Abstract
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the best consolidative treatment for high-risk acute lymphoblastic leukemia (ALL). The Campus ALL study group analyzed the clinical outcomes of patients treated in the real-life with the pediatric-inspired and minimal/measurable residual disease (MRD)-oriented GIMEMA LAL1913 protocol who underwent alloHSCT. Key factors impacting on outcomes were MRD and remission status (1st complete remission vs 2nd complete remission) at transplant. MRD positivity was associated with poorer outcomes, with 3-year overall survival (OS) and disease-free survival (DFS) of 47% and 41% in MRD-positive patients compared to 80% and 70% in MRD-negative patients. Additionally, MRD negativity was associated with improved outcomes also for patients in 2nd complete remission with 3-year OS and DFS rates of 60% and 56%, respectively, compared to only 13% for both outcomes in MRD-positive cases. Patients older than 55 years showed survival rates comparable to younger patients, despite having a slightly higher non-relapse mortality, which remained below 20% at 3 years. These findings underscore the crucial role of alloHSCT in high-risk ALL and emphasize the importance of an early accurate disease risk allocation. The adverse outcome observed with MRD positivity advocates for early pre-transplant intervention with immunotherapy, whenever possible.
Similar content being viewed by others
Introduction
In recent years, the adoption of risk-oriented, pediatric-inspired chemotherapy protocols has significantly improved the clinical outcomes of adult patients affected by acute lymphoblastic leukemia (ALL)/lymphoblastic lymphoma (LBL). Recent clinical trials using this approach reported long-term survival rates of up to 50–70% [1,2,3,4,5,6]. Similarly, the Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) recently published the results of a multicenter phase 2 study, GIMEMA LAL1913 (NCT02067143), showing at 3 years an overall survival (OS) of 67%. Based on these results, this protocol has become the current standard of care for newly diagnosed adult Philadelphia-negative (Ph-) ALL patients in Italy [7]. The LAL1913 protocol provides indications for allogeneic stem cell transplantation (alloHSCT) in first complete remission (CR1), based on a joint assessment of clinical and biological disease characteristics at baseline and measurable residual disease (MRD) evaluation at pre-specified time points during the chemotherapy cycles. The intention-to-transplant analysis of the clinical trial showed that patients deemed eligible for alloHSCT who underwent transplant had a superior 3-year disease-free survival (DFS) rate (75% vs 26%), with a low non-relapse mortality (NRM) [7]. The Campus ALL study group recently analyzed the clinical outcomes of 421 patients treated in the real life according to the GIMEMA LAL1913 protocol. At 3 years, the OS and DFS were similar to those observed in the clinical trial [8]. However, neither the clinical trial [7] nor the real-life study [8] specifically analyzed the clinical outcomes of the subset of patients who underwent an alloHSCT. Most of the scientific evidence regarding the role of alloHSCT in ALL comes from international registry analyses, encompassing patients treated with different chemotherapy protocols and with different indications and timing for transplant allocation. Consequently, the clinical outcomes of patients homogeneously treated according to a modern pediatric-inspired, MRD-oriented chemotherapy protocol and receiving an alloHSCT remain poorly studied.
Based on this background, we analyzed the characteristics and clinical outcomes of adult ALL patients treated in a real-life setting according to the GIMEMA LAL1913 protocol, specifically focusing on those undergoing alloHSCT consolidation.
Subjects and methods
Patient population and study endpoints
We considered consecutive ALL patients treated in a real-life setting according to the GIMEMA LAL1913 protocol who underwent an alloHSCT between August 2016 and January 2023. Data were retrospectively collected from 36 Italian Campus ALL network centers. Outcomes included in the analysis were OS, DFS, cumulative incidence of relapse (CIR), NRM, and graft-versus-host disease and relapse-free survival (GRFS). This observational study was approved by the Ethics Committee of Friuli Venezia Giulia (Ethical Approval Number CEUR-2022-OS-03) and conducted in accordance with the Declaration of Helsinki.
