Abstract
Children with relapsed or refractory (R/R) mature B-cell non-Hodgkin lymphoma (B-NHL) have a poor prognosis with approved therapies. Chimeric antigen receptor (CAR)-T cells are approved for adults with R/R B-NHL, but pediatric data is lacking. We report on 13 children with R/R mature B-NHL enrolled on a clinical trial for CD19 CAR-T cells harboring CD28 costimulation. Twelve patients were infused with CAR-T cells, and one had progressed and died prior to infusion. Toxicities included cytokine release syndrome in 8 patients and neurotoxicity in 6, including two patients with grade 4 neurotoxicity. All patients responded to CAR-T cells, including a complete response in 6, complete metabolic response in 2 and partial response in four. The median event-free survival was 15.2 months and median overall survival was not reached. Outcome differed by disease type, as most patients with primary mediastinal B-cell lymphoma had long term remissions, while only two of seven patients with Burkitt lymphoma were long term survivors. Thus, initial response may suffice for certain patients, but further consolidative strategies should be studied in patients with R/R Burkitt lymphoma.
Similar content being viewed by others
Introduction
Pediatric mature non-Hodgkin lymphoma (NHL) are mostly high-grade aggressive lymphomas of B-cell origin, including Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma (PMBCL) [1,2,3]. These lymphomas frequently present with disseminated disease, often involving the bone marrow or central nervous system. The up-front therapy for children with these aggressive lymphomas is typically short-term, intensive, multiagent chemotherapy with the addition of rituximab [4, 5]. The 5-year outcomes are excellent for this group of patients with most histologies [6]. Children with relapsed or refractory (R/R) mature non-Hodgkin B-cell lymphoma are treated with intensive chemotherapy and autologous stem cell transplantation (ASCT), and usually have a poor prognosis [3, 7, 8]. The outcomes of relapsed disease in children after modern rituximab-based therapy is especially poor [9].
Since first being reported in 2010, CD19-directed chimeric antigen receptor (CAR) T cells have become standard-of-care in 2nd or 3rd line adult mature B-NHL. Adults with R/R DLBCL and PMBCL treated with either Axicabtagene ciloleucel, Lisocabtagene maraleucel and Tisagenlecleucel have a complete response rate of 40–60% and 60–70%, respectively, confirmed also by real-world studies [10,11,12,13,14]. Reports in BL are scarce, but response has been demonstrated in patients treated with CD19 CAR T cells [15,16,17].
Given the excellent outcomes in upfront protocols, the rarity of pediatric R/R B-NHL resulted in slow clinical development. Data in pediatric mature B-cell lymphoma is scarce and limited to a single study from China [18], and two early reports of clinical trials in abstract form [19, 20]. In general, pediatric development of several products has not matured into clinical approval [21, 22]. We hereby report an initial cohort of pediatric ( < 19 years old) patients with relapsed/refractory mature B-cell lymphomas treated on a prospective clinical trial.
Methods
CAR production and study oversight
We conducted a single-center phase 2 clinical trial for CD19-expressing hematologic malignancies, in a tertiary hospital serving national and international patients, between 2016 and 2024. Results of pediatric ALL and adult NHL have been previously reported [23, 24]. Briefly, autologous peripheral blood mononuclear cells were leukapheresed, activated and transduced with an FMC63-CD28-CD3zeta CAR encoded on an MSGV retrovirus (kindly provided by Dr. Steve Rosenberg). Patients received lymphodepletion with fludarabine (25 mg/m2/day for 3 days) and cyclophosphamide (900 mg/m2 for 1 day) prior to an infusion of cells, per protocol guidelines [25]. Release criteria for all products include viability ≥70%, CAR transduction ≥15%, and interferon-gamma release potency assay, and standard sterility and mycoplasma testing. A fresh CAR-T product was infused to all patients. In two cases, apheresis was cryopreserved and production was initiated after bridging chemotherapy. CAR-T cell expansion in the peripheral blood was monitored by flow cytometry (CD19 CAR detection reagent, Miltenyi Biotech, DE) or by qPCR previously described [25].
