Abstract
Immune-mediated rheumatologic and musculoskeletal diseases (RMDs) comprise a heterogeneous group of systemic conditions that affect the connective tissues of the musculoskeletal system and internal organs. Immune-mediated RMDs are driven by chronic autoimmune responses and typically require continuous or repeated administration of immunosuppressive or biologic disease-modifying drugs. Although generally effective, these therapies can cause both short- and long-term side effects and may fail to control the disease with risk of irreversible tissue damage. For such patients, haematopoietic stem cell transplantation (HSCT) has been successfully employed over the past 30 years, but this procedure requires caution due to significant side effects. To address these aspects, updated recommendations for the use of HSCT in RMDs have been developed in collaboration with an international expert panel from the European Society for Blood and Marrow Transplantation (EBMT). The panel reviewed all available evidence regarding HSCT application since 2004. Based on this review, EBMT expert-based consensus recommendations were formulated to guide best practices and ensure high-quality patient care. These recommendations include detailed indications, contraindications, and cautionary notes specific to each RMD, along with comprehensive protocols for diagnostic work-up. They are intended to support clinicians, scientists, patients, and caregivers in the field of RMDs.
Similar content being viewed by others
Introduction and current state-of-the-art
Immune-mediated rheumatologic and musculoskeletal diseases (RMD) may affect joints, muscles, internal organs, skin, vessels or connective tissues. These diseases may result in pain, loss of function and reduced quality-of-life and are among the most disabling and costly conditions in the adult population in the developed world [1,2,3]. RMDs result from a break of immunologic self-tolerance leading to activated autoreactive T and B cells with consequent development of autoantibodies. Most of the classic RMDs are polygenic and exhibit features of the broad spectrum of autoimmune and autoinflammatory mechanisms [4, 5]. Examples for severe forms of autoimmune RMDs are systemic diseases such as systemic sclerosis (SSc) [6], systemic lupus erythematosus (SLE) [7], idiopathic inflammatory myopathies (IIM) [8], vasculitis and rheumatoid arthritis (RA). The use of immunosuppressive, biologic, or targeted synthetic disease-modifying drugs (DMARDs), administered as monotherapies or in combination, is recommended by the European Alliance of Associations for Rheumatology (EULAR) guidelines for treatment, depending on the individual manifestations and severity of the disease [9,10,11,12].
However, not all RMD patients sufficiently respond to conventional or biologic therapies, which can lead to disease flares, progression, and ultimately morbidity and mortality. In this context, restoration of immunologic tolerance with consequent resolution of autoimmune and inflammatory responses against self-antigens is key to provide durable remissions in RMD patients [13]. Over the last four decades, there has been increasing clinical and scientific evidence that immunological tolerance can be achieved by the use of high-dose cytotoxic therapy combined with serotherapy, followed by autologous, or less frequently allogeneic, hematopoietic stem cell transplantation (HSCT). This approach allows for resetting the immune response and re-inducing immunologic self-tolerance, a concept developed and utilized over nearly four decades, beginning with animal models [14, 15]. The clinical application of HSCT has become an integral part of standard-of-care treatment algorithms for certain indications, particularly for patients with rapidly progressive, early severe SSc [9, 16]. Previous European Society for Blood and Marrow Transplantation (EBMT) guidelines and recommendations have recommended that all patients being considered for and treated with HSCT are managed in institutions where relevant autoimmune disease specialists work closely together with transplant hematologists and others in a multidisciplinary team (MDT), in accordance with JACIE (or equivalent) accreditation standards and other regulatory requirements [17].
Given the continually evolving evidence base in the field, with numerous novel treatment options, there is a need for updated EBMT clinical practice guidelines and recommendations to support both the RMD and haematopoietic cell transplantation (HCT) communities and their patients at various levels, including national and international organizations, as well as local clinical teams across rheumatologic specialties who may refer patients. This EBMT consensus aims to promote patient safety and harmonize procedures for AD patient selection, care, follow-up, clinical and immune monitoring before and after treatment delivery, and data collection, in accordance with Good Clinical Practice (GCP), Good Manufacturing Practice (GMP), where applicable, and relevant accreditation and regulatory requirements. As with the previous clinical development of cellular therapies for RMDs [18], the EBMT and the broader autoimmune disease specialist community should continue to play a central role in coordinating retrospective registry-based analyses and prospective studies to evaluate the safety and efficacy of HSCT in patients with RMDs.
Methods
Methodology
These consensus recommendations were developed by an international panel of experts during a 2-day in person workshop held in Lille, France, in September 2024. This workshop was conducted following the methodology published by the EBMT Practice Harmonization and Guidelines Committee, with the aim of providing clear and practical recommendations based on international consensus or, where such consensus is lacking, expert opinion [19]. Nineteen experts from different countries belonging to the EBMT, including the Autoimmune Diseases Working Party (ADWP), and international experts were invited to join the workshop. Two videoconferences were held in preparation of the workshop, during which specific research questions and areas to be covered were developed and confirmed. Previous ADWP guidelines [17, 20,21,22], ADWP best practice recommendations for management of innovative cellular therapies [18], and other international guidelines [23, 24] provided a model for discussions. After the in-person meeting in Lille, a draft paper was generated and further circulated to the experts for review and contribution. They therefore represent the consensus views of all the authors. Levels of evidence have been inserted according to the currently accepted EBMT grading system (Table 1) [16].
Objectives and research questions
These recommendations were developed due to the growing number of autologous HSCT performed for RMDs [14]. They aim to cover detailed indications, contraindications, and cautionary notes specific to each RMD, along with comprehensive protocols for diagnostic work-up, clinical management, and immune monitoring during HSCT. The recommendations focussed on the following research questions: (i) Which RMD patients are candidates for autologous HSCT? (ii) What is the optimal timing for auto-HSCT in RMD patients? (iii) How do we perform autologous-HSCT in RMD patients? (iv) How do we manage RMD patients post-autologous HSCT? They represent the consensus point of view of expert authors from international MDTs. They reflect current best practices in this growing field and aim to help clinicians and other healthcare professionals in providing consistent, high-quality patient care and support local clinical teams delivering these specialized treatments. These EBMT recommendations are intended to be general in scope and applicable to all mentioned RMDs and autologous HSCT adopted within current clinical practice. These guidelines and recommendations should not inhibit progress and innovation but instead used as a basis for further research and development of conditioning regimens, standards of care, inclusion or exclusion of patient groups, biomarkers, heterogeneity in disease, end organ regeneration, or inclusion of artificial intelligence. When administering HSCT for RMD within clinical trials, physicians are advised to follow respective trial protocols.
Search strategy and selection criteria
Data for literature review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “autologous HSCT”, “rheumatological diseases” and “systemic sclerosis” or “SLE”, lupus”, “myositis”, “vasculitis” or “arthritis” (Supplementary material). Only articles written in English from January 2004 until September 2024, including all clinical (single or randomized phase II, or phase III randomized controlled) trials as well as registry analyses and key reviews/guidelines were considered in the evaluation, and served as the basis for the discussions. In rare disease indications, given the lack of high-quality evidence from randomized trials, we also included case series and older data supporting the use of HSCT.
Results
General considerations
General recommendations
Evidence for the feasibility, efficacy and toxicity of HSCT in RMDs has been provided by many clinical trials, large registry analyses and summarized in previous reviews [14, 25, 26]. The risks of toxicity and transplant-related mortality (TRM) may vary between autologous and allogeneic haematopoietic stem cell (HSC), intensity of conditioning regimens, centre experience, patient selection, and RMD category [25, 27, 28]. The potential for safer, yet equally effective, non-HSCT treatments should be actively explored in all cases, including biological therapies, where deliverable. Disease activity, organ damage and organ involvement should be carefully assessed before HSCT in RMDs. All clinical and paraclinical parameters necessary to evaluate all comorbidities associated with the systemic AD activity, damage and severity should be documented, with a detailed work-up dating back to no more than three months before the planned HSCT procedure. Specific indications are given for each RMD in subsequent sections. Clinicians should consider the balance between active disease and the possibility of withdrawing immunosuppressive therapies within the time window required to perform HSCT, as well as the importance of sequelae secondary to RMD. Non-interventional prospective studies offer a means of obtaining meaningful clinical data, where full phase III randomized controlled trials are not feasible, and are the preferred option over ‘ad-hoc’ procedures (level III).