Study design, risk stratification, MRD monitoring, and transplant allocation
The GIMEMA LAL1913 chemotherapy protocol was previously reported [7]. According to the original protocol, patients were stratified at baseline into 3 risk classes: standard risk (SR), high risk (HR), and very high risk (VHR). VHR patients had a white blood cell count (WBC) count >100 × 109/L and/or early/late non-cortical immunophenotype (EGIL T-I/II/IV) and/or expressed an adverse cytogenetics/molecular biology (t(4;11)/KMT2A rearrangement at 11q23, +8, −7, del6q, t(8;14), low hypodiploidy with 30–39 chromosomes, near triploidy with 60–78 chromosomes, complex with >5 unrelated anomalies). Ph-like signature was not systematically evaluated, but did not affect the therapy-oriented risk definition. Early-T precursor ALL (ETP-ALL) was defined according to the following phenotype: cCD3+, CD7+ with low or absent expression of CD5, along with the expression of stem cell and myeloid markers, such as HLA-DR, CD13, CD33, CD34, and CD117. HR subjects showed a pro-B phenotype (EGIL B-I), and/or a WBC > 30 up to 100 × 109/L (if B-precursor ALL), and/or had achieved a late complete remission (CR), i.e., after cycle 2. SR patients had no risk features [7].
MRD was assessed in bone marrow and peripheral blood samples, using real-time quantitative polymerase chain reaction (RT-qPCR) measurement of clonal immunoglobulin (Ig)/T-cell receptor (TCR) gene rearrangements in 3 reference laboratories as in the GIMEMA LAL1913 trial. Multiparameter flow cytometry (MFC) was utilized locally in patients lacking appropriate molecular probes. MRD was evaluated at specific time points (TP) according to the GIMEMA LAL1913 protocol. For patients harboring a clonal Ig/TCR rearrangement, MRD negativity was defined as less than 10−4 at TP2-3 (between weeks 10–16), or undetectable at TP4 (week 23), whereas patients with MRD equal or superior to 10−4 at TP2-3 and/or positive TP4 were defined as MRD-positive.
Transplant allocation in CR1 was decided considering the baseline risk class and MRD evaluation at specific time points: VHR or MRD-positive SR/HR patients were eligible to receive an alloHSCT as consolidation treatment. All patients beyond CR1 were allocated to transplantation. The use of immunotherapy before transplant, with blinatumomab or inotuzumab, for relapses or MRD-positive B-ALL was permitted. The choice of donor, conditioning regimen, and graft versus host disease (GvHD) prophylaxis varied locally based on the standard protocols at each Center.
The intensity of the conditioning regimen, myeloablative conditioning (MAC) or reduced intensity conditioning (RIC), was defined according to the criteria published by Bacigalupo and colleagues [9]. Specifically, the MAC regimens included those with TBI greater than 8 Gy, as well as chemotherapy-based regimens such as Busulfan 4 days, Fludarabine (Bu4Flu), Busulfan, Cyclophosphamide (BuCy), or Thiotepa, Busulfan, Fludarabine (TBuF). On the other hand, the RIC regimens were characterized by TBI ≤ 8 Gy based, or chemotherapy-based regimens like Treosulfan, Fludarabine (TreoFlu), or Thiotepa, Busulfan 2 days, Fludarabine (TBu2F).
Acute GvHD grading was established according to the Mount Sinai Acute GvHD International Consortium (MAGIC) criteria [10]. Chronic GVHD was classified according to the National Institute of Health (NIH) 2015 criteria [11].
