Abstract
In this study, we aimed to compare the engraftment days, graft-versus-host disease (GVHD) development, relapse and overall survival rates in patients using myeloablative/reduced intensity conditioning regimens with posttransplant cyclophosphamide (PTCy) 25 mg/kg x2 with Anti-T lymphocyte Globulin (ATLG) (n = 29) and PTCy 50 mg/kg x2 doses (n = 41) in patients with acute leukemias. Matched related, matched unrelated, 1 mismatched unrelated, and haploidentical donors were selected for the patients. Platelet (median 11 vs 17 days) and neutrophil (median 14 vs 15 days) engraftment times were shorter in ATLG+ PTCy25 treated patients (both p < 0.05); veno-occlusive disease rates, graft failure and poor graft functions were similar between the two approaches (all p > 0.05); cumulative incidences of grade II-IV aGVHD at +100 days, grade III-IV aGVHD at +100 days, and grade II-IV cGVHD at 1-year were comparable between ATLG+PTCy25 and PTCy50 groups (all p > 0.05). Cumulative incidences of relapse and non-relapse mortality at 1-year were similar in two cohorts (both p > 0.05). PTCy50 was associated with a statistically significant benefit in terms of GVHD-free/relapse-free survival (GRFS) at 1-year (p = 0.03). Median GRFS was 115 (95% CI: 42–214) days and 248 (95% CI: 151-not reached) days, respectively [HR was 0.51 (0.28–0.95), p = 0.03; GRFS at 1-year was 20.7% vs 44.3%, respectively]. However, the groups were comparable in terms of PFS and OS. Median PFS was 332 days (95% CI: 182 days-not reached) for ATLG+PTCy25 group. It was not reached (95% CI: 210 days-not reached) for the patients who received PTCy50. Median OS was not reached in either ATLG+PTCy25 (95% CI: 191 days-not reached) or PTCy50 groups (Log rank = 0.42). Our study showed that lowering PTCy dose with ATLG seems to accelerate platelet and neutrophil engraftment rates; confers similar survival and relapse rates, similar acute and chronic GVHD frequency despite increased GRFS at 1-year.
Similar content being viewed by others
Introduction
Despite recent advances, allogeneic stem cell transplantation remains the only curative option for many hematological malignancies today [1]. However, the procedure is not without risk; many infectious and immunological complications, like graft-versus-host disease (GVHD), may arise [2]. GVHD is divided into acute and chronic, mainly based on the timing of occurrence. Acute GVHD (aGVHD) incidence varies among reports, ranging between 10–80% [3]. Like aGVHD, chronic GVHD (cGVHD) frequency differs markedly among publications, ranging 25–80% [4]. Given the problem’s relatively high frequency, many approaches have been suggested to mitigate its effects on outcomes. Cyclophosphamide (Cy) is one of the pivotal agents for aGVHD prophylaxis. It has unique characteristics and effects on T-cells, especially T-regulatory cells [5]. Induction of T-regulatory cells, along with myeloid-derived suppressor cells by the Cy, maintains immune tolerance and prevents tissue damage. Cy is broadly administered post-transplantation on days +3 and +4 [5]. Despite its success in preventing GVHD, Cy is not without severe toxicities and side effects [6]. The most dreadful complication is hemorrhagic cystitis, which is seen in nearly one-quarter of the patients who received post-transplantation Cy (PTCy) [6]. To circumvent these complications, dose reductions with additional immunosuppressive agents were proposed [7]. Anti-T lymphocyte globulin (ATLG) is a polyclonal antibody preparation used in solid organ and marrow transplants. The addition of the ATLG to the conditioning regimen has been shown to decrease aGVHD rates and increase survival [8, 9].
