Abstract
A total of 6767 first hematopoietic cell transplants (HCT), 4121 autologous (61%) and 2646 allogeneic (39%), were reported by 166 teams from 12 Latin American countries that answered the 2022 LABMT/WBMT activity survey. The transplant rate (TR) for Latin America in 2022 was 103 HCT/10 million inhabitants with a wide variation between the different countries. The main indication for allogeneic (allo)-HCT was acute lymphoblastic leukaemia (41%) for the pediatric population and acute myeloid leukemia (32%) for adults. The main indication for autologous (auto)-HCT was neuroblastoma (33%) in children and plasma cell disorders (57%) in adults. In alloHCT, the most used hematopoietic cell source was the bone marrow (54%) in pediatric while peripheral blood stem cells (PBSC) (87%) was in adults. PBSC was the source of choice for autoHCT in both ages. The main trends observed in the period 2019-2022 was a decrease in the number of procedures in 2020 in association with the start of the COVID-19 pandemic, resuming growth in the following years. AlloHCT had a greater growth compared to autoHCT, and it was mainly driven by the utilization of haploidentical related donors, which became the main source from 2020 onwards.
Similar content being viewed by others
Introduction
Hematopoietic stem cell transplantation (HCT) is an established procedure for the treatment of hematological diseases, solid tumors, bone marrow failure syndromes, hemoglobinopathies, inborn errors of metabolism and immunity [1, 2]. The Latin American Bone and Marrow Transplantation Society (LABMT) has the mission of promoting excellence in HCT, stem cell donation, cellular therapy and accreditation in Latin America [3]. Through the Global Transplant Activity (GTA) survey of the Worldwide Network for Blood & Marrow Transplantation (WBMT) [4], LABMT collects data on underlying diagnosis, phase of the disease, types of transplants, indications, donor and graft sources of hematopoietic cells in Latin America. LABMT has previously presented the transplant activity in the 2009–2012 and 2012–2018 periods showing the numbers and trends of HCT in the region [5, 6].
Here we present the third report of HCT activity in Latin America using the WBMT electronic Global Transplant Registry (eGTR: https://gtr.nulyse.com) with data collected from the 2019-2022 period. We report activity data for 2022 as well as trends during 2019–2022. For the first time, the data are reported on adult and pediatric populations.
Subjects and Methods
Study design
This was a retrospective, observational study that included first HCT performed in Latin America in the period 2019–2022. Fifteen countries have known HCT activity in Latin America: Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Ecuador, Guatemala, México, Panama, Paraguay, Peru, Uruguay and Venezuela. All HCT teams were invited by the LABMT to report their activity through the WBMT eGTR. Individual center data were collected with the WBMT GTR survey reporting sheet (available at www.wbmt.org) or with the web-based eGTR that captured information on first transplants performed per year. Information was grouped at a country level in an online database and then extracted for analysis by the WBMT and LABMT.
Definitions
For the first time, HCT were divided according to patient´s age. Adults were considered as those 18 years or older at HCT and pediatric patients those less than 18 years at HCT. HCT were classified as allogeneic or autologous. Allogeneic donors were separated into family members and unrelated donors (URD). URD included HCT from matched or mismatched unrelated donors. Family related donors were divided in HLA-identical siblings and syngeneic (both grouped as familial HLA identical matched related donors or Fam HLA-id MRD) and familial HLA non-identical family donors (Fam HLA non id). The vast majority of the “Fam HLA non id” correspond to haploidentical donors (haplo) although there are certain cases that fall into this category and do not correspond to haplo (e.g., siblings with 1 mismatch due to crossing over). The LABMT/WBMT database does not discriminate this small proportion of Fam HLA non id cases other than haplos. However, this data is collected in the Brazilian registry and during the period 2020–2022 the number of HLA non id not falling into the category of “haplo” was only 3% (personal communication from Anderson Simione, Data Manager, SBTMO registry- Sociedade Brasileira de Terapia Celular e Transplante de Medula Óssea). Therefore, to emphasize that the largest proportion of the group “Fam HLA non id” corresponds to haplo, we indicate this in parentheses.
Stem cell sources were bone marrow (BM), peripheral blood stem cells (PBSC), and cord blood (CB). Indications for HCT were stratified according to underlying disease and disease status at transplant. Disease definitions followed the European Society for Blood and Marrow Transplantation (EBMT) List of Disease Classifications [7]. Population data for each country and the region were obtained from the United Nations’ World Population Prospects [8].