Statistical analysis
All clinical outcomes were calculated from transplant to the first event or the last follow-up. OS was defined as the time from transplant to death from any cause. DFS was defined as the time from transplant to disease relapse or death from any cause. GRFS was defined as survival without grade III or IV acute GvHD, moderate or severe chronic GvHD, disease relapse, or death from any cause. CIR was defined as the occurrence of molecular or morphological relapse after alloHSCT. NRM was defined as death from any cause other than relapse. NRM, CIR, and GvHD incidence were estimated using the cumulative incidence function, considering relapse and death as a competing event for NRM and other incidence, respectively; Gray’s non-parametric test was used to assess group differences. OS and GRFS were estimated using the Kaplan-Meier method, and the log-rank test was applied to test differences between groups. Univariate and multivariable analyses were performed by fitting Fine and Gray models for cumulative incidences and Cox models for survival outcomes; hazard ratio with 95% confidence intervals were reported. A significance level of 0.05 was fixed. All the analyses were performed using the R software (version 4.0.0).
Results
This study included 203 consecutive patients with an initial diagnosis of B- (n = 99) or T-ALL/LBL (n = 104) treated in real-life according to the risk-oriented, pediatric-inspired, MRD-oriented GIMEMA LAL1913 protocol. The main patient, disease, and transplant characteristics are summarized in Table 1. MRD was assessed molecularly using RT-qPCR in 135 patients and by immunophenotype in 59. MFC was used for patients without appropriate molecular probes. The method used for MRD detection was unknown for 9 patients. The transplant was performed during the frontline treatment in 147 (72.4%) patients, mostly (n = 99, 67.3%) in the early consolidation phase. Disease status at transplant was CR1 in 83.2% (n = 169) of patients, second complete remission (CR2) in 16.3% (n = 33), and active disease in 0.5% (n = 1). Patients in CR1 were allocated to transplant due to VHR clinical risk class (n = 113, 66.9%), MRD positivity (n = 40, 23.7%), achievement of CR1 after second-line therapy (n = 5, 3%), HR with MRD unknown (n = 1, 0.5%), SR with MRD unknown (n = 1, 0.5%). The remaining 9 patients (4.4%) were transplanted in CR1 despite SR/HR and MRD negativity. Among patients in CR at transplant, MRD was positive in 30.2% (n = 61), negative in 67.3% (n = 136), and unknown in 2.5% (n = 5). Fifty B-ALL patients received immunotherapy before the transplant. Of these, 32 patients due to MRD positivity (blinatumomab, n = 30; inotuzumab, n = 2), while 18 patients received treatment as salvage therapy for hematological relapse or primary refractory disease (blinatumomab n = 11, inotuzumab n = 2, blinatumomab and inotuzumab n = 4, unknown n = 1). Among them, 24 out of 32 (75%) patients obtained a MRD negativity before transplant. The conditioning regimen was myeloablative in 83.3% (n = 169) and reduced intensity in 16.7% (n = 34) of patients. A total body irradiation (TBI)-based conditioning was adopted for 127 (62.6%) patients. The most common conditioning regimen was TBI-cyclophosphamide in 69 (34%) patients. Donors were matched unrelated (MUD) in 68 (33.5%), HLA-matched sibling (MSD) in 60 (29.6%), haploidentical (haplo) in 40 (19.7%), and mismatched unrelated (MMUD) in 35 (17.2%) patients. The stem cell source was bone marrow in 13 patients and peripheral blood in 186 (missing data in 4 patients). GvHD prophylaxis was based on calcineurin inhibitors (cyclosporine or tacrolimus) in all patients. T-cell depletion was performed in vivo with anti-thymocyte globulin (ATG) in 114 patients (56.2%), post-transplant cyclophosphamide (PTCy) in 52 (25.6%), and both ATG and PTCy in 5 (2.5%).