Response and toxicity criteria
CAR-T related toxicity was graded per ASTCT guidelines [26]. All patients were evaluated for response using FDG-PET on day 28 post CAR-T infusion (± 5 days). Additional imaging were performed per physician’s request. For patients with bone marrow involvement, a bone marrow aspiration with flow-cytometry minimal-residual disease (MRD) was performed at the same time.
Response was evaluated according to Lugano classification criteria [27], the type of response was divided into four subgroups: Complete response (CR) was considered complete anatomical and metabolic response by PET-FDG. For patients with bone marrow involvement of Burkitt leukemia, absence of malignant cells by flow-cytometry MRD monitoring was necessary for definition of CR. Complete metabolic response (CMR) was defined as absence of PET-avid lesions with a partial anatomical response [27]. A partial response (PR) was defined as partial anatomic and metabolic response. Non-response (NR) was considered stable or progressive disease.
Supportive care
All patients received prophylaxis for P. jiroveci. Anti-fungal and anti-viral prophylaxis was not mandatory but per physician’s discretion. Per our institutional guidelines, tocilizumab is given to any patient with grade 3 or greater cytokine release syndrome (CRS), and corticosteroids administered to patients with any kind of immune-effector cell associated neurotoxicity syndrome (ICANS) following CD28-costimulated CAR T-cells. Immunoglobulins were monitored at least monthly after CAR-T cell infusion, till recovery (measurable IgM levels). IVIg supplementation was given if IgG levels were lower than 400 mg/dL.
Statistical analysis
Statistical analyses were performed using Prism v10.4 (GraphPad software). For event-free survival (EFS), an event was considered relapse or death of any cause. Overall survival (OS) and EFS were censored at last assessment date for patients without events or lost to follow-up.
Results
Patients characteristics
We describe 13 children with R/R non-Hodgkin lymphoma enrolled in a trial for the treatment of R/R lymphomas, 12 of whom were treated with CD19 CAR T cells in our institution. One patient did not start lymphodepletion due to disease progression. Patients included 7 patients with Burkitt lymphoma / leukemia, 4 patients with PMBCL and one patient with DLBCL and one had a composite lymphoma of PMBCL and DLBCL.
Patients characteristics are described in Table 1. The median age at CAR-T cell therapy was 11 years (range, 3.7–18.8). Patients had failed a median of 2 previous therapy lines. Responses to the prior therapy line before CAR-T cells included no response in 3 patients, partial response in 5, and complete response in 5. Of the latter, 3 had relapsed prior to CAR-T therapy and 2 were treated with no evidence of active disease. Overall 9 patients entered lymphodepletion with active disease, 1 patient had evidence of minimal residual disease in the bone marrow, and 2 patients were treated in remission. Five patients had elevated LDH prior to lymphodepletion, and two patients had low platelet counts (Table 1).
CAR T therapy and toxicity
The median time between apheresis and CAR-T administration was 11 days (range 10–94). CAR T-cells were successfully manufactured in all patients but 1. That one patient, with relapsed Burkitt lymphoma, had progressive disease following apheresis and died of his disease prior to lymphodepletion, at which point CAR T cell production was stopped. The remaining twelve patients were each infused with 1×106 CAR-T cells/kg and are evaluable for response. Eight patients developed CRS: one had grade 3, one had grade 2 and six had grade 1. Overall, the rate of CRS was 67%, and of grade 3 + CRS 8.3%. Six patients developed ICANS: four patients with grade 1 and two with grade 4. One patient (#4) with massive peritoneal involvement became unarousable on day +6 and developed generalized seizures. CT scan did not show brain edema. He was treated with high-dose steroids, levetiracetam and anakinra. Despite therapy the patient had prolonged clinical and EEG-evident encephalopathy and flaccid paralysis with a diffusely abnormal MRI of the brain 3 and 6 weeks after CAR T cells, and did not recover his baseline neurologic function. A second patient (#5) developed grade 4 ICANS on day +6 including confusion and seizures. Brain CT scan did not reveal any edema. She was treated with high-dose steroids and anti-epileptic drugs along with mannitol and hypertonic saline. Toxicity completely resolved on day +11. The rate of ICANS was 50%, with 16.6% grade 3 or above. Out of 3 patients with a history of CNS involvement, only 1 had grade 3 + ICANS. Overall one patient received tocilizumab, 5 received corticosteroids and 1 received anakinra for CAR related toxicity. Hematologic toxicity was observed in 11 patients: neutropenia was recorded at grade 2 (n = 1), grade 3 (n = 6) and grade 4 (n = 4). Two patients developed grade 4 thrombocytopenia. At day 28 25% of the patients had grade 3 or higher neutropenia, and 8.3% had grade 3 or higher thrombocytopenia. The only patient with prolonged neutropenia was referred to an allogeneic HSCT. B-cell aplasia was documented in all patients at day 28, and was ongoing for 2–6 months (Table 2). Patients were supplemented with IV immunoglobulins for 1–12 months. Two patients who had no further therapy after CAR-T cells experienced late infections, including COVID19 in one, and acute otitis media and herpes simplex in another.