Transplant rates (TR) were computed as the number of HCT per 10 million inhabitants and were not normalized for the population age distribution [9].
The number of transplants reflects first HCT only. Information on additional HCT was not included.
Health and Socio-Economic parameters for the year 2022 (or the closest year if no data was available for 2022) were obtained from the World Bank [10], the World Health Organization [11] and the Economic Commission for Latin America and the Caribbean [12]. Indicators were compared with the 2022 TR of each country by simple linear regression analysis with least squares (R2 test method) with Microsoft Excel v16.92.
Ethics approval and consent to participate
As a polled registry data, no individual patient information was reported and thus, no ethical approval or informed consent was necessary.
Study outcomes
The primary study endpoint was to determine the numbers of first HCT reported in 2022 in each age group and its characteristics (types of transplants, main indications, donor types and stem cell sources). A secondary endpoint was to evaluate the correlation of health and socio-economic indicators with HCT activity (TR) in each country in 2022. Other secondary endpoints were to evaluate trends in the numbers of autologous (autoHCT) and allogeneic (alloHCT) as well as trends in donor types for alloHCT from 2019 to 2022.
Results
The survey was sent during 2022 to the 246 known transplant centers in 15 Latin American countries: Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Ecuador, Guatemala, México, Panama, Paraguay, Perú, Uruguay and Venezuela.
Responses were obtained from 166 transplant centers in 12 countries. Three countries did not answer the survey (Costa Rica, Ecuador and Guatemala). Guatemala has recently started HCT activity.
For each country, the percentage of transplant centers that reported to the survey out of the total number of centers in the country was determined. They were grouped into > 80% of reporting centers (Argentina, Bolivia, Brazil, Cuba, Paraguay, Peru, and Uruguay); between 30% and 80% (Colombia, Chile, Mexico, and Venezuela), and < 30% (Panama). Since the largest national centers responded to the survey, we estimate that we have captured more than 80% of the total transplant activity in Latin America. The participating centers in each country are listed in the “ACKNOWLEDGEMENTS/PARTICIPATING CENTERS” section.
Transplant activity in 2022
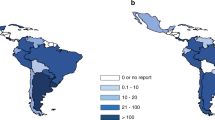
The total number of HCT reported was 6767 of which 2646 were alloHCT (39%) and 4121 were autoHCT (61%). (Supplementary Table S1) The total number of pediatric HCT was 1121 of which 914 were alloHCT (82%) and 207 were autoHCT (18%). (Supplementary Table S2) The total number of adult HCT was 5646 of which 1732 were alloHCT (31%) and 3914 were autoHCT (69%). (Supplementary Table S3) For an estimated Latin American population of 654,148,000 the TR in 2022 was 103 HCT/10 million population (63 TR autoHCT and 40 TR alloHCT).
Table 1 shows the TR and numbers in the different Latin American countries.
Indications for HCT in 2022
The main indications for alloHCT in the pediatric population were acute lymphoblastic leukemia (ALL) n = 379 (41%), acute myeloid leukemia (AML) n = 143 (16%) and bone marrow failure - severe aplastic anemia (BMF-SAA) n = 166 (18%) (Fig. 1a). The main indications for autoHCT were neuroblastoma n = 68 (33%), Hodgkin´s lymphoma (HD) n = 53 (26%) and other solid tumors n = 35 (17%) (Fig. 1b).
The main indications for alloHCT in adults were AML n = 550 (32%), ALL n = 520 (30%) and myelodysplastic neoplasms (MDS) n = 174 (10%) (Fig. 2a). Main indications for autoHCT were plasma cell disorders (PCD) n = 2243 (57%), non-Hodgkin lymphoma (NHL) n = 715 (18%), HD n = 574 (15%) and autoimmune diseases (AID) n = 250 (6%) (Fig. 2b).
Hematopoietic cell source in 2022
The main stem cell source for pediatric alloHCT was the BM n = 494 (54%), followed by PBSC n = 383 (42%) and CB n = 37 (4%). For autoHCT it was mainly PBSC n = 200 (97%). The main stem cell source for alloHCT in adults was PBSC n = 1511 (87%) followed by BM n = 220 (13%). AutoHCT were performed almost exclusively with PBSC n = 3 905 (99.8%). The few CB were almost exclusively used in the pediatric population n = 37 and were mostly from unrelated donors n = 32.