Bullet points:
-
A MDT, with at least one disease and transplant specialist is mandatory to discuss eligibility and follow the patient during and after the transplant procedure (level III).
-
In addition to JACIE certification (or equivalent), centres should specifically train staff (physicians, nurses, allied health staff, data managers) in the management of specific RMDs, since it is linked to improved outcomes (level III).
-
Whenever possible, patients considered for HSCT should be entered into a Research Ethics Committee/Institutional Review Board (REC/IRB) approved clinical trial or prospective non-interventional study (level III).
-
All autologous and allogeneic HSCT cases should be reported to international registries (e.g. EBMT or other equivalent societies) (level III).
-
Age above 65 years should not be a specific limitation for HSCT treatment per se, but co-morbidities may increase with age and should be considered as part of the biological fitness of the patient for HSCT (level II).
-
Impact of the conditioning regimen on short- and long-term outcomes is an important consideration in the planning of HSCT in ADs (level III).
-
Careful and standardized follow-up procedures (for the first 100 days and at least 6 monthly for the first two years, and afterwards annually if medically stable) after HSCT are important to assess and monitor the AD patient, disease evolution and the immunological reconstitution process. Other healthcare structures, including follow-up and rehabilitation care centres, may be involved with other healthcare service providers (i.e. nutrition and medical equipment) (level III).
-
Long-term formalized follow-up is a minimum recommendation. Annual review and data reporting are recommended to capture all outcomes, including late effects of HSCT (level III).
-
Biobanking within current regulatory frameworks allowing to maximize the utility of stored biological samples should be actively sought (level II).
-
Studies using other sources of data (registry and established clinical trials) should be used in evaluating the potential cost-effectiveness of HSCT compared with non-HSCT treatment options (level II).
General eligibility criteria
When using HSCT in patients with RMD, an acceptable balance between the expected benefits and potential side effects should be considered, based on available evidence and considering alternative treatment options. Therefore, potential candidates for HSCT should generally have failed one or more lines of standard (guideline-based and/or regulatory-approved) therapies, a severe rapidly progressive disease with poor prognosis (according to disease specific risk scores for disease related mortality), and a general condition that permits HSCT with minimal risk of morbidity and mortality. Under these considerations, general considerations (Table 2) and contraindications (Table 3) have been developed.
Recommendations on wash-out period before mobilization specifically for RMDs
Washout of DMARDs needs to be considered due to potential impact on mobilization or increased risk of infections. To this end, a discontinuation of the following drugs is recommended at 2–3 weeks before mobilization: mycophenolate mofetil, azathioprine, calcineurin inhibitors, mTOR inhibitors, JAK-inhibitors, cyclophosphamide, methotrexate and subcutaneous tocilizumab and one week before mobilization: CD20-targeting therapies [18]. It is recommended to perform HSCT within 2 weeks and up to 3 months after apheresis, during which time the HSCT work-up does not need to be repeated and ideally wash out of the immunosuppressive treatment should be maintained, provided there is no change in clinical status. The general exceptions are serological testing (e.g., HIV and hepatitis) and pregnancy tests, which should be updated within seven days prior to prior to the initiation of the recipient’s preparative regimen in accordance with FACT-JACIE standards.
Mobilization, stem cell collection and CD34-selection
Over the past decades, mobilized peripheral blood stem cells (PBSCs) have largely replaced bone marrow as the primary source of HSCs for autologous HSCT, because PBSCs contain a significantly higher number of CD34+ cells and also offer a more convenient collection procedure and faster hematologic recovery [29]. However, obtaining a sufficient number of autologous PBSCs depends on the effective mobilization of HSCs from the bone marrow niche into the bloodstream, usually using G-CSF and cyclophosphamide. Purification of HSCs from the stem cell grafts using CD34 selection may be performed to reduce the risk of reinfusing autoreactive lymphocytes into the patient, but this purging is associated with excess infection due to delayed immune reconstitution and the selection procedure adds significantly to the costs and logistics of the procedure [30]. While some post-hoc analyses from clinical studies in SLE and SSc suggested improved outcomes in CD34-selected HSCT transplant recipients [30, 31], others have not shown benefit or are inconclusive [32]. In addition, one pilot randomized study of HSCT in rheumatoid arthritis did not demonstrate superiority of CD34 selection [33]. The intensity of the conditioning regimen used and the use of anti-thymocyte globulin (ATG) have been variable across clinical practice, providing challenges for assessment of the utility of ATG. Based on the current level of evidence and understanding, the following recommendations were developed:
-
Autologous HSC may be derived from peripheral blood or bone marrow. Mobilized PBSC are preferred based on ease of procurement and better engraftment characteristics (level II).
-
Mobilization procedures and stem cell processing should be performed in JACIE or FACT (or equivalent) accredited collection centres (level III).
-
Priming chemotherapy is recommended to enhance mobilization, whilst maintaining disease control and to prevent potential flare, which may be a consequence of G-CSF alone (level I). The most commonly used dosage for mobilization is cyclophosphamide at 2 g/m2 with uromixetan (Mesna) and cautious hyperhydration followed by G-CSF 5–10 µg/kg/day (level II).
-
A minimum dose of 2 × 106/kg CD34+ cells should be reinfused, irrespective of any graft manipulation (level II). A higher yield of 2.5–5 × 106 CD34+ cells/kg have been associated with faster neutrophil and platelet recovery. The target dose of HSC to be collected should be a minimum of 5 × 106 CD34+ cells/kg in case of graft manipulations (e.g. CD34-selection) and a minimum of 2 × 106 CD34+ cells/kg in the absence of graft manipulation.
-
Back-up harvest can be considered, especially when graft manipulation has been undertaken (level II).
-
When cyclophosphamide primed mobilization exceptionally fails, a second attempt at PBSC mobilization or bone marrow harvest should be considered. Despite the lack of evidence in patients with RMD, the use of plerixafor and G-CSF may be reasonable in poor mobilizers after weighing up the benefits and risks, and glucocorticoids may be considered to prevent disease flare (level II).
-
There is no consistent evidence from prospective or randomized trials or registry data to support superiority of CD34+ or other ex-vivo graft manipulation, although decisions can be made on an individual patient basis (level II). Further research is warranted to determine the benefits, risks and economic aspects of CD34+ or other selection based on specific conditioning regimens.
Conditioning regimen
Over the past decade, EBMT centres have adopted various conditioning regimens for RMDs (Supplementary material). Three randomized trials comparing conventional therapy of SSc (cyclophosphamide) to autologous HSCT (ASSIST, ASTIS, SCOT) [34,35,36] demonstrated the superiority of HSCT. An accurate comparison of the different conditioning regimens used in these prospective randomized trials is not feasible due to differences between populations, endpoints [37] and HSCT techniques and would require a randomized trial of the different conditioning regimens. The following recommendations have been developed considering all the relevant literature, including clinical trials [34,35,36, 38,39,40,41,42,43], registry studies [27, 44], review/metanalysis [45,46,47], recommendations/guidelines [17, 48], book chapters [49], and the expertise of the panellists included in the workshop:
-
The most commonly used regimen for rheumatologic indications is cyclophosphamide 200 mg/kg (cyclophosphamide 50 mg/kg/day on days –5, –4, –3, –2) in combination with rabbit ATG. Standard conditioning is recommended in presence of: normal cardiac function as assessed by EBMT guidelines [21], normal electrocardiogram (ECG), normal 24 h Holter-ECG, LVEF > 45% at cardiography, absence of right pulmonary hypertension, and no septal bounce on cardiac MRI (level II).
