Abstract
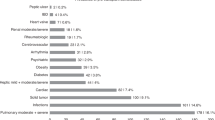
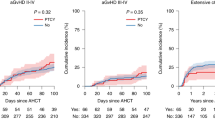
Post-transplant cyclophosphamide (PTCY) is increasingly used as effective graft-versus-host disease (GvHD) prophylaxis in allogeneic hematopoietic-cell transplantation (allo-HCT). However, PTCY is associated with toxicities. Whether patients with specific comorbidities are more vulnerable to cyclophosphamide-induced toxicity is unclear. We retrospectively evaluated the impact of individual organ dysfunctions for non-relapse mortality (NRM) risk and overall survival (OS) among 5888 adults who underwent PTCY-based allo-HCT for acute myeloid leukemia between 2010 and 2023. In multivariable analyses 5 of the comorbidities (renal, moderate/severe hepatic, cardiac including arrhythmia/valvular disease, severe pulmonary, infection) were independently associated with adverse NRM and OS without influencing relapse rate. A simplified model using the absence (n = 4390), presence of 1 (n = 1229) or presence of 2 or 3 (n = 269) of the comorbidities which were determined individually to contribute to NRM stratified patients into 3 NRM risk (16.2% vs. 21.6% vs. 36%, retrospectively) and OS categories (64% vs. 56% vs. 36.4%, retrospectively). In Cox model, recipients with 2 or 3 comorbidities had an increased hazard ratio for NRM of 2.38 (95% confidence interval [CI], 1.89–3) and for OS of 1.96 (95% CI 1.64–2.33). Whether patients with concomitant diagnoses, as determined here, may benefit from a reduced PTCY dose remains to be evaluated in prospective clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data sharing is available through the EBMT ALWP office.
References
Rimando J, McCurdy SR, Luznik L. How I prevent GVHD in high-risk patients: posttransplant cyclophosphamide and beyond. Blood. 2023;141:49–59.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol OncolJ Hematol Oncol. 2018;11:40.
Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–47.
Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol J Am Soc Clin Oncol. 1991;9:1215–23.
Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141:758–63.
Kurauchi K, Nishikawa T, Miyahara E, Okamoto Y, Kawano Y. Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res Notes. 2017;10:406.
Rambaldi B, Kim HT, Reynolds C, Chamling Rai S, Arihara Y, Kubo T, et al. Impaired T- and NK-cell reconstitution after haploidentical HCT with posttransplant cyclophosphamide. Blood Adv. 2021;5:352–64.
Ruggeri A, Roth-Guepin G, Battipaglia G, Mamez A-C, Malard F, Gomez A, et al. Incidence and risk factors for hemorrhagic cystitis in unmanipulated haploidentical transplant recipients. Transpl Infect Dis. 2015;17:822–30.
Mohty R, Brissot E, Battipaglia G, Ruggeri A, Dulery R, Bonnin A, et al. Infectious complications after post-transplantation cyclophosphamide and anti-thymocyte globulin-based haploidentical stem cell transplantation. Br J Haematol. 2019;187:e64–8.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Sorror ML, Logan BR, Zhu X, Rizzo JD, Cooke KR, McCarthy PL, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transpl. 2015;21:1479–87.
Shouval R, Fein JA, Shouval A, Danylesko I, Shem-Tov N, Zlotnik M, et al. External validation and comparison of multiple prognostic scores in allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3:1881–90.
Penack O, Peczynski C, Mohty M, Yakoub-Agha I, de la Camara R, Glass B, et al. Association of pre-existing comorbidities with outcome of allogeneic hematopoietic cell transplantation. A retrospective analysis from the EBMT. Bone Marrow Transpl. 2022;57:183–90.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Shouval R, Fein JA, Cho C, Avecilla ST, Ruiz J, Tomas AA, et al. The simplified comorbidity index: a new tool for prediction of nonrelapse mortality in allo-HCT. Blood Adv. 2022;6:1525–35.
Hahn T, Sucheston-Campbell LE, Preus L, Zhu X, Hansen JA, Martin PJ, et al. Establishment of definitions and review process for consistent adjudication of cause-specific mortality after allogeneic unrelated-donor hematopoietic cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:1679–86.
Fein JA, Shimoni A, Labopin M, Shem-Tov N, Yerushalmi R, Magen H, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia. 2018;32:1787–94.
Farhadfar N, Dias A, Wang T, Fretham C, Chhabra S, Murthy HS, et al. Impact of pretransplantation renal dysfunction on outcomes after allogeneic hematopoietic cell transplantation. Transpl Cell Ther. 2021;27:410–22.
