutated or KMT2A-Rearranged AML. J Clin Oncol. 2025:Jco2500914. https://doi.org/10.1200/JCO-25-00914.
Issa GC, Aldoss I, DiPersio J, Cuglievan B, Stone R, Arellano M, et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature. 2023;615:920–4.
Zeidan AM, Fathi A, Issa G, Erba H, Mackey JA, Corum D, et al. Pb1885: Phase 1 Study of Ziftomenib in Combination with Venetoclax, Venetoclax/Azacitidine, or Standard Induction (7 + 3) Chemotherapy in Patients with Acute Myeloid Leukemia. Hemasphere. 2023;7:e56956b1 https://doi.org/10.1097/01.HS9.0000974364.56956.b1.
Wang ES, Issa GC, Erba HP, Altman JK, Montesinos P, DeBotton S, et al. Ziftomenib in relapsed or refractory acute myeloid leukaemia (KOMET-001): a multicentre, open-label, multi-cohort, phase 1 trial. Lancet Oncol. 2024;25:1310–24.
Wei AH RJ, Garciaz S, Aldoss I, Pierola AA, Allred A, et al. RP2D Determination of bleximenib in combination with VEN+AZA: phase 1B study in ND & R/R AML with KMT2A/NPM1 alterations. HemaSphere. 2025;9.
Issa GC, Cuglievan B, Daver N, DiNardo CD, Farhat A, Short NJ, et al. Phase I/II Study of the All-Oral Combination of Revumenib (SNDX-5613) with Decitabine/Cedazuridine (ASTX727) and Venetoclax (SAVE) in R/R AML. Blood. 2024;144:216.
Patel SA. Precision and strategic targeting of novel mutation-specific vulnerabilities in acute myeloid leukemia: the semi-centennial of 7 + 3. Leuk Lymphoma. 2023;64:1503–13.
Author information
Authors and Affiliations
Contributions
NG and CDD wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
NG has served on the Advisory Board for Agios and DISC Medicine. CDD: Consultant/Advisory Boards: Abbvie, AstraZeneca, Astellas, BMS, Genentech, GenMab, GSK, Notable Labs, Rigel, Ryvu, Schrodinger, Servier. CDD is supported by the LLS Scholar in Clinical Research Award.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gangat, N., Dinardo, C.D. Newly diagnosed acute myeloid leukemia in unfit patients: 2026 treatment algorithms. Blood Cancer J. 15, 139 (2025). https://doi.org/10.1038/s41408-025-01346-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-025-01346-1