There is a need for clinicians to improve the transplantation outcomes such as increased survival, decreased the relapse, GVHD, and cytomegalovirus (CMV) infection rates [10]. There is no universally accepted standard conditioning regimen and GVHD prophylaxis approaches due to the heterogeneity of the hematologic neoplasms and the transplantation biology itself [11]. As PTCy50-based prophylaxis has gained widespread use in allogeneic stem cell transplantation across different donor types due to its efficacy in preventing GVHD. However, PTCy50 has been associated with undesirable toxicities, including delayed engraftment and increased susceptibility to infections. This underscores the importance of exploring whether reducing the PTCy dose can achieve comparable GVHD prevention while minimizing transplant-related toxicities [12,13,14].
In the literature, no study compares PTCy 25 mg/kg x2 with ATLG (ATLG+PTCy25) to PTCy 50 mg/kg x2 without ATLG (PTCy50). Therefore, in this study, we aimed to compare the engraftment days, GVHD development, relapse and overall survival (OS) rates in patients using myeloablative/reduced intensity conditioning (MAC/RIC) regimens with ATLG+PTCy25 and PTCy50 without ATLG in patients with acute leukemias.
Subjects and methods
Subjects
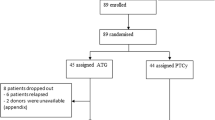
We conducted this multicenter retrospective non-randomized study and analyzed 70 patients who received their first transplantation for acute leukemias in their first remission and had PTCy dosing of 25 mg/kg x2 plus ATLG (ATLG+PTCy25, n = 29) or PTCy dosing of 50 mg/kg x2 without ATLG (PTCY50, n = 41) between 2018 and 2024.
All patients included in the study were 18 and older. Exclusion criteria included patients with incomplete or missing key information, such as treatment details, engraftment data, GVHD status, relapse data, or survival outcomes. Patients were also excluded if they did not receive the specified conditioning regimens (myeloablative or reduced intensity) or if they were administered PTCy or ATLG doses outside the defined protocols. Additional exclusions applied to patients with other hematologic or non-hematologic malignancies outside the study criteria, those who had undergone prior allogeneic hematopoietic stem cell transplantation, and those treated with experimental therapies, investigational drugs, or adjunctive treatments not included in the study protocol (e.g., post-transplant maintenance therapies). Furthermore, patients who received stem cells from sources not specified in the study (e.g., umbilical cord blood or ex vivo T-cell–depleted grafts) were excluded.
Our center, Ankara Oncology Hospital, has standard operation procedures using ATLG for all conditioning regimens, and all of the patients receive PTCy regardless of the donor type. Patients’ data were obtained from this center who have had ATLG+PTCy25. For comparisons, PTCy50 patients’ data were obtained from the other two centers, Istanbul and Inonu Medical Faculties. All the patients had cardiology, pulmonology, gynecology (for females), psychiatry, and dental evaluation for transplant preparation.
Ethics approval and consent
Ethical approval was obtained from Ankara Oncology Training and Research Hospital in accordance with the Declaration of Helsinki (Approval number: 2024-12/200), and written informed consents were obtained from all patients authorizing the use of their data for research purposes prior to the transplantation.
Donor selection
Matched related, one mismatched related, matched unrelated, one mismatched unrelated, and haploidentical donors were selected for the patients. Firstly, the patients underwent transplantation if they had matched or one mismatched related donor; if there were no matched or one mismatched related donor, donor search was initiated from the national donor pool named TURKOK, a Ministry of Health-based organization. Unrelated donors were selected if a matched unrelated or one mismatched unrelated donor was found from the TURKOK pool. If no donor is found at the TURKOK, then patients undergo haploidentical transplantation.
Conditioning regimens and transplantation procedure
All of the laboratory values obtained prior to the initiation of the conditioning regimen and peripheral collected stem cells were used for all transplants. All patients received MAC or RIC conditioning regimens according to the EBMT definitions [15, 16]. Fludarabine 150 mg/m2, busulfan 12.8 mg/kg, treosulfan 42 gr/m2, fractionized 12 Gray total body irradiation (TBI) for MAC and fludarabine 150 mg/m2, busulfan 6.4 or 9.6 mg/kg, treosulfan 30 or 36 gr/m2, fractionized 8 Gray TBI were administered for RIC regimens. Fludarabine/Busulfan/ATLG, Fludarabine/Treosulfan/ATLG for AML and Fludarabine/TBI/ATLG combinations for ALL were given in patients for ATLG + PTCy 25 cohort; Fludarabine/Busulfan, Fludarabine/Treosulfan for AML and Fludarabine-TBI combinations for ALL were given in patients for PTCy 50 cohort.