Donor source for alloHCT in 2022
The main transplant type for alloHCT (including BM and PBSC but excluding CB) was Fam HLA non id (haplo) n = 1256 (48%), followed by HLA-id MRD n = 908 (34%) and URD n = 444 (17%); additionally, n = 38 patients (1%) received CB HCT. In the pediatric population the main transplant type (BM and PBSC) resulted Fam HLA non id (haplo) n = 449 (49%), followed by URD n = 217 (24%) and HLA-id MRD n = 211 (23%); n = 37 were CB (4%). Similar was the distribution in adults with Fam HLA non id (haplo) n = 807 (47%) followed by HLA-id MRD n = 697 (40%) and URD n = 227 (13%).
Correlation of health and Socio-Economic indicators with HCT activity in 2022
Figure 3 shows the scatter diagram of the gross domestic product at purchasing power parity (GDP PPP) per capita and TR among 9 of the 12 reporting countries (for this indicator, Cuba and Venezuela have no data and Panama was excluded as an outlier, probably due to underreporting). A strong positive linear association between these two variables was found (R2 = 0.73; F = 13; p = 0.01). Other variables that were assessed in relation to TR were: universal health coverage index, hospital beds per 10,000 inhabitants, medical doctors per 10,000 inhabitants, life expectancy at birth, health spending as a percentage of gross domestic product, health expenditure per capita, cost of HCT, index cost of HCT/per capita gross national income (GNI), poverty and extreme poverty. Tables and graphics as well as definitions and data sources for each indicator are depicted in Supplementary Figure S1.
HCT hematopoietic cell transplantation, LABMT Latin American Bone and Marrow Transplantation Society, TR Transplant Rate (number of HCT per 10 million population), GDP gross domestic product, PPP purchasing power parity, International $ International dollar (An international dollar would buy in the cited country a comparable amount of goods and services a U.S. dollar would buy in the United States).
Trends in the period 2019–2022
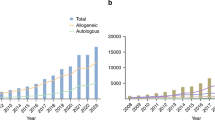
A total of 23,206 first HCT (14,237 autologous and 8969 allogeneic, pediatric and adult) were reported to the LABMT through the WBMT GTA survey in the period 2019–2022. The total number of first HCT during this period was as follows: n = 5841 in 2019, decreased in 2020 to n = 4859 and increased again in 2021 to n = 5739 and in 2022 to n = 6767. This represents an increase of 16% in 2022 as compared to 2019 (Fig. 4a).
a Numbers of autologous, allogeneic and total HCT reported to LABMT in 2019–2022. b Donor sources for first allogeneic HCT in the period 2019–2022. HCT hematopoietic cell transplantation, LABMT Latin American Bone and Marrow Transplantation Society, Fam HLA-id MRD human leukocyte antigen identical matched-related donor (HLA identical sibling and syngeneic), Fam HLA non id (haploidentical and other mismatched family donors), URD unrelated donor, CB cord blood, PB peripheral blood, BM bone marrow.
After a decrease of autoHCT from 2019 (n = 3736) to 2020 (n = 2932), there was a continuous annual increase in 2021 (n = 3448) and 2022 (n = 4121) of a total of 10% for the period 2019-2022. The number of alloHCT showed a less pronounced decrease from 2105 in 2019 to 1927 in 2020 and then recovered to 2291 in 2021 and 2646 in 2022, showing a total increase of 26% from 2019 to 2022 (Fig. 4a).
The trends in donor sources for alloHCT are depicted in Fig. 4b. While in 2019 the main donor source was HLA-id MRD, transplants from Fam HLA non id (haplo) were the predominant source from 2020 onwards. It was the only donor source without a decrease during the COVID-19 pandemic. During 2019-2022, Fam HLA non id (haplo) increased from 772 to 1256 transplants showing a 63% increase, HLA-id MRD from 834 to 908 (9%) while URD decreased from 472 to 444 (-6%).
Discussion
HCT numbers continue to increase in all world regions with Latin America having an intermediate TR, between the most developed regions of the world and those with lower TR [13, 14]. The 2019–2022 survey data confirm this growing trend and also the upward trend compared to previous LABMT publications [5, 6], although caution should be exercised when comparing with previous publications since they were carried out with the same tool (WBMT/LABMT survey) but with some differences in methodology.