-
Reduced dose of cyclophosphamide or combinations with fludarabine plus rituximab (ie. cyclophosphamide 60 mg/kg on day –2, fludarabine 30 mg/m2/day on days –5 –4 –3 –2, rituximab 500 mg on days –6 and +2) or thiotepa (i.e. cyclophosphamide 2 × 50 mg/kg and thiotepa 2 x 5 mg/kg) [38], and various rabbit ATG dosing combinations (6.0–7.5 mg/kg), are increasingly used (Supplementary material) (level II). Similar short-term treatment responses have been demonstrated using these regimens with reduction in TRM, likely due to the reduced cardiotoxicity of the conditioning, shorter duration of neutropenia and less transfusion requirement. So-called ‘cardiac safe’ conditioning [38, 39] can be considered in patients: age >50 years, abnormalities on ECG and/or 24 h Holter-ECG [35] and/or echocardiography (left or right ventricular dysfunction, pulmonary hypertension, auricular dysfunction), abnormal right heart catheterization (RHC) and/or MRI criteria for myocarditis [50] or septal bounce on cardiac MRI (level II). Further research and randomized trials are warranted.
-
As part of the conditioning regimen, the use of ATG has varied in type and dose in both clinical trials and registry data.
-
In the EBMT ASTIS clinical trial, Thymoglobulin® (Sanofi) rabbit ATG 7.5 mg/kg (administered in equal amounts over 3 consecutive days) was used.
-
Based on the ASSIST and related ‘cardiac safe’ protocols, a reduced total dosage of Thymoglobulin® rabbit ATG, i.e. 6.0–6.5 mg/kg rabbit ATG (administered as 0.5 mg/kg on day –5, 1.0 or 1.5 mg/kg on day –4, and 1.5 mg/kg/day on days –3, –2, –1) is progressively used (level II).
-
Grafalon® (Neovii) rabbit ATG i.e. 10 mg/kg/day on days –3, –2, –1, total dose 30 mg/kg) has been used in some centres (level III).
-
Horse ATG (hATG, ATGAM®, Pfizer) 10 mg/kg on Days –6, –5, –4 and –3 has been used [51] as an alternative to rabbit ATG in conditioning. It may be a consideration in centres where preference over rabbit ATG can be justified.
-
Further research is necessary to determine the optimal type and dosing of ATG and other serotherapy in HSCT conditioning regimens in RMDs (level II).
-
-
Administration of ATG should be done in a slow rate during at least 12 h (ideally 18 h), accompanied by an intermediate dose of glucocorticoids [45]. Details for the dosage, the duration and the tapering of glucocorticoids may vary according to local practise. Based on a recent EBMT-ADWP survey, majority of centres use methylprednisolone (or equivalent, with or without taper) 1 mg/kg/day (37.8% of centres) [45]. Fever and other reactions should be promptly treated according to centre policy and protocols (level III).
-
Alemtuzumab is not recommended as serotherapy within the conditioning regimen as it is associated with increased mortality and increased risk of developing secondary autoimmune diseases after HSCT (level II).
-
Total body irradiation (TBI) is not recommended because it increases TRM and the risks for myeloid as well as solid malignancies (level II).
-
It is recommended to perform HSCT within 2 weeks and up to 3 months after apheresis (level II).
-
The interval between the last dose of cyclophosphamide and infusion of the graft should be at least 48 h (level II).
-
Patients receiving high doses of cyclophosphamide should receive uromixetan (Mesna) and cautious intravenous hydration as prophylaxis of hemorrhagic cystitis (level I).
-
The minimum number of CD34+ cells reinfused should be 2.0 × 106/kg, irrespective of graft manipulation (level II).
-
Consideration should be given to the short- and long-term toxicities of the various type of serotherapy, including serum sickness (level II).
-
Otherwise, supportive care should be based on institutional HSCT practice and standard operating procedures, including maintenance of venous access via central venous catheter from the start of conditioning as below.
Supportive care, prophylaxis and follow-up
MDT management of RMD patients undergoing HSCT is highly recommended, with a minimum of one disease specialist and one HCT specialist per centre working closely together [25]. In-patient accommodation for HSCT period is recommended: all patients should be accommodated in isolation facilities, with appropriate clean air facilities in accordance with JACIE accreditation standards during aplasia/severe neutropenia (level II). The risk of infection depends on the underlying AD and degree of immunosuppression [25], and management should be carefully discussed upfront in a MDT meeting (disease specialist, infection-disease specialist, and HSCT specialists). A follow-up of potential infectious complications is considered mandatory. Sufficiently long anti-infectious prophylaxis should be maintained according to patient individual risk and in line with institutional guidelines. Management of these patients should follow specific indications (Table 4), considering all aspects related to the underlying AD and HSCT procedure. Hematologists should be continued to be involved in monitoring of side effects according to ADWP [17] and EBMT Handbook recommendations [52] for the first 100 days and at least 6 monthly for the first two years, and afterwards annually if medically stable with data collection and reporting in the EBMT registry.
Immunological monitoring after HSCT for RMDs
Immune monitoring should include a baseline assessment (before mobilization and before conditioning) and at intervals after HSCT such as at 3, 6 and 12 months for the first year, then every 6 months for the second year and then annually up to 5 years [20]. Recommended tests include:
• Flow cytometric immunophenotyping of peripheral blood mononuclear cells to measure changes in CD3+, CD4+ and CD8+ T cells, CD19+ B cells, NK cells (CD16+ and/or CD56+) and monocytes (CD14+ and/or CD16+)
• Serum immunoglobulin electrophoresis (IgG, IgA and IgM quantitation, including monoclone, if present)
Recommendations for the assessment of disease biomarkers are provided in the specific sections below.
Disease-specific considerations
Systemic sclerosis
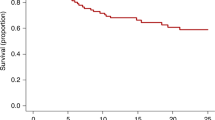
Systemic sclerosis (SSc) is a rare and devasting autoimmune disease characterized by tissue fibrosis, an immune mediated endothelial vasculopathy and autoantibody production [6]. Despite recent improvements in the disease management [9], mortality is still high, especially in patients with heart involvement, interstitial lung disease (ILD), renal crisis or high modified Rodnan skin score (mRSS) [53, 54]. Over the past years, rapidly progressive diffuse SSc has become the main indication for HSCT among RMD patients. Three RCTs comparing autologous HSCT to intravenous cyclophosphamide demonstrated significant survival benefit, improvements in skin scores, lung-function, and quality of life [34,35,36]. The European (ASTIS) [35] and American (SCOT) [36] multicentre trials reported 5-year progression-free survival rates of 70–74%, which remained superior to those achieved with monthly cyclophosphamide in the ASTIS trial over a 10-year follow-up period. Based on this evidence, SSc is recommended as a standard indication for autologous HSCT (level I) by the EULAR since 2009 [55], and further supported by updated international 2020 CIBMTR [23] and 2023 EULAR [9] recommendations.
The higher early toxicity of autologous HSCT in SSc compared to other conditions is primarily linked to right ventricular cardiac dysfunction from SSc-related cardiac involvement, especially in cases with pulmonary arterial hypertension, left ventricular diastolic dysfunction from a non-relaxing stiff myocardium, and pericardial constriction or tamponade. Myocardial alterations are at high risk of exacerbation during HSCT by cyclophosphamide cardiotoxicity, fluid hydration and fever. Improved pre-transplant cardiac screening protocols [21], use of conditioning regimens that minimize cyclophosphamide dose [38, 39], and maintaining baseline fluid balance have reduced TRM from 10% in the early ASTIS study [35] to 6% in a prospective non-interventional EBMT study (NISSC-1) [31], 3% in the SCOT study [36], and 2.4% in the CAST study [39].