For ATLG + PTCy 25 cohort, ATLG (Grafalon) was given −5 to −3 at a total dose of 15 (5 mg/kg x3) mg/kg for matched related donor and 30 (10 mg/kg x3) mg/kg days (total 3 days) for unrelated or haploidentical donors based on our center’s standard operation procedures. All transplants were performed using the doses of PTCy 25 or 50 mg/kg x2 on days +3 and +4. G-CSF was given to all patients starting on day +5 at 5 µg/kg/day until absolute neutrophil count recovery.
Definitions
Neutrophil engraftment was defined as a measurable neutrophil count >0.5 × 109/L for 3 consecutive days. Primary graft failure was defined as failure to recover neutrophil count by day +28 post-HSCT without evidence of relapsed disease. Secondary graft failure was defined as graft failure occurring after either initial partial or complete engraftment and was depicted by recurrent pancytopenia with a neutrophil count <0.5 × 109/L without evidence of relapsed disease. Patients with 100% host chimerism were considered to have graft rejection, and all others had graft failure [17].
Non-relapse mortality (NRM) was defined as deaths not related to relapse. Graft-versus-host relapse-free survival (GRFS) was described as the time elapsed between transplantation and aGVHD, cGVHD, relapse, death, or last contact. Progression-free survival (PFS) was estimated as the time between transplantation, relapse, death, or last contact. OS was defined as the time between the transplantation and death or last contact.
GVHD prophylaxis and assessment
Based on standard operation procedure in our center, cyclosporine alone was administered for GVHD prophylaxis for ATLG+PTCy25 cohort; whereas cyclosporine plus mycophenilate mophetil, cyclosporine, or tacrolimus alone was administered for PTCy50 cohort. The physician confirmed the diagnosis of aGVHD based on clinical, radiologic, and laboratory results. The Mount Sinai Acute GVHD International Consortium (MAGIC) standards were used to assess the staging and grading of aGVHD [18, 19]. National Institutes of Health (NIH) consensus criteria were used for the diagnosis and staging/grading of cGVHD [20].
Endpoints
Primary endpoint of this analysis was GRFS. Secondary endpoints included OS, PFS, cumulative incidence functions (CIF) of grade II-IV aGVHD at day +100, grade III-IV aGVHD at day +100, relapse at 1-year, and NRM at 1-year.
Sample size calculation
The sample size was calculated to compare 1-year GRFS between the PTCy50 group (50% GRFS) and the ATLG+PTCy25 group (20% GRFS). The following assumptions were applied: Type I error (α): 0.05 (two-tailed), power (1-β): 80%, and allocation ratio: 1:1 (N1 = N2). Using the exponential assumption, HR = ln(S2)/ln(S1). S1 is 0.5 (50%), S2 is 0.2 (20%). So ln(0.2)/ln(0.5) ≈ −1.609/−0.693 ≈ 2.32.
Using Schoenfeld’s formula to find the number of events needed, the formula is D = (Zα/2 + Zβ)^2 / (ln(HR))^2 * φ(1-φ), where φ is the proportion in each group (0.5 for equal allocation) [21]. Zα/2 for two-tailed 0.05 is 1.96, Zβ for 0.80 power is 0.84. A total of 45 events are needed.