Response
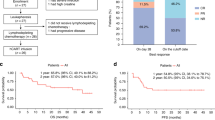
Eleven patients had evidence of CAR-T cell expansion in the peripheral blood, and all patients had subsequent B-cell aplasia. All 12 evaluable patients had a response to CAR-T cell therapy within 30 days (Fig. 1a): Six patients achieved complete response (CR), two patients (both with PMBCL) had a CMR and four had a partial response. Both patients with complete metabolic response had a complete response by 3–6 months. Two patients with a partial response treated for PMBCL and BL had progressed 6 and 12 months after treatment, respectively. Two additional patients with a partial response proceeded to complete response at 3 and 6 months evaluation.
Relapse and survival
Two patients had undergone allogeneic-SCT in remission for Burkitt leukemia/lymphoma, using a total-body irradiation based conditioning. Both patients relapsed within 30 days of HSCT. One patient was referred to palliative care and died of his disease, and the second had a CD19-negative relapse and was referred to antibody-based therapy (inotuzumab and glofitamab) but eventually passed away of his disease. The remaining 10 patients had no subsequent therapy while in remission. Three of these patients relapsed: One with Burkitt lymphoma, who died of his disease (Fig. 1a). One patient with PMBCL who had a PR had relapsed and was treated with brentuximab vedotin and nivolumab (BV+Nivo). One patient with DLBCL relapsed at 6 months after having a complete response. This patient was found to have a germline ZAP70 mutation that is related to lymphoma risk, and was further treated with salvage therapy and an allogeneic hematopoietic stem-cell transplant (allo-HSCT), and is still alive.
One patient with BL had prolonged disability after severe neurotoxicity and had died in remission 15 months after CAR-T cell infusion. At last follow-up 8 patients are alive and 6 are in continuous complete remission without additional therapy (Fig. 1a).
The median event-free survival is 15.2 months and the median overall survival was not reached (Fig. 1b). With a median follow-up of 21.5 months, the estimated 2-year EFS is 40% and OS is 61%.
Discussion
Despite being commercially available for adult B-NHL and pediatric ALL, data on CAR-T therapy in pediatric R/R B-NHL is lacking. We present our experience with CD19 CAR-T cell therapy for children with R/R mature B-NHL. Standard therapy for such patients includes multi-agent chemotherapy and rituximab, and typically consolidated with an autologous stem cell transplant. Response to salvage chemotherapy for R/R mature B-NHL is reported to be 50–75% [28], and many patients do not reach transplant [8]. Published data on children with first relapse of BL shows that survival with this approach is 30% or even lower [8, 9, 28, 29], especially when rituximab is administered in 1st line [8, 9], as is the current standard-of-care. Thus, R/R BL is a challenge requiring novel therapeutic measures.
Development of CD19 CAR T cells in adult NHL had brought CAR-T cells to approval in 2nd relapse and later in 1st relapse [11]. Interestingly, while in pediatric ALL the need for persistence has shown superiority of 4-1BB costimulated CAR-T cells, this is not the case for mature B-NHL. Moreover, non-randomized studies of 2nd relapse, CD28-costimulated CAR-T cells have shown better outcomes compared to 4-1BB costimulated CAR-T cells in adults with B-NHL [30, 31]. Our hypothesis was that the need for durable CAR-T cells in these diseases is limited, and that CD28 costimulation may overcome the aggressive nature of these lymphomas.