Eligibility for HSCT varies by protocol but risk factors for disease related mortality and aggressive diseases have been identified. They include progressive SSc (increasing skin score or declining pulmonary function), age at onset <40 years, male gender, antibodies against topoisomerase I or RNA polymerase III, elevated level of C-reactive protein (>10 mg/l), recurrent digital ulcers, tendon friction rubs, weight loss (>10% during the past three months), active myositis on MRI or elevated creatine kinase levels (>2 x ULN), early (during the first year) lung manifestations with DLCO <60% and/or FVC <70% and cardiac manifestations with elevated troponin levels and/or myocardial involvement [54, 56].
Several conditioning regimens have been used for autologous HSCT in SSc, mostly comprising cyclophosphamide and ATG at different doses with a general trend towards lower-intensity regimens. Expert consensus suggests that regimen need to be adapted to the individual cardiopulmonary function of patients. While cyclophosphamide at doses of 200 mg/kg is considered the standard, reduced doses need to be considered in case of cardiac involvement, e.g. using the protocol from the CAST study [39] or thiotepa-based regimens [38], where available. In highly active and severely affected patients with transient contraindications (e.g. active myocarditis), data support pretreatment with RTX and MMF [57]. The CAST regimen was initially applied for patients excluded from high-dose cyclophosphamide transplant due to cardiac parameters of pulmonary artery systolic pressure (PASP) >45 mmHg or mean pulmonary artery pressure (mPAP) >24 mmHg on right heart catheterization, and/or MRI criteria for active myocarditis [50]. Due to the CAST regimen’s reduced toxicity in high cardiac risk transplants, several centres have extended the CAST regimen to include standard cardiac risk patients (level II) [38, 39].
Other concerns unique to HSCT for SSc are gastric antral vascular ectasia (GAVE), renal crises and a general concern of regimen-related post-transplant lymphoproliferative disease (PTLD). GAVE, often referred to as “watermelon stomach” due to the characteristic red streaking of submucosal telangiectasias, is more commonly observed in patients with anti-RNA polymerase III antibodies at the onset of the disease. Iron deficiency anaemia is a common consequence of GAVE and endoscopy should be performed in such patients to assess for GAVE (or any other upper GI cause) before HSCT, given the bleeding risks of thrombocytopenia. Renal crises may be precipitated by the use of glucocorticoids with ATG or cyclophosphamide. Prophylaxis with an angiotensin-converting enzyme inhibitor can be considered, particularly in patients with anti-RNA polymerase III antibodies. Disease-specific considerations are summarized in Table 5.
Systemic lupus erythematosus (SLE)
SLE is a prototypic autoimmune disease with heterogenous clinical manifestations, characterized by the development of antibodies directed at a variety of nuclear antigens causing multi-organ inflammation and damage [7]. Despite recent advances in the SLE treatment with approved biologics, such as belimumab and anifrolumab, mortality is still higher than in the general population, especially in youngest patients [58]. According to current EULAR recommendations, treatment should aim at remission [59] or lupus low-disease activity state (LLDAS) [60] with a daily prednisolone maintenance dosage not exceeding 5 mg [10]. Patients not achieving or not maintaining these validated treat-to-target endpoints have a higher risk for developing disease flares, damage accrual and mortality [61, 62].
Data on HSCT is available from single-centre phase I/II clinical trials and retrospective EBMT analyses [30, 63], together covering more than 300 severely affected SLE patients worldwide. Data from these trials indicate a disease-free survival of ~50–65% at 5 years despite discontinuation of DMARDs and responding patients are usually free of clinical symptoms and may regain seronegativity for antinuclear antibodies (ANA) [41, 49, 64]. Early use of HSCT has also been demonstrated to protect against organ-failure and toxicity-related morbidity and to provide improvement in health-related quality-of-life [43]. As for other RMD, cyclophosphamide/ATG based regimens are most commonly used for conditioning (Supplementary material). Current data suggest HSCT in SLE as clinical option in patients with active disease despite immunosuppressive and/or biologic therapies (level II) [14]. Disease-specific considerations are summarized in Table 6.
Vasculitis
Vasculitides are a group of rare conditions that are characterized by inflammation of blood vessels that demonstrate a wide range of organ involvement and severity, and may lead to long-term sequelae including vision loss, aneurysms or renal failure. The Chapel Hill Consensus definitions, originally published in 1994 and updated in 2012 [65], categorized vasculitis according to the size of affected vessels into large, medium or small vessel vasculitis. Subsequently, modern practical classification criteria for major systemic vasculitides have been developed and endorsed by ACR/EULAR initiatives, including criteria for granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), giant cell arteritis (GCA) and Takayasu arteritis (TAK) [66]. Similarly, EULAR recommendation for management of the diseases were developed specifically for ANCA-associated vasculitis (AAV) [11] and large vessel vasculitis [67].
In the era of biologic therapies, refractory cases are rare, but rituximab resistance has been reported in patients with GPA [68] and relapse development in GCA under tocilizumab treatment [69]. HSCT has been used as salvage therapy over the past years, including 29 transplants reported to the EBMT registry (Supplementary material). Retrospective multicentre analyses from the registry summarizing the outcomes specifically for TAK [70], Behçet’s disease [71] and AAV [72] demonstrated an overall beneficial response to HSCT, but relapses and TRM have been reported. Therefore, HSCT represents a clinical option (level II) in patients with severe and refractory disease, as outlined in Table 6. The recently described VEXAS syndrome, which is associated with vasculitis, polychondritis and other RMD manifestations, should be considered and excluded with molecular (UBA-1) testing as this would be a potential indication for allogeneic HSCT (rather than autologous HSCT) in carefully selected patients [73].
Idiopathic inflammatory myopathy
IIM, also commonly referred to as myositis, are a family of rare conditions that are characterized by chronic inflammation of muscle of unknown cause and often include extramuscular manifestations, including rash, arthritis, ILD and cardiac involvement [8]. One of the earliest and most widely used criteria for classifying the IIM has been the Bohan and Peter criteria, but newer EULAR/ACR criteria exist now [74]. However, these criteria have limitations to categorize patients into IIM subtypes, usually comprising dermatomyositis (DM), overlap myositis, anti-synthetase syndrome (ASyS), immune-mediated necrotizing myopathy (MMNM), and sporadic inclusion body myositis (sIBM). The identification of myositis-specific antibodies, which are present in up to 60% of patients, are more useful to classify IIM into homogenous phenotypic subtypes [8]. Treatment of IIM can be challenging as many patients poorly respond to first-line treatment with glucocorticoids, methotrexate, azathioprine or even second-line therapy with cyclophosphamide, rituximab or JAK-inhibitors [75]. For such refractory patients, HSCT was utilized only sporadically over the past decades, delivering mixed results (Supplementary material). The majority of the available literature on HSCT in IIM comes from reports of juvenile DM with impressive findings [76, 77], but these were excluded from the current literature search. Disease-specific considerations for IIM are summarized in Supplementary Table 1, and HSCT can only be considered as a ‘clinical option’ (level III) (Table 7).
Rheumatoid arthritis and other inflammatory arthritis
Modern biologic and targeted-synthetic DMARDs revolutionized the treatment landscape in rheumatoid arthritis (RA) [12], and severely refractory patients, usually referred to as difficult-to-treat [78], are the exception. Early in its evolution, HSCT was investigated in severe forms of RA, predominantly in the pre-biologic era. Data from the last retrospective analysis from the EBMT and the Autologous Blood and Marrow Transplant Registry including 73 patients treated between 1996 and 2000 using a cyclophosphamide-based regimen demonstrated an ACR-50 response in only 67% of patients [79]. Treatment with JAK inhibitors achieves almost similar response rates with less toxicity, not justifying continued use of HSCT in RA with primary joint manifestations. Exceptions could be systemic forms of rheumatoid arthritis, including Felty syndrome or Adult-onset Still’s disease (AOSD) with life- or organ-threatening involvement, where positive results have been obtained from individual cases [80]. However, most of these reports derived from juvenile arthritis published before interleukin-1 or interleukin-6 receptor antagonists became available. Therefore, eligibility for HSCT should carefully weigh the risks and benefits according to criteria summarized in Supplementary Table 1, where HSCT could represent a ‘clinical option’ (level III) (Table 7).