Statistical analysis
IBM SPSS Statistics version 26 and Stata 14.2 for Mac (StataCorp, TX, USA) were used for statistical analyses. Continuous variables were expressed as medians and interquartile ranges, whereas categorical variables were expressed as numbers and percentages. Kolmogorov-Smirnov test was applied to test for data distribution. Independent Sample T test, Mann Whitney U, and Pearson Chi Square/Fisher Exact tests were used for numerical and categorical data for comparisons, respectively. Competitive risk analyses were performed for all CIF calculations. In the CIF analyses of grade II-IV aGVHD at day +100, grade III-IV aGVHD at day +100, and grade II-IV cGVHD at day +100, relapse and death were regarded as the competitive risk factors. On the other hand, death was the only competitive risk factor for CIFs of relapse at 1-year and NRM at 1-year. Patients who did not experience failure event within 100 days and 1-year for the cumulative analyses of grade II-IV aGVHD, grade III-IV aGVHD, and grade II-IV cGVHD; and CIFs of relapse and NRM, GRFS, PFS, and OS, respectively, were censored. Fine-Gray’s test was performed to determine the differences in the CIFs between groups. Confidence intervals (CI) were used as an estimate of variation within each group. Sub-distributional, cause-specific hazard ratios (HR) with corresponding CI were presented. Survival function was assessed by the Kaplan Meier method. Groups were compared by the Log-rank test. Variance was assessed indirectly via the proportional hazards assumption. HRs with CIs were estimated by Cox proportional regression analyses. A two-sided p-value of ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
The demographical and clinical features of the patients’ data are shown in Table 1. In ATLG+PTCy25 cohort; 14 (48.3%) patients had AML, 15 (51.7%) had ALL. They had median 1-line treatment prior to the transplantation. Three patients had RIC regimen and 26 patients had MAC. Patients who received RIC regimens had AML, and all of the patients’ ages were over 50 years. Of patients who received MAC regimens, 11 had AML and 15 had ALL; 4 AML patients’ ages were over 50 years, and none of the ALL patients’ ages were over 50 years.
In PTCy50 cohort, 31 (75.6%) patients had AML, 10 (24.4%) had ALL. They had also median 1-line treatment prior to the transplantation. Thirteen patients had RIC regimen and 28 patients had MAC. Twelve patients who received RIC regimens had AML, and only 1 had ALL, and all of the RIC patients’ ages were also over 50 years old. Of patients who received MAC regimens, 19 had AML and 9 had ALL; 2 AML patients’ and 1 ALL patient’s ages were over 50 years.
Engraftment and graft failure information
In ATLG+PTCy25 cohort and PTCy 50 cohort, the median platelet engraftment times were 11 days and 17 days; median neutrophil engraftment times were 14 days and 15 days, respectively. Platelet and neutrophil engraftment were more rapid in ATLG+PTCy25 cohort, and these were statistically significant (p < 0.001 and p = 0.027, respectively). Also, there was no engraftment syndrome in PTCy50 cohort, whereas 13.8% of patients (n = 4) in ATLG+PTCy25 cohort had engraftment syndrome. In ATLG+PTCy25 cohort, 3.4% (n = 1) of the patients had secondary graft failure (age: 58, AML and had MAC regimen with Fludarabine/Treosulfan/ATLG) whereas 2.4% (n = 1) of PTCy50 cohort patients had primary graft failure (age: 36, AML and had MAC regimen with Fludarabine/Busulfan). In addition, 10.3% of ATLG+PTCy25 cohort (n = 3; 1 primary, 2 secondary) and 19.5% of PTCy50 cohort patients (n = 8; 3 primary, 5 secondary) had poor graft function after the transplant. Both poor graft function and engraftment failure were similar across groups (both p > 0.05) (Table 2).
Study endpoints
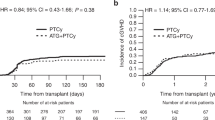
aGVHD and cGVHD patients’ characteristics are presented in Tables 3 and 4. Cumulative incidences of grade II-IV aGVHD at +100 days, grade III-IV aGVHD at +100 days, and grade II-IV cGVHD at 1-year were comparable between ATLG+PTCy25 and PTCy50 groups (Table 5, Figs. 1–3). Cumulative incidences of relapse and NRM at 1-year were similar in patients who received ATLG+PTCy25 and PTCy50 (Table 5, Figs. 4 and 5).