The overall response rate in our cohort was 100%. Despite high response rates, only two patients treated for BL survived long-term. Two patients had undergone consolidative allogeneic HSCT and relapsed within 1 month of transplant. One patient relapsed 1 year after therapy, and a fourth one had died of subsequent complications of CAR-T cells.
There are a few reports on CAR-T cells in pediatric patients with B-NHL. A study from Seattle Children’s Hospital reported on 8 children with R/R B-NHL treated with CD19 CAR T cells. Five patients responded, including two patients with DLBCL having a CR. Nevertheless, responses were not durable [19]. Tisagenlecleucel, a 4-1BB costimulated CD19 CAR-T product approved for R/R pediatric ALL and adult NHL, was studied in pediatric B-NHL, and early results were presented at the 2022 European Hematology Association annual meeting. Overall, 33 children and young adults with B-NHL were infused with tisagenlecleucel. The ORR was 32% and 7% had a CR, leading to a 12-month EFS and OS of 23% and 47%, respectively. Seven of the eighteen patients treated for BL were alive, though only 2 are in remission without further therapy [20]. In a report of 23 patients with R/R BL from a single center in China treated with CD19 CAR-T cells with 4-1BB costimulation, 9 had ongoing CR after CD19 CAR-T cells. The remaining patients had received subsequent CD22 and CD20 CAR-T cells. Altogether they report an 18-month progression-free survival of 78% [18]. All the aforementioned trials used 4-1BB costimulation. In three case reports in adult BL treated with CD28-costimulated CAR-T cells (two with FMC63 ScFv and one with a humanized ScFv), all 3 patients had achieved a CR, and two had long term remissions [15, 17]. A study of six adult patients with BL treated with a 3rd generation CAR, combining CD28 and 4-1BB for costimulation reported 1 CR and 2 PR that were not maintained [16]. Altogether, data is inconsistent between trials, and awaits further confirmatory multi-center studies.
The toxicity in our cohort was significant, however high grade CRS was reported in 1 patient (8%) and high grade ICANS in 16% of patients. These outcomes are similar to other published cohorts of CD28 costimulated CAR-T cells [10, 11, 31].
Outcomes of R/R PMBCL in children are better compared to BL, with a 3-year EFS of 58% [32]. Novel therapies such as BV+Nivo are used in R/R PMBCL, and excellent outcomes were reported in adults [33]. In these reports, many patients underwent a consolidative HSCT, or required prolonged therapy for more than 1 year. Outcomes in patients with PMBCL treated in our study were better than with BL: Three patients in were remission without subsequent therapy, and all patients surviving. Importantly, all four patients with PMBCL had poor responses BV+Nivo prior to enrolling on our trial, suggesting CAR-T cells as either salvage therapy or consolidation for this approach.
Our data supports further development of CAR-T cells for pediatric B-NHL. Since numbers are small we cannot further analyze risk factors associated with benefit from this therapy. Large prospective studies have been difficult to complete with rarity of patients. Outcomes in R/R PMBCL are excellent, similar to adult data in this disorder [13], and perhaps current approval of commercial CAR-T cells for this disorder may be extended to pediatric and adolescent population. Expanding access of current approved CAR-T products to adolescents should be considered in such rare disorders. Long-term outcomes in patients with R/R Burkitt lymphoma are still not satisfactory. Current approaches with CD19 CARs for heavily relapsed patients still yield few long-term responders. Additional therapy, either through bi-specific CAR-T cells (NCT06508931), sequential infusions of CAR-T cells directed against different antigens [18], or addition of bispecific antibodies targeting CD20 [34] currently studied in children, may be future strategies fit for further investigation for treating children with relapsed disease.
Data availability
Data will be provided upon request.
References
Sandlund JT, Martin MG. Non-Hodgkin lymphoma across the pediatric and adolescent and young adult age spectrum. Hematol Am Soc Hematol Educ Progr. 2016;2016:589–97.