Conclusion and future directions
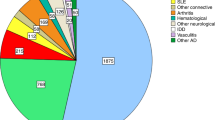
For over three decades, autologous HSCT has been successfully delivered to RMD patients with severe and treatment-refractory disease courses with more than 3000 cases reported to the EBMT registry [14]. Over time, outcomes have gradually improved due to better patient selection, improved centre experience and optimized supportive care [25]. With accumulating data, the current EBMT recommendations aim to further improve clinical practice by facilitating harmonized procedures for patient selection, transplant management and follow-up. These recommendations reflect currently available evidence, coupled with expert opinion, and will continue to be revised according to necessary modifications in practice. We recommend that decision making is delivered within a MDT, in accordance with GCP and GMP, and appropriate accreditation and regulatory requirements, including JACIE or equivalent accreditation of centres. Data reporting to EBMT and/or equivalent HCT registries is recommended. Where possible, patients should be included into prospective non-interventional studies of the EBMT to evaluate the safety and efficacy in addition to the retrospective registry studies that EBMT regularly performs on available data within the Registry.
The main indication for HSCT in RMDs remains systemic sclerosis, where evidence from RCTs demonstrate autologous HSCT as a ‘standard of care’ (S) indication. Available evidence supports the use of HSCT early in the disease course, especially in those patients with red flags for high SSc-related mortality. Reduced-intensity regimens provided superior safety outcomes and should be considered after thorough pre-transplant work-up with focus on cardiac dysfunction. SLE, vasculitis, IIM and inflammatory arthritis represent indications with clinical option based on evidence from phase I/II trials or smaller cohort studies. Due to the dynamic nature of alternative, highly effective therapies, indications and eligibility criteria for HSCT may change rapidly. Promising results from chimeric antigen receptor (CAR)- T cell studies [81, 82] and first experiences with the utilization of bispecific antibodies [83,84,85] in major rheumatic diseases have been reported. However, further research is needed to integrate these therapies in current and future treatment algorithms supported by the evidence base. In parallel, scientific studies will be crucial in elucidating the mechanisms of action in HSCT versus CAR-T versus non-cellular therapies in relation to response to treatment, particularly regarding the degree of depletion of autoreactive lymphocytes in inflamed tissues, the reduction of autoantibodies, and the consideration of short-term toxicities alongside long-term risks.
Data availability
The final analysis dataset will be available upon specific request to the Working Party chair.
References
Global, regional, and national burden of rheumatoid arthritis, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023: e594-e610. https://doi.org/10.1016/s2665-9913(23)00211-4.
Watts RA, Hatemi G, Burns JC, Mohammad AJ. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2022;18:22–34. https://doi.org/10.1038/s41584-021-00718-8.
Barber MRW, Drenkard C, Falasinnu T, Hoi A, Mak A, Kow NY, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17:515–32. https://doi.org/10.1038/s41584-021-00668-1.
Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584–9. https://doi.org/10.1038/nature03723.
McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3:e297 https://doi.org/10.1371/journal.pmed.0030297.
Volkmann ER, Andréasson K, Smith V. Systemic sclerosis. Lancet. 2023;401:304–18. https://doi.org/10.1016/s0140-6736(22)01692-0.
Hoi A, Igel T, Mok CC, Arnaud L. Systemic lupus erythematosus. Lancet. 2024;403:2326–38. https://doi.org/10.1016/s0140-6736(24)00398-2.
Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Prim. 2021;7:86 https://doi.org/10.1038/s41572-021-00321-x.
Del Galdo F, Lescoat A, Conaghan PG, Bertoldo E, Čolić J, Santiago T, et al. EULAR recommendations for the treatment of systemic sclerosis: 2023 update. Ann Rheum Dis. 2024. https://doi.org/10.1136/ard-2024-226430.
Fanouriakis A, Kostopoulou M, Andersen J, Aringer M, Arnaud L, Bae S-C, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024;83:15–29. https://doi.org/10.1136/ard-2023-224762.
Hellmich B, Sanchez-Alamo B, Schirmer JH, Berti A, Blockmans D, Cid MC, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2024;83:30–47. https://doi.org/10.1136/ard-2022-223764.
Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18. https://doi.org/10.1136/ard-2022-223356.
Maschmeyer P, Chang HD, Cheng Q, Mashreghi MF, Hiepe F, Alexander T, et al. Immunological memory in rheumatic inflammation - a roadblock to tolerance induction. Nat Rev Rheumatol. 2021;17:291–305. https://doi.org/10.1038/s41584-021-00601-6.
Alexander T, Greco R. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: overview and future considerations from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2022;57:1055–62. https://doi.org/10.1038/s41409-022-01702-w.
Swart JF, Delemarre EM, van Wijk F, Boelens JJ, Kuball J, van Laar JM, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol. 2017;13:244–56. https://doi.org/10.1038/nrrheum.2017.7.
Snowden JA, Sanchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transpl. 2022;57:1217–39. https://doi.org/10.1038/s41409-022-01691-w.
Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2012;47:770–90. https://doi.org/10.1038/bmt.2011.185.
Greco R, Alexander T, Del Papa N, Muller F, Saccardi R, Sanchez-Guijo F, et al. Innovative cellular therapies for autoimmune diseases: expert-based position statement and clinical practice recommendations from the EBMT practice harmonization and guidelines committee. eClinicalMedicine. 2024;69:102476 https://doi.org/10.1016/j.eclinm.2024.102476.
Yakoub-Agha I, Greco R, Onida F, de la Camara R, Ciceri F, Corbacioglu S, et al. Practice harmonization workshops of EBMT: an expert-based approach to generate practical and contemporary guidelines within the arena of hematopoietic cell transplantation and cellular therapy. Bone Marrow Transpl. 2023;58:696–700. https://doi.org/10.1038/s41409-023-01958-w.
Alexander T, Bondanza A, Muraro PA, Greco R, Saccardi R, Daikeler T, et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone Marrow Transpl. 2015;50:173–80. https://doi.org/10.1038/bmt.2014.251.
Farge D, Burt RK, Oliveira MC, Mousseaux E, Rovira M, Marjanovic Z, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transpl. 2017;52:1495–503. https://doi.org/10.1038/bmt.2017.56.
Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transpl. 2020;55:283–306. https://doi.org/10.1038/s41409-019-0684-0.
Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transpl. 2020;26:1247–56. https://doi.org/10.1016/j.bbmt.2020.03.002.
Illei GG, Cervera R, Burt RK, Doria A, Hiepe F, Jayne D, et al. Current state and future directions of autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:2071–4. https://doi.org/10.1136/ard.2010.148049.
Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1:2742–55. https://doi.org/10.1182/bloodadvances.2017010041.
Alexander T, Greco R, Snowden JA. Hematopoietic stem cell transplantation for autoimmune disease. Annu Rev Med. 2021;72:215–28. https://doi.org/10.1146/annurev-med-070119-115617.
Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on autoimmune diseases. Haematologica. 2010;95:284–92. https://doi.org/10.3324/haematol.2009.013458.
van Bijnen S, de Vries-Bouwstra J, van den Ende CH, Boonstra M, Kroft L, Geurts B, et al. Predictive factors for treatment-related mortality and major adverse events after autologous haematopoietic stem cell transplantation for systemic sclerosis: results of a long-term follow-up multicentre study. Ann Rheum Dis. 2020;79:1084–9. https://doi.org/10.1136/annrheumdis-2020-217058.
Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Cámara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transpl. 2021;56:1651–64. https://doi.org/10.1038/s41409-021-01227-8.
Alchi B, Jayne D, Labopin M, Demin A, Sergeevicheva V, Alexander T, et al. Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: data from the European Group for Blood and Marrow Transplantation registry. Lupus. 2013;22:245–53. https://doi.org/10.1177/0961203312470729.