PTCy50 was associated with a statistically significant benefit in terms of GRFS at 1 year. Median GRFS was 115 (95% CI: 42–214) days and 248 (151-NR) days, respectively. HR was 0.51 (0.28–0.95), p = 0.03. GRFS at 1-year was 20.7% vs 44.3%, respectively (Table 5, Fig. 6). However, the groups were comparable in terms of PFS and OS. Median PFS was 332 days (95% CI: 182 days-not reached) for ATLG+PTCy25 group. It was not reached (95% CI: 210 days-not reached) for the patients who received PTCy50. Median OS was reached in neither ATLG+PTCy25 (95% CI: 191 days-not reached) nor PTCy50 groups (Table 5, Figs. 7 and 8).
Other complications
The CIFs of post-transplantation CMV reactivation at 100 days were comparable between the groups. However, there was a trend for higher CIFs of post-transplantation CMV reactivation at 1-year in PTCy50 group [(76.0% (60.4–88.9%) vs 51.8% (33.4–73.1%), p = 0.06 (Table 2, supplementary Figs. 1 and 2)]. Veno-occlusive disease rates were similar across groups (Table 2).
Discussion
This is the first study investigated ATLG+PTCy25 versus PTCy50 in patients with acute leukemias, and the main findings of our study were; (i) platelet and neutrophil engraftment times were shorter for ATLG+PTCy25 treated patients, (ii) engraftment syndrome was just seen in ATLG+PTCy25 patients, (iii) VOD rates, graft failures and poor graft functions were similar between two approaches, (iv) CIFs of grade II-IV aGVHD at +100 days, grade III-IV aGVHD at +100 days, and grade II-IV cGVHD at 1-year were comparable between groups, (v) post-transplantation CMV reactivation was comparable between the groups, (vi) CIFs of relapse and non-relapse mortality at 1-year were similar in two cohorts, (vii) PTCy50 was associated with a significant benefit in terms of GRFS at 1 year, (viii) PFS and OS rates were similar across groups.
There are few reports in the literature regarding the use PTCy with anti-thymocyte globulin (ATG) and engraftment kinetics. Zu et al. evaluated the addition of the ATG to PTCy, aiming for decreased doses of Cy and ATG while preserving efficacy in matched unrelated donors. In this trial, PTCy 20 mg/kg x2 plus 1.5 mg/kg dose ATG resulted in faster platelet and neutrophil engraftment [22]. Wang et al. demonstrated that lower doses of ATG did not affect platelet engraftment time in haploidentical hematopoietic stem cell transplants [23]. Our study used ATLG instead of ATG and resulted in faster platelet and neutrophil engraftment compared to the PTCy50 cohort.
Transplantation success is affected mainly by post-transplant events, like viral infections and endothelium-originated adverse events, like VOD. Goldsmith et al. proposed increased CMV infection risk with PTCy in seropositive patients [13]. In a retrospective study, Tischer et al. compared PTCy 50 mg/kg x2 dose with rabbit ATG at a dose of 20 mg/kg for 5 days. Authors concluded that prophylaxis with ATG caused a marked increase in CMV reactivation [24]. Dulery et al reported that a reduced PTCy dose of 40 mg/kg x2 does not mean reduced CMV infection rates [25]. Tian et al. found no statistical difference between ATLG and ATG regarding the CMV viremia [26]. Conflicting knowledge in literature that ATG/ATLG increased CMV infections; our data showed post-transplantation CMV reactivation at 100 days was comparable between two cohorts. However, there was a trend for higher CIFs of post-transplantation CMV reactivation at 1 year in PTCy50 group.