Shouval R, de Jong CN, Fein J, Broers AEC, Danylesko I, Shimoni A, et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol Blood Marrow Transpl. 2018;24:1685–91.
Whelan R, Hingorani S. More than creatinine but less than perfect: challenges of estimated kidney function in HCT patients. Transpl Cell Ther. 2021;27:355–6.
Juma FD. Effect of liver failure on the pharmacokinetics of cyclophosphamide. Eur J Clin Pharm. 1984;26:591–3.
Yeh J, Whited L, Saliba RM, Rondon G, Banchs J, Shpall E, et al. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021;5:5599–607.
Duléry R, Mohty R, Labopin M, Sestili S, Malard F, Brissot E, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncology. 2021;3:250–9.
Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172:384–90.
Coffey DG, Pollyea DA, Myint H, Smith C, Gutman JA. Adjusting DLCO for Hb and its effects on the hematopoietic cell transplantation-specific comorbidity index. Bone Marrow Transpl. 2013;48:1253–6.
Cheng G-S. Pulmonary function and pretransplant evaluation of the hematopoietic cell transplant candidate. Clin Chest Med. 2017;38:307–16.
Hunter MR, Sabo RT, McCarty JM, Newland AM. Effectiveness and toxicity of high-dose cyclophosphamide in obese versus non-obese patients receiving allogeneic hematopoietic stem cell transplant. J Oncol Pharm Pr. 2016;22:54–9.
de Jonge ME, Mathôt RA, van Dam SM, Beijnen JH, Rodenhuis S. Extremely high exposures in an obese patient receiving high-dose cyclophosphamide, thiotepa and carboplatin. Cancer Chemother Pharm. 2002;50:251–5.
Saandeep K, Vikram A, Tripathi DN, Ramarao P, Jena G. Influence of hyperglycaemia on chemical-induced toxicity: study with cyclophosphamide in rat. Basic Clin Pharm Toxicol. 2009;105:236–42.
Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Kanakry CG. Optimized timing of post-transplantation cyclophosphamide in MHC-haploidentical murine hematopoietic cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2020;26:230–41.
McAdams MJ, Hyder M, Dimitrova D, Sadler JL, McKeown C, Steinberg SM, et al. Phase I/II study of reduced dosing of post-transplantation cyclophosphamide (PTCy) after HLA-haploidentical bone marrow transplantation. Blood. 2021;138:101.
Duléry R, Goudet C, Mannina D, Bianchessi A, Granata A, Harbi S, et al. Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malignancies. Bone Marrow Transpl. 2023;58:386–92.
Sugita J, Kamimura T, Ishikawa T, Ota S, Eto T, Kuroha T, et al. Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transpl. 2021;56:596–604.
Duléry R, Malard F, Brissot E, Banet A, Sestili S, Belhocine R, et al. Reduced post-transplant cyclophosphamide dose with antithymocyte globulin in peripheral blood stem cell haploidentical transplantation. Bone Marrow Transpl. 2023;58:1215–22.
Fuji S, Sugita J, Najima Y, Konishi T, Tanaka T, Ohigashi H, et al. Low‐ versus standard‐dose post‐transplant cyclophosphamide as GVHD prophylaxis for haploidentical transplantation. Br J Haematol. 2024;204:959–66.
Juárez A, Salas MQ, Pedraza A, Suárez-Lledó M, Rodríguez-Lobato LG, Solano MT, et al. Reduced dose of post-transplant cyclophosphamide with tacrolimus for the prevention of graft-versus-host disease in HLA-matched donor peripheral blood stem cell transplants: a prospective pilot study. Cancers. 2024;16:2567.
Acknowledgements
We thank the ALWP EBMT staff in the Paris office for data management. The study was accomplished thanks to the contributing centers of the EBMT registry which provided patient data; a complete list appears in the Supplementary Appendix.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the manuscript. AS, ML, FC, MM and BPS contributed to the study conception and design as well as to data analysis, or interpretation. ML provided statistical oversight and guidance. AK, DB, AB, SS, AMR, JV, GC, MR, JS, MIR, PB, AE, YK, EB, AN provided patient data and critical feedback. AS drafted the manuscript and all authors critically revised and approved it. Following EBMT publication rules, co-authorship was offered to centers contributing the highest number of patients.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spyridonidis, A., Labopin, M., Savani, B.P. et al. The impact of individual comorbidities in transplant recipients receiving post-transplant cyclophosphamide. Bone Marrow Transplant 60, 499–506 (2025). https://doi.org/10.1038/s41409-025-02514-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02514-4