Main clinical outcomes
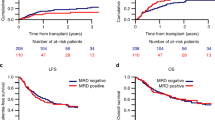
The median follow-up duration for the study population was 32 months (range 0–88). At 3 years, OS and DFS for the entire study population were 68% and 60%, respectively. Transplant in CR1, compared to CR2, was strongly associated with superior outcomes: 3-year OS 75% vs 35%, respectively, p < 0.0001, DFS 65% vs 34%, respectively, p < 0.0001, and CIR 25% vs 44%, respectively, p = 0.01 (Fig. 1a–c). Similarly, pre-transplant MRD negativity was strongly associated with improved outcomes. MRD negative patients showed 3-year OS and DFS rates of 80% and 70%, compared to 47% and 41% in MRD positive patients (p < 0.0001) and a lower CIR (22% versus 42%, respectively, p = 0.0008) (Fig. 2a–c). Notably, MRD negativity conferred a significant survival benefit even in patients transplanted in CR2 with a 3-year OS of 60% and DFS of 56%, in contrast to those transplanted in CR2 with MRD positivity that showed a poor prognosis with both OS and DFS rates at only 13% (Fig. 3a, b). We observed similar OS and DFS among patients aged <55 vs ≥55 years with a 3-year OS 68% in both groups, p = 0.81 and DFS 61% versus 55%, respectively, p = 0.49 (Fig. 4a, b). The 3-year NRM was 12% across the entire cohort, with a slightly higher risk observed in older patients (19% in those aged 55 years or older compared to 10% in younger patients, p = 0.08) (Fig. 4c). The main outcomes were similar between cell lineages (B-ALL versus T-ALL), and there were no significant differences when comparing TBI 8 Gy to TBI 12 Gy or TBI-fludarabine to TBI-alkylating agents. It is important to note that patients older than 55 years who received TBI experienced a significantly higher NRM compared to younger patients (33% versus 5%, p = 0.001). Among patients transplanted in CR1 early, specifically within the first 5 cycles of the chemotherapy due to VHR characteristics at diagnosis or MRD positivity at TP2, we found that these patients had a higher risk of relapse compared to those who were transplanted later, after the fifth cycle (the CIR was 25% in the early group vs 11% in the late group, p = 0.0384). This increased risk is likely attributed to the less favorable characteristics of the early group. However, the two groups had no significant differences in OS and NRM. Additionally, no differences in clinical outcomes were observed based on the Sorror comorbidity index. A total of 83 out of 202 (41.1%) patients experienced acute GvHD, of grade I in 31 (15.3%), grade II in 41 (20.3%), and grade ≥III in 11 (5.4%) patients. Chronic GvHD was documented in 52 patients (25.7%) classified as mild in 23 (11.4%), moderate in 24 (11.9%), and severe in 5 (2.5%). The 3-year GRFS was 40% of the study population. When stratifying patients according to the type of GvHD prophylaxis, there was a trend indicating a better GRFS in patients receiving PTCy compared to those receiving ATG, attributed to a non-significant higher DFS with PTCy and similar incidences of acute and chronic GvHD in both groups. For patients who experienced a disease relapse, the median OS was 9 months. Patients who underwent a second alloHSCT after achieving a new CR had a significantly improved 3-year OS of 36% compared to 20% for those not receiving a second transplant (p = 0.02).
By multivariable analysis, remission and MRD status were the only factors retaining statistical significance for OS and DFS (Fig. 5).
Discussion
This multicenter real-life study documents the feasibility and benefit of alloHSCT consolidation in Ph-ALL patients, uniformly treated following a risk-oriented strategy based on clinical characteristics at diagnosis and/or persistent MRD positivity during chemotherapy. Consistent with previous reports and guidelines [12], we confirm the crucial role played by alloHSCT as a consolidative treatment for VHR ALL patients. Patients who underwent a transplant in CR1 experienced significantly better survival rates than those allografted in CR2, suggesting that an appropriate timing for alloHSCT is crucial to obtain better results. Notably, we observed a significant improvement in NRM compared to historical data [13], likely due to the early transplant allocation based on the specific risk-oriented treatment strategy adopted. Most patients in this study received alloHSCT after 3 to 4 cycles of chemotherapy due to VHR characteristics at baseline and/or MRD positivity during treatment, according to the GIMEMA LAL1913 protocol indications. This approach resulted in a significant reduction in the number of cycles of chemotherapy prior to transplant. The low NRM also explains the comparable benefit of alloHSCT observed between patients younger and older than 55 years. In addition, our analysis confirms that persistent MRD positivity negatively impacts on all post-transplant outcomes. This is in keeping with previous studies reporting a higher risk of relapse in patients with MRD positivity before transplant [14,15,16]. Therefore, considering the availability of immunotherapy—namely blinatumomab - that in B-lineage ALL can convert to MRD negativity a high proportion of MRD-positive cases [17, 18], every effort should be made to eradicate MRD before alloHSCT. When eradication is not possible, post-transplant preemptive treatments should be implemented.