Issues related to graft health significantly affect survival and morbidity rates. Engraftment syndrome complicates the acute phases of the transplant course and has the potential to be a life-threatening complication. In a review article, the year of the transplant and CNI-based GVHD prophylaxis were regarded as the most important determinants for engraftment syndrome [27]. Combination prophylaxis with low-dose PTCy and ATLG led to more engraftment syndrome in our cohort; this is the first-ever reported data in this setting. Further data is needed to confirm this correlation and the mechanism responsible for this finding also needs to be explained.
Reducing GVHD rates while improving tolerability and limiting toxicities could be regarded as the main target of a prophylaxis regimen. Prem et al. demonstrated lower acute and chronic GVHD rates when 50 mg/kg x2 dosing of PTCy combined with ATG at a total dose of 4.5 mg/kg [28]. In a phase 2 trial conducted by Yang et al., patients received 1 day 50 mg/kg dose of PTCy in combination with ATG. This trial published the first results of combined lower dose Cy and ATG and yielded encouraging results with lower acute and chronic GVHD rates, improved engraftment, and less viral reactivation [29]. Zhang et al. further improved the regimen by relocating the ATG at a dose of 2.5 mg/kg to +8 days of transplant and PTCy 40 mg/kg/d on +3 and +4 days. Compared with the standard 10 mg/kg dosing of ATG, the novel regimen resulted in lower acute grade 2–4 GVHD in haploidentical transplants [30]. Xue et al. introduced a regimen that contains the post-transplant 5th day of ATLG in combination with PTCy +3 and +4 days dose of 50 mg/kg. This regimen significantly decreased non-severe cGVHD but aGVHD rates did not significantly reduce compared with PTCy alone [31]. In our study, we found that CIFs of grade II-IV aGVHD at +100 days, grade III-IV aGVHD at +100 days, and grade II-IV cGVHD at 1-year were comparable between groups.
Another success of PTCy is reduced relapse rates, particularly in haploidentical transplants. Two studies evaluating the role of PTCy in haploidentical settings showed similar relapse and survival rates to calcineurin (CNI)-based GVHD prophylaxis [32, 33]. Additionally, some studies have shown lower relapse risk with PTCy compared to CNI-based GVHD prophylaxis [34, 35]. A retrospective study of Gallardo-Perez et al. revealed similar relapse rates with 25 mg/kg x2 dose of PTCy compared to the standard dosing scheme [36]. Adding ATLG to PTCy doesn’t seem to impact survival and relapse in matched-related and unrelated donor peripheral blood-derived stem cell transplant [31]. In our study, dose reduction in PTCy and addition of ATLG did not lead to more CIFs of relapse and non-relapse mortality at 1 year both.
Certain limitations should be considered like retrospective non-randomized study design, relatively small number of the patients, and the follow-ups of the both ATLG+PTCy25 and PTCy50 cohorts were short.
In conclusion, our study compared lower-dose PTCy plus ATLG with standard dose PTCy. Lowering PTCy dose with ATLG seems to accelerate platelet and neutrophil engraftment rates; confers similar survival and relapse rates, similar acute and chronic GVHD frequency despite increased GRFS at 1-year. The results should be interpreted with caution and using/choosing these different regimens should be considered based on the patients’ clinical and disease characteristics prior to the transplantation. The evidence gap is broad in this field, and further high-quality data and performing prospective randomized clinical trials are paramount for validating and implementing lower PTCy dose plus ATLG to routine care.
Data availability
Requests for materials on reasonable request should be addressed to Turgay Ulas.