For Latin America, similar to what is observed worldwide, there is a predominance of auto over alloHCT in the general population, but this difference is only observed in adults, while in the pediatric population alloHCT predominates over autoHCT. This is explained because the main indication for transplant in children is acute leukemia (ALL and AML), while in adults it is plasma cell disorders (PCD), mainly multiple myeloma (MM), followed by lymphomas (NHL and HL). PCD remains the main indication for autoHCT in the region. In previous LABMT reports, MM accounted for 48% in 2012 and 50% in 2018. This indication has increased especially in Latin America compared to other world regions, but it is still far from the rates observed in Europe and North America, clearly indicating that the number of procedures should be higher to guaranty an equal access around the world [15].
The role of upfront autoHCT in MM has been a matter of debate since the introduction of novel therapeutic regimens that achieve high response rates. However, comparative studies with or without autoHCT show an advantage particularly in terms of progression free survival establishing that this is the preferred option in eligible patients [16,17,18,19,20,21]. Therefore, until challenged by clinical trials, PCD/MM is expected to remain one of the main indications for autoHCT in Latin America. Furthermore, autoHCT is available in most countries in the region while accessibility to novel drugs and specific cellular therapies is limited due to lack of availability and their high cost [22, 23].
The second indication for autoHCT is lymphomas. The indication for autoHCT in lymphomas, particularly in relapsed/refractory diffuse large B-cell lymphomas (DLBCL) has recently been challenged by the utilization of chimeric antigen receptor (CAR) -T cell therapy, bispecific antibodies and other novel treatments [24,25,26] and the increasing utilization of CAR-T cells may at least partially explain the decrease in the indication for autoHCT in DLBCL observed in North America and Europe [27, 28]. However, this is not the case in our region where the access to these therapies is still incipient. In Latin America, facilitating access to new therapies in lymphomas such as CAR-T cells is of great importance. The first treatments have been implemented in Brazil and there are projects under development in other Latin American countries, expecting that this will be an area of important development in the coming years. On the other hand, new therapies are available in the region for Hodgkin lymphoma, improving results on first line treatment as brentuximab, pembrolizumab and nivolumab, which might spare patients from getting an autoHCT. Increased availability of imaging studies, particularly PET/CT, may also have an impact on the number of patients referred to autoHCT in the region. Therefore, the trend in lymphoma HCT will have to be observed in the coming years, highlighting that until 2022, the observed trend continued to show an increase in absolute numbers although with a progressive reduction in the percentage compared to other indications (Supplemental Figure S2).
As in previous LAMBT publications, the number of autoHCT for autoimmune conditions continues to increase (there were 159 procedures reported in 2018 and there are 263 reported in 2022) [29].
For alloHCT acute leukemias are the main indication both in pediatrics and in adults. Similarly to other indications, absolute numbers of alloHCT for acute leukemias (AML and ALL, pediatric and adults) are reported more frequently over time (1324 in 2018 to 1592 in 2022). The percentage of acute leukemias among the total number of alloHCT remained unchanged over time (60% in 2022).
The use of HCT in AML has been addressed recently and the utilization in the region remains low, estimated to be 4.5% in 2016, far from what was observed in Europe (17.9%) and North America (18.4%) during the same year. This indicates that there is still much room for growth in this indication [30].
Between 2019 and 2022 there was a marked decrease in HCT numbers during the year 2020 (−17% compared to 2019). As expected, this can be attributed to the COVID-19 pandemic and the limitations that were imposed to the centers; in some cases, limiting the activity only to urgent procedures or in extreme cases with situations that forced the temporary closure of transplant units to focus the hospital’s activity and resources on responding to the pandemic. The decline of the activity during 2020 compared to 2019 was more pronounced in autoHCT (−22%) compared to alloHCT (−8%). This difference corresponds mainly to a drop in the main indication for autoHCT in the region, which, as mentioned above, represents PCD/MM. It should be noted that in many Latin American countries, HCT activity was limited in non-urgent pathologies or situations, and this particularly affected the number of transplants in MM (-29%), and to a lesser extent, in lymphomas (-9%), which were deferred, or the transplant indication was replaced by other therapeutic modalities. However, in allogeneic transplantation, where most of the indications are urgent and correspond to acute leukemia, activity was not affected (−0,6%). Transplant activity recovered during 2021 compared to 2019 and continued to increase in 2022 (Supplementary Figure S2).