The use of TBI has been consolidated for decades in ALL transplantation, as established in a position statement of the ALWP of the EBMT [19]. On this topic, conflicting results have been reported with retrospective analyses showing a higher risk of disease relapse with a chemotherapy-based conditioning regimen when compared to TBI [20,21,22]. In contrast, a recent randomized clinical trial in adults comparing TBI vs busulfan-cyclophosphamide did not show a clear benefit for TBI use. However, in this latter study, the absence of advantage with TBI may be explained by the inclusion of patients with standard-risk ALL, with only a minority of MRD-positive patients in both arms [23]. In contrast, in the pediatric setting, a randomized clinical trial showed that very high-risk patients achieved a clear survival benefit with the use of TBI compared to chemotherapy-based conditioning [24]. We did not observe significant differences in relapse incidence between 8 Gy and 12 Gy, although this analysis was limited by the small number of patients receiving TBI 8 Gy. These results are consistent with the existing limited retrospective data [19, 25]. Similar findings have been reported in patients with AML, where a randomized trial prospectively compared these two doses of TBI [26]. However, randomized trials in the specific setting of ALL are needed to provide clinically relevant data on the optimal dose of TBI. Another noteworthy result was that 40% of patients were free from immunosuppressive therapy and disease relapse 3 years after transplant. Most patients in this analysis received ATG, which aligns with a report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT), which demonstrated improved GRFS in patients receiving ATG [27]. Furthermore, a recent study demonstrated no differences in relapse incidence between ATG and PTCy, while showing lower rates of GvHD in the ATG group [28].
Disease relapse was the leading cause of death, with most relapses occurring within the first two years after the transplant. Patients who experienced a relapse had a poor prognosis, with a median OS of less than 12 months. A minority of patients could benefit from a second alloHSCT, with a 3-year OS of 36%. With the growing availability of CAR-T cells, the role of a second alloHSCT remains to be defined.
The strengths of this study include the use of the same pediatric-inspired chemotherapy protocol [6, 7] for all patients, the adoption of a centrally MRD-driven strategy, the high number of patients, and the relatively long follow-up period. The limitations of our study arise from its retrospective nature, which introduces factors that could influence the transplant strategy, such as the choice of the donor, the conditioning regimen (reduced-intensity conditioning vs. myeloablative conditioning), and the GvHD prophylaxis, which were determined at the discretion of each center. Moreover, we lack information on specific high-risk subsets, such as Ph-like ALL.
In conclusion, our multicenter analysis of adult patients with Ph-negative ALL who received a pediatric-inspired and MRD-oriented chemotherapy protocol emphasizes the importance of an accurate disease stratification at baseline and during clinical follow-up. This approach is essential in order to perform an alloHSCT in CR1 when clinically indicated and, ideally, after achieving a status of MRD negativity before the transplant. This strategy resulted in a low NRM, suggesting that transplant should also be offered to older patients.
Data availability
The datasets generated and analysed during the current study are not publicly available due to patient privacy concerns but are available from the corresponding author upon reasonable request.
References
Boissel N, Huguet F, Leguay T, Mathilde HB, Graux C, Chalandon Y, et al. In adults with Ph-negative acute lymphoblastic leukemia (ALL), age-adapted chemotherapy intensity and MRD-driven transplant indication significantly reduces treatment-related mortality (TRM) and improves overall survival - results from the Graall-2014 Trial. Blood. 2022;140:112–4. https://doi.org/10.1182/blood-2022-157903.