References
Abedin S, Hamadani M. Contemporary updates in the prevention and treatment of graft-versus-host disease. Curr Hematol Malig Rep. 2024;19:246–55. https://doi.org/10.1007/s11899-024-00741-y
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18:1150–63. https://doi.org/10.1016/j.bbmt.2012.04.005
Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. Haemato-oncology task force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. https://doi.org/10.1111/j.1365-2141.2012.09129.x
Min CK. The pathophysiology of chronic graft-versus-host disease: the unveiling of an enigma. Korean J Hematol. 2011;46:80–7. https://doi.org/10.5045/kjh.2011.46.2.80
Nunes NS, Kanakry CG. Mechanisms of graft-versus-host disease prevention by post-transplantation cyclophosphamide: an evolving understanding. Front Immunol. 2019;10:2668 https://doi.org/10.3389/fimmu.2019.02668
Ibrahim M, Bhyravabhotla K, Khalaf B, Van Meter K, Saba NS, Safah H, et al. The utility of hyperbaric oxygen therapy in post-transplant cyclophosphamide-induced hemorrhagic cystitis: a case report and review of the literature. J Med Case Rep. 2021;15:1 https://doi.org/10.1186/s13256-020-02580-w
Juárez A, Salas MQ, Pedraza A, Suárez-Lledó M, Rodríguez-Lobato LG, Solano MT, et al. Reduced dose of post-transplant cyclophosphamide with tacrolimus for the prevention of graft-versus-host disease in HLA-matched donor peripheral blood stem cell transplants: a prospective pilot study. Cancers. 2024;16:2567 https://doi.org/10.3390/cancers16142567
Valdez-Ortiz R, Bestard O, Llaudó I, Franquesa M, Cerezo G, Torras J, et al. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transplant Int. 2015;28:108–19. https://doi.org/10.1111/tri.12448
Admiraal R, Nierkens S, Bierings MB, Bredius RGM, van Vliet I, Jiang Y, et al. Individualised dosing of anti-thymocyte globulin in paediatric unrelated allogeneic haematopoietic stem-cell transplantation (PARACHUTE): a single-arm, phase 2 clinical trial. Lancet Haematol. 2022;9:e111–e120. https://doi.org/10.1016/S2352-3026(21)00375-6
Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020;188:129–46. https://doi.org/10.1111/bjh.16355
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e159. https://doi.org/10.1016/S2352-3026(23)00342-3
Nakamae H. Graft-versus-tumor effect of post-transplant cyclophosphamide-based allogeneic hematopoietic cell transplantation. Front Immunol. 2024;15:1403936 https://doi.org/10.3389/fimmu.2024.1403936
Goldsmith SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P, et al. Posttransplant cyclophosphamide is associated with increased cytomegalovirus infection: a CIBMTR analysis. Blood. 2021;137:3291–305. https://doi.org/10.1182/blood.2020009362
Al Malki MM, Tsai NC, Palmer J, Mokhtari S, Tsai W, Cao T, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv. 2021;5:2650–9. https://doi.org/10.1182/bloodadvances.2021004192
Jethava YS, Sica S, Savani B, Socola F, Jagasia M, Mohty M, et al. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transplant. 2017;52:1504–11. https://doi.org/10.1038/bmt.2017.83
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55:1114–25. https://doi.org/10.1038/s41409-020-0803-y. Erratum in: Bone Marrow Transplant. 2020; 55(6): 1213 10.1038/s41409-020-0835-3
Llaurador G, Nicoletti E, Prockop SE, Hsu S, Fuller K, Mauguen A, et al. Donor-host lineage-specific chimerism monitoring and analysis in pediatric patients following allogeneic stem cell transplantation: influence of pretransplantation variables and correlation with post-transplantation outcomes. Transplant Cell Ther. 2021;27:780.e1–780.e14. https://doi.org/10.1016/j.jtct.2021.05.020
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10. https://doi.org/10.1016/j.bbmt.2015.09.001
Malard F, Huang XJ, Sim JPY. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia. 2020;34(May):1229–40. https://doi.org/10.1038/s41375-020-0804-2
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001
Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503.