It is noteworthy that in alloHCT, the decrease was observed in HLA-id MRD and URD, however, Fam HLA non id (haplo) continued to increase even during the pandemic year. Fam HLA non id (haplo) surpassed HLA-id MRD as the main donor source since 2020 and was the first source for both pediatric and adult alloHCT in 2022. This is probably due to the affordability and easy access of cyclophosphamide, the lack of effective local unrelated donor registries in most Latin-American countries and the high costs, longer waiting time and regulatory and administrative burden associated with international donor procurement in an environment of increased access to family donors due to the population pyramid and socio-demographic differences common to low and middle-income regions in comparison to North-America or Europe.
Finally, to better understand the population’s accessibility to transplantation, we analyzed TR. For the year 2022, the TR in Latin America was 103 HCT/10 million inhabitants; although an increasing trend observed, there was a wide variation between countries. This different TR distribution has several causes with a significant impact associated with socio economic parameters [31,32,33]. The analysis of the TR per country in 2022 with health and socio-economic indicators shows that the situation remains unchanged and is depicted in Supplementary Figure S1. There is a strong correlation between the GDP PPP as well as the average cost per HCT with the TR of Latin American countries. Another socioeconomic variable that was positively associated with an increase in TR was the expense of medical care per capita, while a weak negative correlation was associated with the percentage of the population living in poverty or extreme poverty in the country. Interestingly, equity in the distribution of the country’s income expressed by the Gini coefficient does not seem to have an impact on the TR in our region. The number of medical doctors per 10,000 inhabitants showed a weak positive correlation with the TR of the country, but no significant correlation was established between the TR and other parameters that evaluate the performance of health systems, such as the universal health coverage index, life expectancy at birth, the number of beds per inhabitants or the spending in health expressed as a percentage of the GDP. The affordability of the transplant in each country can be evaluated with the ratio of cost of HCT to GNI per capita. Since the GNI per capita is disproportionately lower in low- and middle- income compared to high-income countries, this index is useful to estimate the economic impact of the cost of the procedure in each country [34]. However, in Latin America, this index does not seem to have a significant influence on TR.
In summary, the wealth of the country relative to its inhabitants, expressed by GDP per capita, seems to be a significant determinant of TR, probably because it allows for higher health expenditure per individual (in absolute values) and particularly, it allows for higher payment per transplant procedure, which is strongly associated with higher TR. Therefore, among the positive impacts of improved economic conditions in countries, an increase in HCT activity and greater access to the procedure by the population can be expected.
It is relevant to acknowledge some of the limitations of this study. First, it relies on survey data, meaning the results can vary significantly depending on the number of centers responding and their accuracy in reporting. Although all Latin American countries were invited, the study has not included all of them, which limits the generalization of our findings. Furthermore, given that the study involves low- and middle-income countries, it is important to consider that resource constraints heavily influence both decision-making and the types of procedures performed and reported in this study. However, the findings are remarkable since the most active HCT centers were included, providing a relevant overview of the practices in Latin America. This study helps to recognize what has been accomplished with the available resources, offering valuable insight into regional healthcare and HCT strategies.
In conclusion, the 2019–2022 LABMT activity survey confirms the sustained growth in transplant numbers and rates in the Latin American region, only decreased during 2020 in association with the COVID-19 pandemic. For the first time, data are shown separately for pediatric and adult populations, highlighting differences in the use of autologous or allogeneic HCT, indications and graft sources. Of note, in both adult and pediatric HCT in Latin America, there is a particularly high use of Fam HLA non id donors (haplo) for alloHCT.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–39.
Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26:1247–56.
Latin American Bone Marrow Transplantation Society - LABMT. https://www.wbmt.org/member-societies-of-wbmt/labmt/ Accessed 05 May 2024.
Worldwide Network for Blood & Marrow Transplantation - WBMT. https://www.wbmt.org Accessed 05 May 2024.
Jaimovich G, Martinez Rolon J, Baldomero H, Rivas M, Hanesman I, Bouzas L, et al. Latin America: the next region for haematopoietic transplant progress. Bone Marrow Transplant. 2017;52:671–7.
Correa C, Gonzalez-Ramella O, Baldomero H, Basquiera AL, Baena R, Arcuri L, et al. Latin American Bone Marrow Transplantation Group (LABMT); Worldwide Network for Blood and Marrow Transplantation (WBMT). Increasing access to hematopoietic cell transplantation in Latin America: results of the 2018 LABMT activity survey and trends since 2012. Bone Marrow Transplant. 2022;57:881–8.