Henes J, Oliveira MC, Labopin M, Badoglio M, Scherer HU, Del Papa N, et al. Autologous stem cell transplantation for progressive systemic sclerosis: a prospective non-interventional study from the European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. Haematologica. 2021;106:375–83. https://doi.org/10.3324/haematol.2019.230128.
Oliveira MC, Labopin M, Henes J, Moore J, Del Papa N, Cras A, et al. Does ex vivo CD34+ positive selection influence outcome after autologous hematopoietic stem cell transplantation in systemic sclerosis patients? Bone Marrow Transpl. 2016;51:501–5. https://doi.org/10.1038/bmt.2015.299.
Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 2002;46:2301–9. https://doi.org/10.1002/art.10495.
Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378:498–506. https://doi.org/10.1016/s0140-6736(11)60982-3.
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490–8. https://doi.org/10.1001/jama.2014.6368.
Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med. 2018;378:35–47. https://doi.org/10.1056/nejmoa1703327.
Ait Abdallah N, Wang M, Lansiaux P, Puyade M, Berthier S, Terriou L, et al. Long term outcomes of the French ASTIS systemic sclerosis cohort using the global rank composite score. Bone Marrow Transpl. 2021;56:2259–67. https://doi.org/10.1038/s41409-021-01355-1.
Henes JC, Koetter I, Horger M, Schmalzing M, Mueller K, Eick C, et al. Autologous stem cell transplantation with thiotepa-based conditioning in patients with systemic sclerosis and cardiac manifestations. Rheumatology. 2014;53:919–22. https://doi.org/10.1093/rheumatology/ket464.
Burt RK, Han X, Quigley K, Arnautovic I, Shah SJ, Lee DC, et al. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: a pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transpl. 2021;56:50–9. https://doi.org/10.1038/s41409-020-0978-2.
Helbig G, Widuchowska M, Koclega A, Kopinska A, Kopec-Medrek M, Gawel WB, et al. Safety profile of autologous hematopoietic stem cell mobilization and transplantation in patients with systemic sclerosis. Clin Rheumatol. 2018;37:1709–14. https://doi.org/10.1007/s10067-017-3954-5.
Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–35. https://doi.org/10.1001/jama.295.5.527.
Statkute L, Oyama Y, Barr WG, Sufit R, Ho S, Verda L, et al. Autologous non-myeloablative haematopoietic stem cell transplantation for refractory systemic vasculitis. Ann Rheum Dis. 2008;67:991–7. https://doi.org/10.1136/ard.2007.070227.
Burt RK, Han X, Gozdziak P, Yaung K, Morgan A, Clendenan AM, et al. Five year follow-up after autologous peripheral blood hematopoietic stem cell transplantation for refractory, chronic, corticosteroid-dependent systemic lupus erythematosus: effect of conditioning regimen on outcome. Bone Marrow Transpl. 2018;53:692–700. https://doi.org/10.1038/s41409-018-0173-x.
Ismail A, Nitti R, Sharrack B, Badoglio M, Ambron P, Labopin M, et al. ATG and other serotherapy in conditioning regimens for autologous HSCT in autoimmune diseases: a survey on behalf of the EBMT Autoimmune Diseases Working Party (ADWP). Bone Marrow Transpl. 2024. https://doi.org/10.1038/s41409-024-02383-3.
Shouval R, Furie N, Raanani P, Nagler A, Gafter-Gvili A. Autologous hematopoietic stem cell transplantation for systemic sclerosis: a systematic review and meta-analysis. Biol Blood Marrow Transpl. 2018;24:937–44. https://doi.org/10.1016/j.bbmt.2018.01.020.
Burt RK, Muraro PA, Farge D, Oliveira MC, Snowden JA, Saccardi R, et al. New autoimmune diseases after autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transpl. 2021;56:1509–17. https://doi.org/10.1038/s41409-021-01277-y.
Burt RK, Marmont A, Oyama Y, Slavin S, Arnold R, Hiepe F, et al. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: the evolution from myeloablative to lymphoablative transplant regimens. Arthritis Rheum. 2006;54:3750–60. https://doi.org/10.1002/art.22256.
Farge D, Pugnet G, Allez M, Castilla-Llorente C, Chatelus E, Cintas P, et al. French protocol for the diagnosis and management of hematopoietic stem cell transplantation in autoimmune diseases. Rev Med Interne. 2024;45:79–99. https://doi.org/10.1016/j.revmed.2023.12.008.
Burt RK, Farge D, Ruiz MA, Saccardi R, Snowden JA. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases. 2021; eBook: 978-1-315-15136-6: https://www.routledge.com/Hematopoietic-Stem-Cell-Transplantation-and-Cellular-Therapies-for-Autoimmune/Burt-Farge-Ruiz-Saccardi-Snowden/p/book/978113855855.
Keret S, Chutko B, Dobrecky-Mery I, Wolak A, Hardak E, Slobodin G, et al. Cardiac safe hematopoietic stem cell transplantation protocol for systemic sclerosis with myocarditis- a two-step approach. Rheumatology. 2024. https://doi.org/10.1093/rheumatology/keae268.
Moore J, Englert H, Furlong T, Poon T, Milliken S, Ma D. Auto-HSCT induces sustained responses in severe systemic sclerosis patients failing pulse cyclophosphamide. Bone Marrow Transpl. 2012;47:1486–7. https://doi.org/10.1038/bmt.2012.67.
In: Sureda A, Corbacioglu S, Greco R, Kroger N, Carreras E (eds). The EBMT handbook: hematopoietic cell transplantation and cellular therapies, 8th ed. Cham (CH); 2024.
Volkmann ER, Fischer A. Update on morbidity and mortality in systemic sclerosis-related interstitial lung disease. J Scleroderma Relat Disord. 2021;6:11–20. https://doi.org/10.1177/2397198320915042.
Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir-Gurman A, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis. 2017;76:1897–905. https://doi.org/10.1136/annrheumdis-2017-211448.
Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009;68:620–8. https://doi.org/10.1136/ard.2008.096677.
Becker M, Graf N, Sauter R, Allanore Y, Curram J, Denton CP, et al. Predictors of disease worsening defined by progression of organ damage in diffuse systemic sclerosis: a European Scleroderma Trials and Research (EUSTAR) analysis. Ann Rheum Dis. 2019;78:1242–8. https://doi.org/10.1136/annrheumdis-2019-215145.
Keret S, Chutko B, Dobrecky-Mery I, Wolak A, Hardak E, Slobodin G, et al. Cardiac safe hematopoietic stem cell transplantation protocol for systemic sclerosis with myocarditis-a two-step approach. Rheumatology. 2024;63:e328–e330. https://doi.org/10.1093/rheumatology/keae268.
Zen M, Salmaso L, Barbiellini Amidei C, Fedeli U, Bellio S, Iaccarino L, et al. Mortality and causes of death in systemic lupus erythematosus over the last decade: data from a large population-based study. Eur J Intern Med. 2023;112:45–51. https://doi.org/10.1016/j.ejim.2023.02.004.
van Vollenhoven RF, Bertsias G, Doria A, Isenberg D, Morand E, Petri MA, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med. 2021; 8. https://doi.org/10.1136/lupus-2021-000538.
Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis. 2016;75:1615–21. https://doi.org/10.1136/annrheumdis-2015-207726.
Kandane-Rathnayake R, Golder V, Louthrenoo W, Chen YH, Cho J, Lateef A, et al. Lupus low disease activity state and remission and risk of mortality in patients with systemic lupus erythematosus: a prospective, multinational, longitudinal cohort study. Lancet Rheumatol. 2022;4:e822–e830. https://doi.org/10.1016/s2665-9913(22)00304-6.
Ugarte-Gil MF, Hanly J, Urowitz M, Gordon C, Bae SC, Romero-Diaz J, et al. Remission and low disease activity (LDA) prevent damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis. 2022;81:1541–8. https://doi.org/10.1136/ard-2022-222487.
Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13:168–76. https://doi.org/10.1191/0961203304lu525oa.
Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214–23. https://doi.org/10.1182/blood-2008-07-168286.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. https://doi.org/10.1002/art.37715.
Ecclestone T, Watts RA. Classification and epidemiology of vasculitis: emerging concepts. Best Pract Res Clin Rheumatol. 2023;37:101845 https://doi.org/10.1016/j.berh.2023.101845.
Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79:19–30. https://doi.org/10.1136/annrheumdis-2019-215672.
Machet T, Quémeneur T, Ledoult E, Mesbah R, Lebas C, Hachulla E, et al. Rituximab resistance at 3months of induction therapy in newly diagnosed or relapsing ANCA-associated vasculitis: a French multicentre retrospective study in 116 patients. Jt Bone Spine. 2023;90:105591 https://doi.org/10.1016/j.jbspin.2023.105591.
Samec MJ, Rakholiya J, Langenfeld H, Crowson CS, Abril A, Wang B, et al. Relapse risk and safety of long-term tocilizumab use among patients with giant cell arteritis: a single-enterprise cohort study. J Rheumatol. 2023;50:1310–7. https://doi.org/10.3899/jrheum.2022-1214.
Laurent C, Marjanovic Z, Ricard L, Henes J, Dulery R, Badoglio M, et al. Autologous hematopoietic stem cell transplantation with reduced-intensity conditioning regimens in refractory Takayasu arteritis: a retrospective multicenter case-series from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2020;55:2109–13. https://doi.org/10.1038/s41409-020-0907-4.
Puyade M, Patel A, Lim YJ, Blank N, Badoglio M, Gualandi F, et al. Autologous hematopoietic stem cell transplantation for Behçet’s disease: a retrospective survey of patients treated in europe, on behalf of the autoimmune diseases working party of the European Society for Blood and Marrow Transplantation. Front Immunol. 2021;12:638709 https://doi.org/10.3389/fimmu.2021.638709.
Alexander T, Samuelson C, Daikeler T, Henes J, Akil M, Skagerlind L, et al. Autologous haematopoietic stem cell transplantation (HSCT) for anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis: a retrospective survey of patients reported to European Society for Blood and Marrow Transplantation (EBMT) registry. Bone Marrow Transpl. 2020;55:1512–5. https://doi.org/10.1038/s41409-019-0763-2.
Gurnari C, Koster L, Baaij L, Heiblig M, Yakoub-Agha I, Collin M, et al. Allogeneic hematopoietic cell transplantation for VEXAS syndrome: results of a multicenter study of the EBMT. Blood Adv. 2024;8:1444–8. https://doi.org/10.1182/bloodadvances.2023012478.
Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76:1955–64. https://doi.org/10.1136/annrheumdis-2017-211468.
Lundberg IE. Expert perspective: management of refractory inflammatory myopathy. Arthritis Rheumatol. 2021;73:1394–407. https://doi.org/10.1002/art.41762.
Holzer U, van Royen-Kerkhof A, van der Torre P, Kuemmerle-Deschner J, Well C, Handgretinger R, et al. Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand J Rheumatol. 2010;39:88–92. https://doi.org/10.3109/03009740903096622.
Zhu J, Su G, Lai J, Dong B, Kang M, Li S, et al. Long-term follow-up of autologous hematopoietic stem cell transplantation for refractory juvenile dermatomyositis: a case-series study. Pediatr Rheumatol Online J. 2018;16:72 https://doi.org/10.1186/s12969-018-0284-3.
Nagy G, Roodenrijs NM, Welsing PM, Kedves M, Hamar A, van der Goes MC, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31–5. https://doi.org/10.1136/annrheumdis-2020-217344.
Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31:482–8.
Lanza F, Dominici M, Govoni M, Moretti S, Campioni D, Corte RL, et al. Prolonged remission state of refractory adult onset Still’s disease following CD34-selected autologous peripheral blood stem cell transplantation. Bone Marrow Transpl. 2000;25:1307–10. https://doi.org/10.1038/sj.bmt.1702435.
Müller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Völkl S, et al. CD19 CAR T-cell therapy in autoimmune disease - a case series with follow-up. N Engl J Med. 2024;390:687–700. https://doi.org/10.1056/NEJMoa2308917.
Auth J, Müller F, Völkl S, Bayerl N, Distler JHW, Tur C, et al. CD19-targeting CAR T-cell therapy in patients with diffuse systemic sclerosis: a case series. Lancet Rheumatol. 2024. https://doi.org/10.1016/s2665-9913(24)00282-0.
Alexander T, Krönke J, Cheng Q, Keller U, Krönke G. Teclistamab-induced remission in refractory systemic lupus erythematosus. N Engl J Med. 2024;391:864–6. https://doi.org/10.1056/NEJMc2407150.
Hagen M, Bucci L, Böltz S, Nöthling DM, Rothe T, Anoshkin K, et al. BCMA-targeted T-cell-engager therapy for autoimmune disease. N Engl J Med. 2024;391:867–9. https://doi.org/10.1056/NEJMc2408786.
Siegert E, Biesen R, Dzamukova M, Furth C, Probst M, Doellinger F, et al. Teclistamab in relapsed systemic sclerosis after autologous haematopoietic stem cell transplantation. Ann Rheum Dis. 2025. https://doi.org/10.1016/j.ard.2025.01.043.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. https://doi.org/10.1182/blood-2005-05-2004.
Alexander T, Tassy N, Domenech A, Kramer E, Jessop H, Kenyon M, et al. Patient-reported outcomes in HSCT for autoimmune diseases: considerations on behalf of the EBMT ADWP, PAC, and Nurses Group. J Allergy Clin Immunol Glob. 2024;3:100283 https://doi.org/10.1016/j.jacig.2024.100283.
Hantel A, Hibbs SP, Merz LE, Abel GA. The duffy null phenotype - addressing a source of discrimination in cancer care. N Engl J Med. 2024;391:1969–72. https://doi.org/10.1056/NEJMp2409329.
Maertens J, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, et al. ECIL guidelines for preventing Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother. 2016;71:2397–404. https://doi.org/10.1093/jac/dkw157.
Aerts R, Mehra V, Groll AH, Martino R, Lagrou K, Robin C, et al. Guidelines for the management of Toxoplasma gondii infection and disease in patients with haematological malignancies and after haematopoietic stem-cell transplantation: guidelines from the 9th European Conference on Infections in Leukaemia, 2022. Lancet Infect Dis. 2024;24:e291–e306. https://doi.org/10.1016/S1473-3099(23)00495-4.
Greco R, Alexander T, Burman J, Del Papa N, de Vries-Bouwstra J, Farge D, et al. Hematopoietic stem cell transplantation for autoimmune diseases in the time of COVID-19: EBMT guidelines and recommendations. Bone Marrow Transpl. 2021. https://doi.org/10.1038/s41409-021-01326-6.
Snowden JA, Sharrack B, Akil M, Kiely DG, Lobo A, Kazmi M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases - a guide for the generalist. Clin Med. 2018;18:329–34. https://doi.org/10.7861/clinmedicine.18-4-329.
Colton H, Greenfield DM, Snowden JA, Miller PDE, Morley NJ, Wright J, et al. Long-term survivors following autologous haematopoetic stem cell transplantation have significant defects in their humoral immunity against vaccine preventable diseases, years on from transplant. Vaccine. 2021;39:4778–83. https://doi.org/10.1016/j.vaccine.2021.07.022.
Alexander T, Badoglio M, Labopin M, Daikeler T, Farge D, Kazmi M, et al. Monitoring and management of CMV and EBV after autologous haematopoietic stem cell transplantation for autoimmune diseases: a survey of the EBMT Autoimmune Diseases Working party (ADWP). Bone Marrow Transpl. 2025;60:110–3. https://doi.org/10.1038/s41409-024-02461-6.