Marks DI, Kirkwood AA, Rowntree CJ, Aguiar M, Bailey KE, Beaton B, et al. Addition of four doses of rituximab to standard induction chemotherapy in adult patients with precursor B-cell acute lymphoblastic leukaemia (UKALL14): a phase 3, multicentre, randomised controlled trial. Lancet Haematol. 2022;9:e262–75. https://doi.org/10.1016/S2352-3026(22)00038-2.
Rijneveld AW, van der Holt B, de Weerdt O, Biemond BJ, van de Loosdrecht AA, van der Wagen LE, et al. Clofarabine added to intensive treatment in adult patients with newly diagnosed ALL: the HOVON-100 trial. Blood Adv. 2022;6:1115–25. https://doi.org/10.1182/bloodadvances.2021005624.
Ribera JM, Morgades M, Ciudad J, Montesinos P, Esteve J, Genescà E, et al. Chemotherapy or allogeneic transplantation in high-risk Philadelphia chromosome–negative adult lymphoblastic leukemia. Blood. 2021;137:1879–94. https://doi.org/10.1182/blood.2020007311.
Huguet F, Chevret S, Leguay T, Thomas X, Boissel N, Escoffre-Barbe M, et al. Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL-2005 clinical trial. J Clin Oncol. 2018;36:2514–23. https://doi.org/10.1200/JCO.2017.76.8192.
Goekbuget N, Baumann A, Beck J, Brueggemann M, Diedrich H, Huettmann A, et al. PEG-asparaginase intensification in adult acute lymphoblastic leukemia (ALL): significant improvement of outcome with moderate increase of liver toxicity in the german multicenter study group for adult ALL (GMALL) study 07/2003. Blood. 2010;116:494. https://doi.org/10.1182/blood.V116.21.494.494.
Bassan R, Chiaretti S, Della Starza I, Spinelli O, Santoro A, Paoloni F, et al. Pegaspargase-modified risk-oriented program for adult acute lymphoblastic leukemia: results of the GIMEMA LAL1913 trial. Blood Adv. 2023;7:4448–61. https://doi.org/10.1182/bloodadvances.2022009596.
Lazzarotto D, Cerrano M, Papayannidis C, Chiaretti S, Mosna F, Fracchiolla N, et al. Outcome of 421 adult patients with Philadelphia-negative acute lymphoblastic leukemia treated under an intensive program inspired by the GIMEMA LAL1913 clinical trial: a Campus ALL study. Haematologica. 2025;110:55–67. https://haematologica.org/article/view/haematol.2024.285638
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10. https://doi.org/10.1016/j.bbmt.2015.09.001.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001.
DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: updated 2019 evidence-based review from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019;25:2113–23. https://doi.org/10.1016/j.bbmt.2019.08.014.
Giebel S, Labopin M, Socié G, Beelen D, Browne P, Volin L, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:139–49.
Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125:2486–96. https://doi.org/10.1182/blood-2014-09-599894.
Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–76. https://doi.org/10.1182/blood-2011-09-377713.
Pavlů J, Labopin M, Niittyvuopio R, Socié G, Yakoub-Agha I, Wu D, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019;12:108. https://doi.org/10.1186/s13045-019-0790-x.
Gokbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
Bassan R, Chiaretti S, Starza I, Della, Spinelli O, Santoro A, et al. Preliminary results of the GIMEMA LAL2317 sequential chemotherapy-blinatumomab front-line trial for newly diagnosed adult PH-negative B-lineage ALL patients. EHA Libr. 2021;5:S114.
Giebel S, Marks DI, Boissel N, Baron F, Chiaretti S, Ciceri F, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2019;54:798–809. https://doi.org/10.1038/s41409-018-0373-4.
Hirschbühl K, Labopin M, Polge E, Blaise D, Bourhis JH, Socié G, et al. Total body irradiation versus busulfan based intermediate intensity conditioning for stem cell transplantation in ALL patients >45 years—a registry-based study by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2023;58:874–80. https://doi.org/10.1038/s41409-023-01966-w.