Minard-Colin V, Brugieres L, Reiter A, Cairo MS, Gross TG, Woessmann W et al. Non-Hodgkin Lymphoma in Children and Adolescents: Progress Through Effective Collaboration, Current Knowledge, and Challenges Ahead. J Clin Oncol 2015; 33. https://doi.org/10.1200/JCO.2014.59.5827.
Egan G, Goldman S, Alexander S. Mature B-NHL in children, adolescents and young adults: current therapeutic approach and emerging treatment strategies. Br J Haematol. 2019;185:1071–85.
Minard-Colin V, Aupérin A, Pillon M, Burke GAA, Barkauskas DA, Wheatley K, et al. Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med. 2020;382:2207–19.
Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28:3115–21.
Hochberg J, Flower A, Brugieres L, Cairo MS. NHL in adolescents and young adults: A unique population. Pediatr Blood Cancer 2018;65. https://doi.org/10.1002/pbc.27073.
Jourdain A, Auperin A, Minard-Colin V, Aladjidi N, Zsiros J, Coze C, et al. Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Société Française des Cancers de l’Enfant study. Haematologica. 2015;100:810–7.
Woessmann W, Zimmermann M, Meinhardt A, Müller S, Hauch H, Knörr F, et al. Progressive or relapsed Burkitt lymphoma or leukemia in children and adolescents after BFM-type first-line therapy. Blood. 2020;135:1124–32.
Manji F, Chow E, Gerrie AS, Chua N, Puckrin R, Stewart DA, et al. Outcomes in relapsed/refractory burkitt lymphoma: a multi-centre Canadian experience. Blood. 2021;138:2525.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–44.
Locke FL, Miklos DB, Jacobson CA, Perales M-A, Kersten M-J, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022;386:640–54.
Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene ciloleucel in the non-trial setting: Outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38:3095–106.
Crombie JL, Nastoupil LJ, Redd RA, Tang K, Shouse G, Herrera AF, et al. Real-world outcomes of axicabtagene ciloleucel in adult patients with primary mediastinal B-cell lymphoma. Blood Adv. 2021;5:3563–7.
Schubert M-L, Bethge WA, Ayuk FA, von Bonin M, Vucinic V, Wagner-Drouet EM, et al. Outcomes of axicabtagene ciloleucel in PMBCL compare favorably with those in DLBCL: a GLA/DRST registry study. Blood Adv. 2023;7:6191–5.
Avigdor A, Shouval R, Jacoby E, Davidson T, Shimoni A, Besser M, et al. CAR T cells induce a complete response in refractory Burkitt Lymphoma. Bone Marrow Transpl. 2018;53:1583–5.
Zhou X, Ge T, Li T, Huang L, Cao Y, Xiao Y, et al. CAR19/22 T cell therapy in adult refractory Burkitt’s lymphoma. Cancer Immunol Immunother. 2021;70:2379.
Seitter SJ, McClelland PH, Ahlman MA, Goff SL, Yang JC, McIntyre L et al. Durable remissions in two adult patients with Burkitt lymphoma following anti-CD19 CAR T-cell therapy: a single center experience. Taylor and Francis Ltd., 2022.
Liu Y, Deng B, Hu B, Zhang W, Zhu Q, Liu Y, et al. Sequential different B-cell antigen-targeted CAR T-cell therapy for pediatric refractory/relapsed Burkitt lymphoma. Blood Adv. 2022;6:717–30.
Rivers J, Annesley C, Summers C, Finney O, Pulsipher MA, Wayne AS, et al. Early Response Data for Pediatric Patients with Non-Hodgkin Lymphoma Treated with CD19 Chimeric Antigen Receptor (CAR) T-Cells. Blood. 2018;132:2957–2957.
Minard-Colin V, Buechner J, Locatelli F, Gonzalez Martinez B, Vormoor BJ, Cooper S, et al. S255: EFFICACY AND SAFETY OF TISAGENLECLEUCEL IN PEDIATRIC AND YOUNG ADULT PATIENTS (PTS) WITH RELAPSED OR REFRACTORY (R/R) MATURE B-CELL NON-HODGKIN LYMPHOMA (NHL): THE PHASE II BIANCA STUDY. HemaSphere. 2022;6:156–7.