List of Disease Classifications - EBMT: https://www.ebmt.org/sites/default/files/2023-09/EBMT%20List%20of%20disease%20classifications%20v1.0.pdf Accessed 05 May 2024.
United Nations - World Population Prospects 2022. https://population.un.org/wpp/Download/Standard/Population/ Accessed 05 May 2024.
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Worldwide Network of Blood and Marrow Transplantation. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–24.
World Bank Open Data, World Bank Group: https://data.worldbank.org/indicator Accessed 21 December 2024.
The Global Health Observatory, World Health Organization: https://www.who.int/data/gho/data/indicators. Accessed 05 March 2025.
Statistical Databases and Publications – CEPALSTAT, Economic Commission for Latin America and the Caribbean (ECLAC): https://statistics.cepal.org/portal/cepalstat/index.html Accessed 05 March 2025.
Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022;107:1045–53.
Atsuta Y, Baldomero H, Neumann D, Sureda A, DeVos JD, Iida M, et al. Continuous and differential improvement in worldwide access to hematopoietic cell transplantation: activity has doubled in a decade with a notable increase in unrelated and non-identical related donors. Haematologica. 2024 May 9. https://doi.org/10.3324/haematol.2024.285002.
Cowan AJ, Baldomero H, Atsuta Y, Mikhael J, Aljurf M, Seber A, et al. The Global State of Hematopoietic Cell Transplantation for Multiple Myeloma: An Analysis of the Worldwide Network of Blood and Marrow Transplantation Database and the Global Burden of Disease Study. Biol Blood Marrow Transplant. 2020;26:2372–7.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. IFM 2009 Study. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376:1311–20.
Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7:e456–e468.
Gay F, Musto P, Rota-Scalabrini D, Bertamini L, Belotti A, Galli M, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22:1705–20.
Yong K, Wilson W, de Tute RM, Camilleri M, Ramasamy K, Streetly M, et al. Upfront autologous haematopoietic stem-cell transplantation versus carfilzomib-cyclophosphamide-dexamethasone consolidation with carfilzomib maintenance in patients with newly diagnosed multiple myeloma in England and Wales (CARDAMON): a randomised, phase 2, non-inferiority trial. Lancet Haematol. 2023;10:e93–e106.
de Moraes Hungria VT, Martínez-Baños DM, Peñafiel CR, Miguel CE, Vela-Ojeda J, Remaggi G, Duarte FB, et al. Multiple myeloma treatment patterns and clinical outcomes in the Latin America Haemato-Oncology (HOLA) Observational Study, 2008-2016. Br J Haematol. 2020;188:383–93.
Pessoa de Magalhães Filho RJ, Crusoe E, Riva E, Bujan W, Conte G, Navarro Cabrera JR, et al. Analysis of Availability and Access of Anti-myeloma Drugs and Impact on the Management of Multiple Myeloma in Latin American Countries. Clin Lymphoma Myeloma Leuk. 2019;19:e43–e50.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022;386:640–54.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med. 2022;386:629–39.
Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141:1675–84.
Passweg JR, Baldomero H, Ciceri F, de la Cámara R, Glass B, Greco R, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: a report from the EBMT. Bone Marrow Transplant. 2024;59:803–12.
D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–e182.
Murrieta-Álvarez I, Cantero-Fortiz Y, León-Peña AA, Olivares-Gazca JC, Priesca-Marín JM, Ruiz-Delgado GJ, et al. The 1000th Transplant for Multiple Sclerosis and Other Autoimmune Disorders at the HSCT-México Program: A Myriad of Experiences and Knowledge. Front Neurol. 2021;12:647425.
Tokaz MC, Baldomero H, Cowan AJ, Saber W, Greinix H, Koh MBC, et al. An Analysis of the Worldwide Utilization of Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Transplant Cell Ther. 2023;29:279.e1–279.e10.
Gale RP, Seber A, Bonfim C, Pasquini M. Haematopoietic cell transplants in Latin America. Bone Marrow Transplant. 2016;51:898–905.
Jaimovich G, Gale RP, Hanesman I, Vazquez A, Hammerschlak N, Simoes BP, et al. The paradox of haematopoietic cell transplant in Latin America. Bone Marrow Transplant. 2021;56:2382–8.