Zu Y, Li Z, Gui R, Liu Y, Zhang Y, Yu F, et al. Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial. Bone Marrow Transplant. 2022;57:1573–80. https://doi.org/10.1038/s41409-022-01754-y
Wang Y, Liu QF, Lin R, Yang T, Xu YJ, Mo XD, et al. Optimizing antithymocyte globulin dosing in haploidentical hematopoietic cell transplantation: long-term follow-up of a multicenter, randomized controlled trial. Sci Bull. 2021;66:2498–505. https://doi.org/10.1016/j.scib.2021.06.002
Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol. 2015;94:1677–88. https://doi.org/10.1007/s00277-015-2423-y
Duléry R, Goudet C, Mannina D, Bianchessi A, Granata A, Harbi S, et al. Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malignancies. Bone Marrow Transplant. 2023;58:386–92. https://doi.org/10.1038/s41409-022-01908-y
Tian Z, Man Q, Yang Y, Guan H, Wang Y, Luo R, et al. Comparison of rabbit ATLG and ATG for GVHD prophylaxis in hematological malignancies with haploidentical hematopoietic stem cell transplantation. Ann Hematol. 2024;103:1729–36. https://doi.org/10.1007/s00277-024-05724-w
Spitzer TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50:469–75. https://doi.org/10.1038/bmt.2014.296
Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low rates of acute and chronic GVHD with ATG and PTCy in matched and mismatched unrelated donor peripheral blood stem cell transplants. Eur J Haematol. 2019;102:486–93. https://doi.org/10.1111/ejh.13230
Yang J, Jiang J, Cai Y, Li S, Wan L, Zhu J, Liu H, et al. Low-dose anti-thymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transplant. 2019;54:1049–57. https://doi.org/10.1038/s41409-018-0382-3
Zhang W, Gui R, Zu Y, Zhang B, Li Z, Zhang Y, et al. Reduced-dose post-transplant cyclophosphamide plus low-dose post-transplant anti-thymocyte globulin as graft-versus-host disease prophylaxis with fludarabine-busulfan-cytarabine conditioning in haploidentical peripheral blood stem cell transplantation: A multicentre, randomized controlled clinical trial. Br J Haematol. 2023;200:210–21. https://doi.org/10.1111/bjh.18483
Xue E, Lorentino F, Lupo Stanghellini MT, Giglio F, Piemontese S, Clerici DT, et al. Addition of a single low dose of anti T-lymphocyte globulin to post-transplant cyclophosphamide after allogeneic hematopoietic stem cell transplant: a pilot study. J Clin Med. 2022;11:1106 https://doi.org/10.3390/jcm11041106
Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–47. https://doi.org/10.1182/blood-2015-09-671834
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40. https://doi.org/10.1182/blood-2015-04-639831
Marco-Ayala J, Sanz J, Gómez-Seguí I, Balaguer-Rosello A, Montoro J, Guerreiro M, et al. Impact of post-transplantation cyclophosphamide on transfusion requirements in HLA-matched sibling peripheral blood stem cell transplantation. Transplant Cell Ther. 2023;29:313.e1–313.e10. https://doi.org/10.1016/j.jtct.2023.01.009
Maurer K, Ho VT, Inyang E, Cutler C, Koreth J, Shapiro RM, et al. Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD. Blood Adv. 2023;7:3903–15. https://doi.org/10.1182/bloodadvances.2023009791
Gallardo-Pérez MM, Gutiérrez-Aguirre CH, Olivares-Gazca JC, Ruiz-Argüelles GJ. More about post-transplant cyclophosphamide in haploidentical grafts: full or reduced doses? Hematology. 2024;29:2313357 https://doi.org/10.1080/16078454.2024.2313357
Author information
Authors and Affiliations
Contributions
AK, MSD, TU, FA designed the study; AK, TT, TU wrote the manuscript; AK, TT, TU, MSD revised the manuscript; TT, TU performed the statistical analysis; AD, UH, MAK, IYH, TOT, DI, BUU, TNY, MAE were the principal investigators at the centers recruiting the highest number of patients for the study; all authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Karakus, A., Toptas, T., Dal, M.S. et al. Anti-T lymphocyte globulin plus posttransplant cyclophosphamide 25 mg/kg versus posttransplant cyclophosphamide 50 mg/kg in patients with acute leukemias. Bone Marrow Transplant 60, 864–872 (2025). https://doi.org/10.1038/s41409-025-02564-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02564-8