Hsieh EM, Rouce RH. Chimeric antigen receptor T cells for mature B-cell lymphoma and Burkitt lymphoma. Hematology. 2020;20:487–93.
Pearson ADJ, Scobie N, Norga K, Ligas F, Chiodin D, Burke A, et al. ACCELERATE and European Medicine Agency Paediatric Strategy Forum for medicinal product development for mature B-cell malignancies in children. Eur J Cancer. 2019;110:74–85.
Jacoby E, Bielorai B, Hutt D, Itzhaki O, Adam E, Bar D, et al. Parameters of long-term response with CD28-based CD19 chimaeric antigen receptor-modified T cells in children and young adults with B-acute lymphoblastic leukaemia. Br J Haematol. 2022;197:475–81.
Kedmi M, Shouval R, Fried S, Bomze D, Fein J, Cohen Z, et al. Point-of-care anti-CD19 CAR T-cells for treatment of relapsed and refractory aggressive B-cell lymphoma. Transpl Cell Ther. 2022;28:251–7.
Jacoby E, Bielorai B, Avigdor A, Itzhaki O, Hutt D, Nussboim V, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol. 2018;93:1485–92.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Ricard F, Cheson B, Barrington S, Trotman J, Schmid A, Brueggenwerth G, et al. Application of the lugano classification for initial evaluation, staging, and response assessment of hodgkin and non-Hodgkin Lymphoma: The PRoLoG Consensus Initiative (Part 1—Clinical). J Nucl Med. 2023;64:102–8.
Moleti ML, Testi AM, Foà R. Treatment of relapsed/refractory paediatric aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2020;189:826–43.
Cairo M, Auperin A, Perkins SL, Pinkerton R, Harrison L, Goldman S, et al. Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: A report from the FAB/LMB 96 study group. Br J Haematol. 2018;182:859–69.
Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–54.
Jacobson CA, Munoz J, Sun F, Kanters S, Limbrick-Oldfield EH, Spooner C, et al. Real-world outcomes with chimeric antigen receptor T cell therapies in large B cell lymphoma: a systematic review and meta-analysis. Transpl Cell Ther. 2024;30:77.e1–77.e15.
Vardhana S, Hamlin PA, Yang J, Zelenetz A, Sauter CS, Matasar MJ, et al. Outcomes of relapsed and refractory primary Mediastinal (Thymic) Large B cell lymphoma treated with second-line therapy and intent to transplant. Biol Blood Marrow Transpl. 2018;24:2133–8.
Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab combined with brentuximab vedotin for R/R primary mediastinal large B-cell lymphoma: a 3-year follow-up. Blood Adv. 2023;7:5272–80.
Falchi L, Vardhana SA, Salles GA. Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities. Blood. 2023;141:467–80.
Acknowledgements
The authors thank the clinical, apheresis and nursing teams of the division of pediatric hematology and oncology, as well as the supporting teams from the Edmond and Lily Safra Children’s Hospital, Sheba Medical Center. We thank production teams at the Ella Institute of Immuno-oncology and Advanced Biotherapy Center who produced the clinical CAR-T cells.
Funding
Open access funding provided by Tel Aviv University.
Author information
Authors and Affiliations
Contributions
AA, EA and EJ conceived the study. AA, EA, AS, DH, OI, BB, AT and EJ provided clinical care and collected data. AA, EA, BB and EJ analyzed the data and wrote the first draft. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Sheba Medical Center institutional review board and by the Israeli Ministry of Health, and registered at clinicaltrials.gov (NCT02772198). This study was conducted according to GCP guidelines. Informed consent was obtained from all participants or legal guardians. No identifiable images are shared.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abramovich, A., Adam, E., Shapira, A. et al. CD28-costimulated CD19 CAR-T cells for pediatric mature non-Hodgkin B-cell lymphoma. Bone Marrow Transplant 60, 1045–1051 (2025). https://doi.org/10.1038/s41409-025-02615-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02615-0