Okamoto S, Iida M, Hamad N, Duarte FB, Sureda A, Srivastava A, et al. American Society of Transplantation and Cellular Therapy International Affair Committee: Report of the Third Workshop on Global Perspective to Access to Transplantation at the 2022 Tandem Meeting. Transplant Cell Ther. 2023;29:410–7.
Rizvi SS, Rondelli D. An Analysis and Comparison of the Cost of Blood and Marrow Transplantation (BMT) As a Factor of Gross National Income (GNI) in Low-Middle Income Countries and High-Income Countries: Key Takeaways Regarding Global Access to BMT. Transplantation and Cellular Therapy, Volume 31, Issue 2, Supplement, 2025, Page S325.
Acknowledgements
LIST OF PARTICIPATING CENTERS: ARGENTINA: Centro de Hematología y Trasplante, Rosario; Clínica Conciencia, Neuquén; Fundación Favaloro, CABA; FUNDALEU, CABA; Hospital Alemán, Bs As; Hospital Británico, Bs As; Hospital de Clínicas José de San Martín, CABA; Hospital de Niños Santísima Trinidad, Córdoba; Hospital de Niños Sor María Ludovica La Plata, Bs As; Hospital de Niños Víctor J Vilela, Rosario; Hospital de Pediatría Juan P Garrahan, CABA; Hospital El Cruce Florencia Varela, Bs As; Hospital Interzonal General de Agudos Prof. Dr. Rodolfo Rossi La Plata, Bs As; Hospital Italiano de Buenos Aires. Unidad de Adultos. CABA; Hospital Italiano de Córdoba, Córdoba; Hospital Italiano de San Justo, Unidad de Adultos. Bs As; Hospital Privado Universitario de Córdoba, Córdoba; Hospital Ramón Madariaga, Misiones; Hospital San Martín de La Plata, Bs As; Hospital Universitario Austral, Pilar, Bs As; Instituto Alexander Fleming, CABA; Instituto de Trasplantes de Alta Complejidad, CABA; Instituto Médico de Alta Complejidad, Salta; Sanatorio Allende, Córdoba; Sanatorio Sagrado Corazón, CABA; Sanatorio Anchorena, CABA. BRAZIL: AC Camargo; CEPON; Complexo Hospitalar de Niterói; DF Star; Einstein; Fundação Centro Médico de Campinas; GPACI; GRAAC; HC-FMRP-USP; Hopital Universitário Professor Edgar Santos, Universidade Federal da Bahia; Hopital Vera Cruz; Hospital A Beneficência Portuguesa de São Paulo; Hospital Amaral Carvalho; Hospital Beneficiente de Senhoras - Hospital Sírio Libanês; Hospital Brasília; Hospital Brigadeiro; Hospital Casa Hospital do Câncer; Hospital Cassems; Hospital da Criança de Brasília; Hospital das Clínicas - FMUSP; Hospital das Clínicas da Universidade Federal de Minas Gerais; Hospital de Amor; Hospital de Base da Fundação Faculdade Regional de Medicina de São José do Rio Preto; Hospital de Clínicas da UFPR; Hospital de Clínicas de Itajubá; Hospital de Clínicas de Porto Alegre; Hospital de Clínicas de Uberlândia; Hospital dos Fornecedores de Cana de Piracicaba; Hospital e Maternidade Brasil - Rede D’Or São Luiz; Hospital e Maternidade São Luiz Itaim; Hospital Erastinho; Hospital Icaraí; Hospital Israelita Albert Einstein; Hospital Leforte Liberdade; Hospital Luxemburgo; Hospital Miguel Soeiro - Unimed Sorocaba; Hospital Moinhos de Ventos; Hospital Monte Klinikum; Hospital Monte Sinai; Hospital Naval Marcílio Dias; Hospital Nossa Senhora das Graças - IP; Hospital Nove de Julho; Hospital Pequeno Príncipe; Hospital Real Portugues; Hospital Regional da Unimed; Hospital Rio Grande; Hospital Samaritano; Hospital Santa Genoveva; Hospital Santa Joana; Hospital Santa Rita de Cássia; Hospital Santos Dummont - Unimed São José dos Campos; Hospital São Lucas-RJ; Hospital São Lucas - Aracaju; Hospital São Rafael; Hospital Saúde da Mulher; Hospital Sírio Libanês Brasília; Hospital Universitário Clementino Fraga Filho; Hospital Universitário de Londrina - UEL; Hospital Universitário de Santa Maria; Hospital Universitário Pedro Ernesto - UERJ; Hospital Universitário Walter Cantídio; Hospital Vila Nova Star; IBCC Oncologia; Institutito Nacional do Cancer (INCA); Instituto da Criança - HCFMUSP; Instituto de Cardiologia e Transplantes do Distrito Federal; Núcleo de Hematologia e Transplante de Medula Óssea; Real Sociedade Portuguesa de Beneficência; Santa Casa de Montes Claros; Santa Casa de Porto Alegre; São Camilo - Mooca; São Camilo - Pompéia; São Camilo - Santana; UNESP; União Oeste Paranaense de Estudos e Combate ao Câncer; UNICAMP; UNIFESP; UNIMED - Volta Redonda; Universidade Federal de Juiz de Fora; Universidade Federal do Triângulo Mineiro. BOLIVIA: Los Olivos; Oncoclinic; Instituto Boliviano de Oncohematología. CHILE: Hospital Del Salvador (adults); Dr. Luis Calvo Mackenna; Catholic University of Chile (pediatrics); Clínica Alemana; Clínica Las Condes; Hospital Regional Valdivia; Clínica Dávila (adults); Clínica Dávila (pediatrics). COLOMBIA: Fundación HOMI Hospital de la Misericordia; Centro Médico Imbanaco; FOSCAL-UNAB; Instituto de Cancerología, Clínica Las Américas; Clínica Nogales; Hospital San Vicente Fundación. CUBA: Hospital Ameijeiras; Instituto de Hematología e Inmunología; Hospital CIMEQ; Hospital Arnaldo Milián; Hospital Lucía Íñiguez. MEXICO: Hospital Angeles Acoxpa; Hospital Angeles Chihuahua; Hospital Angeles Lomas; Hospital Angeles Puebla; Centro Medico Nacional 20 de Noviembre; Centro Médico del Noreste; Doctors Hospital Auna; Centro HOPE; Hospital Central Chihuahua; Hospital Central Militar; Hospital Regional de Alta Especialidad del Bajio; Hospital Juarez de México; Hospital Regional de Alta Especialidad de Ixtapaluca; Hospital Infantil De Oncologia HITO Teleton; Instituto Nacional de Pediatria; Centro Médico Nacional Siglo XXI; Centro Médico Nacional La Raza; IMSS - Unidad Médica de Alta Especialidad No. 25; INCMNSZ-Instituto Nacional de Ciencias Médicas y Nutrición; Centro Medico Nacional Manuel Ávila Camacho IMSS; Hospital Universitario Dr José Eleuterio Gonzalez; Hospital Puerta de Hierro Andares; Hospital Infantil Federico Gomez; Clinica Gomez Almaguer; Clinica Ruiz - Centro de Hematología y Medicina Interna. PANAMA: Caja Seguro Social. PERU: Hospital Almanzor Aguinaga Asenjo; Hospital Edgardo Regabliati Martins; Hospital Guillermo Almenara Irigoyen; Instituto Nacional de Enfermedades Neoplásicas; Instituto Nacional de Salud del Niño San Borja. PARAGUAY: Instituto de Previsión Social; Hospital de Clínicas; Hospital Pediátrico Niños de Acosta Ñu. URUGUAY: Fundación Pérez Scremini, Hospital Británico, CITMO-Servicio Médico Integral, Hospital Maciel. VENEZUELA: Ciudad Hospitalaria Dr. Enrique Tejera.
Author information
Authors and Affiliations
Contributions
SG conceived the analysis and drafted the manuscript. CB, AG-D, AS, CC, HB, DN, ALB, AA, JP, BP, MS, JMH, CH, FGM, NM, AW, MH, DN and CF were responsible for the data collection and assembly. AK, GJ, GB, MB, GR, FB, AS, FB, DG-A, GR-A, MLB, CP and DR were responsible for the integrity of the data and gave scientific input.
Corresponding author
Ethics declarations
Competing interests
Andrés Gómez-De León: Honoraria: Janssen, Sanofi, Abbvie, Astellas, Amgen, BMS, Novartis. Advisory board: Pfizer, Janssen.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Galeano, S., Bonfim, C., Karduss, A. et al. Results of the Latin American Bone Marrow Transplantation Society (LABMT) activity survey 2019-2022: the impact of the COVID-19 pandemic and the increase in related haploidentical donors. Bone Marrow Transplant 60, 971–977 (2025). https://doi.org/10.1038/s41409-025-02600-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02600-7