Mehra V, Rhone E, Widya S, Zuckerman M, Potter V, Raj K, et al. Epstein-Barr virus and monoclonal gammopathy of clinical significance in autologous stem cell transplantation for multiple sclerosis. Clin Infect Dis. 2019;69:1757–63. https://doi.org/10.1093/cid/ciz047.
Cesaro S, Ljungman P, Tridello G, Mikulska M, Wendel L, Styczynski J, et al. New trends in the management of cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a survey of the Infectious Diseases Working Pary of EBMT. Bone Marrow Transpl. 2023;58:203–8. https://doi.org/10.1038/s41409-022-01863-8.
Massey J, Artuz C, Dyer Z, Jackson K, Khoo M, Visweswaran M, et al. Diversification and expansion of the EBV-reactive cytotoxic T lymphocyte repertoire following autologous haematopoietic stem cell transplant for multiple sclerosis. Clin Immunol. 2023;254:109709 https://doi.org/10.1016/j.clim.2023.109709.
Rotz SJ, Bhatt NS, Hamilton BK, Duncan C, Aljurf M, Atsuta Y, et al. International recommendations for screening and preventative practices for long-term survivors of transplantation and cellular therapy: a 2023 update. Bone Marrow Transpl. 2024;59:717–41. https://doi.org/10.1038/s41409-023-02190-2.
Daikeler T, Labopin M, Di Gioia M, Abinun M, Alexander T, Miniati I, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118:1693–8. https://doi.org/10.1182/blood-2011-02-336156.
Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10:220–7. https://doi.org/10.1016/j.hemonc.2017.05.009.
Rotz SJ, Bhatt NS, Hamilton BK, Duncan C, Aljurf M, Atsuta Y, et al. International recommendations for screening and preventative practices for long-term survivors of transplantation and cellular therapy: a 2023 update. Transpl Cell Ther. 2024;30:349–85. https://doi.org/10.1016/j.jtct.2023.12.001.
Tichelli A, Rovo A. Fertility issues following hematopoietic stem cell transplantation. Expert Rev Hematol. 2013;6:375–88. https://doi.org/10.1586/17474086.2013.816507.
Maciejewska M, Snarski E, Wiktor-Jedrzejczak W. A preliminary online study on menstruation recovery in women after autologous hematopoietic stem cell transplant for autoimmune diseases. Exp Clin Transpl. 2016;14:665–9. https://doi.org/10.6002/ect.2015.0336.
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–55. https://doi.org/10.1136/annrheumdis-2013-204424.
Lescoat A, Bellando-Randone S, Campochiaro C, Del Galdo F, Denton CP, Farrington S, et al. Beyond very early systemic sclerosis: deciphering pre‑scleroderma and its trajectories to open new avenues for preventive medicine. Lancet Rheumatol. 2023;5:e683–e694. https://doi.org/10.1016/s2665-9913(23)00212-6.
Spierings J, van Rhenen A, Welsing PM, Marijnissen AC, De Langhe E, Del Papa N, et al. A randomised, open-label trial to assess the optimal treatment strategy in early diffuse cutaneous systemic sclerosis: the UPSIDE study protocol. BMJ Open. 2021;11:e044483 https://doi.org/10.1136/bmjopen-2020-044483.
Furst DE, Clements PJ, Steen VD, Medsger TA Jr, Masi AT, D’Angelo WA, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25:84–8.
Steen VD, Medsger TA Jr. The value of the health assessment questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum. 1997;40:1984–91. https://doi.org/10.1002/art.1780401110.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151–9. https://doi.org/10.1136/annrheumdis-2018-214819.
Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology. 2005;44:902–6. https://doi.org/10.1093/rheumatology/keh624.
Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91.
Jesus D, Matos A, Henriques C, Zen M, Larosa M, Iaccarino L, et al. Derivation and validation of the SLE Disease Activity Score (SLE-DAS): a new SLE continuous measure with high sensitivity for changes in disease activity. Ann Rheum Dis. 2019;78:365–71. https://doi.org/10.1136/annrheumdis-2018-214502.
Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. https://doi.org/10.1002/art.1780390303.
Chakka S, Krain RL, Concha JSS, Chong BF, Merola JF, Werth VP. The CLASI, a validated tool for the evaluation of skin disease in lupus erythematosus: a narrative review. Ann Transl Med. 2021;9:431 https://doi.org/10.21037/atm-20-5048.
Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–8.
Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. https://doi.org/10.1002/art.1780400222.
Acknowledgements
We are grateful for the support of EBMT and ADWP, without which this work would not have been possible. The authors thank the EBMT practice harmonization and guidelines committee, Manuela Badoglio and Myriam Labopin in the EBMT Paris Office, EBMT centres for their contributions to the EBMT registry and those active in the ADWP.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: RG, TA; Investigation and creation of recommendations: all authors; Final Analysis and Visualization: TA, ER, DF, JH, ZM, MP, NdP, JAS, JS, JVB, MB, RB, RC, AD, JM, MCO, GP, DR, MS; Methodology: TA, RG, ISO, AR, FO, IYA; Writing Original Draft: TA, ER, RG; Writing Review and Editing: all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
TA received Honoria from Abbvie, Amgen, AstraZeneca and GSK study support from Johnson & Johnson. RG discloses speaking honoraria from Biotest, Pfizer, Medac, Neovii, Kyverna and Magenta. RB is CEO of Genani Corporation, Chicago, Illinois. FM received honoraria & Travel Support from BMS, Janssen, Kite/Gilead, Miltenyi, Novartis, Received Research Support from Kite/Gilead. JAS discloses consultancy for BMS, Medac, Vertex and Jazz. JH discloses consultancy for Miltenyi and Neovii. JS discloses research support from Boehringer Ingelheim and Miltenyi. RC discloses consultancy for AstraZeneca, Celgene, GSK, Janssen, ElyLilly, Pfizer, UCB, Rubió and Werfen. PA reports no conflict of interest; he discloses travel support and speaker honoraria from unrestricted educational activities organized by Novartis, Bayer HealthCare, Bayer Pharma, Biogen Idec, Merck-Serono and Sanofi Aventis and consulting to Magenta Therapeutics, Jasper Therapeutics and Cellerys AG. ER discloses speaking honoraria by Janssen Pharmaceutica. AR discloses speaker honoraria from Gilead. The remaining authors have nothing to declare. JM discloses speaker honoria from Medac and Pfizer. JVR discloses speaker honoraria from Janssen-Cilag, Boehringer-Ingelheim, Pfizer, AstraZeneca, BMS, UCB; consultancy honoraria from Abbvie, Janssen, Boehringer- Ingelheim, and grant/research support from ReumaNederland, Janssen-Cilag, Galapagos, Roche; all payments were made to the institution. GP reports no conflict of interest; he discloses travel support and speaker honoraria from Abbvie, Astra-Zeneca, Novartis, Amgen, GSK, Pfizer, Boehringer Ingelheim, Amicus. None of the mentioned conflicts of interest were related to financing of the content of this manuscript.
Ethical approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the Autoimmune Diseases Working Party of the EBMT.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alexander, T., Roldan, E., Del Papa, N. et al. Autologous haematopoietic stem cell transplantation for rheumatic diseases: best practice recommendations from the EBMT Practice Harmonization and Guidelines Committee. Bone Marrow Transplant 60, 1451–1464 (2025). https://doi.org/10.1038/s41409-025-02695-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02695-y
This article is cited by
-
The future of autologous stem cell transplantation in systemic sclerosis
Nature Reviews Rheumatology (2025)
-
Indications for haematopoietic cell transplantation and CAR-T for haematological diseases, solid tumours and immune disorders: 2025 EBMT practice recommendations
Bone Marrow Transplantation (2025)
-
Studienlage und aktualisierte Leitlinie der European Society for Blood and Marrow Transplantation zur autologen hämatopoetischen Stammzelltransplantation bei entzündlich rheumatischen Erkrankungen
Zeitschrift für Rheumatologie (2025)