Eder S, Canaani J, Beohou E, Labopin M, Sanz J, Arcese W, et al. Thiotepa-based conditioning versus total body irradiation as myeloablative conditioning prior to allogeneic stem cell transplantation for acute lymphoblastic leukemia: A matched-pair analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Am J Hematol. 2017;92:997–1003. https://doi.org/10.1002/ajh.24823.
Kebriaei P, Anasetti C, Zhang MJ, Wang HL, Aldoss I, de Lima M, et al. Intravenous Busulfan Compared with Total Body Irradiation Pretransplant Conditioning for Adults with Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant [Internet]. 2018;24:726–33. https://doi.org/10.1016/j.bbmt.2017.11.025.
Zhang H, Fan Z, Huang F, Han L, Xu Y, Xu N, et al. Busulfan plus cyclophosphamide versus total body irradiation plus cyclophosphamide for adults acute B lymphoblastic leukemia: an open-label, multicenter, phase III trial. J Clin Oncol. 2022;41:343–53. https://doi.org/10.1200/JCO.22.00767.
Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority phase III study. J Clin Oncol. 2020;39:295–307. https://doi.org/10.1200/JCO.20.02529.
Steiner N, Massoud R, Richter J, Perekhrestenko T, Gagelmann N, Niederwieser C, et al. Comparable results between 8 and 12 gray TBI in combination with fludarabine and post-transplant cyclophosphamide in MRD-negative but not in MRD-positive acute lymphoblastic leukemia patients transplanted in first complete remission. Eur J Haematol. 2025;114:79–88. https://doi.org/10.1111/ejh.14305.
Bornhäuser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–44. https://doi.org/10.1016/S1470-2045(12)70349-2.
Czerw T, Labopin M, Giebel S, Socié G, Volin L, Fegueux N, et al. Anti-thymocyte globulin improves survival free from relapse and graft-versus-host disease after allogeneic peripheral blood stem cell transplantation in patients with Philadelphia-negative acute lymphoblastic leukemia: an analysis by the Acute Leukemia Working Party of the EBMT. Cancer [Internet]. 2018;124:2523–33. https://doi.org/10.1002/cncr.31354.
Giebel S, Labopin M, Salmenniemi U, Socié G, Bondarenko S, Blaise D, et al. Posttransplant cyclophosphamide versus antithymocyte globulin in patients with acute lymphoblastic leukemia treated with allogeneic hematopoietic cell transplantation from matched unrelated donors: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer [Internet]. 2023;129:3735–45. https://doi.org/10.1002/cncr.35004.
Acknowledgements
We are grateful to all Campus ALL Centers in Italy.
Author information
Authors and Affiliations
Contributions
GC and FV collected data, collaborated in data interpretation, wrote the manuscript, and gave final approval before manuscript submission. CP performed statistical analysis, collaborated in data interpretation, revised the manuscript, and gave the final approval before manuscript submission. DL, AG, CPa, MC, NF, FG, MD, ML, SI, MdP, ST, MF, PZ, PS, MD, CP, FM, BS, FF, PC, CS, BC, MD, GL, EM, MB, CM, LS, AM, VM, PM, GB, AC, LA collaborated in data collection and interpretation, revised the manuscript, and gave the final approval before manuscript submission. FL, SC, AC, and RF designed the study, supervised the data analysis, provided major intellectual contributions to the manuscript, and gave the final approval before manuscript submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cavallaro, G., Lazzarotto, D., Pavoni, C. et al. Outcomes of allogeneic stem cell transplant in adult Philadelphia negative acute lymphoblastic leukemia patients treated with the pediatric-inspired GIMEMA 1913 protocol. A Campus ALL study. Bone Marrow Transplant 60, 1228–1235 (2025). https://doi.org/10.1038/s41409-025-02632-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02632-z