Abstract
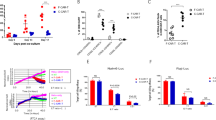
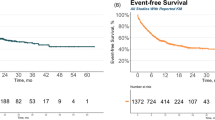
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment landscape of relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (B-ALL), with high remission rates across various CAR T-cell constructs. However, the durability of these responses remains a major challenge, with many patients experiencing relapse after an initial remission. This systematic review and meta-analysis aimed to compare the efficacy and safety of different CAR T-cell constructs across 40 clinical trials, including a total of 1540 R/R B-ALL patients. We assessed the impact of patient demographics, prior treatment exposure, and construct characteristics on treatment outcomes. The pooled complete remission rate (CRR) was 83.4% (I2 = 49%), with a minimal residual disease-negative complete remission (MRDneg-CR/CRi) rate of 92.7% (I2 = 48%). 4-1BB co-stimulatory domain constructs showed higher MRDneg-CR/CRi rates compared with CD28 (94.0% vs. 84.4%p = 0.048) and a lower incidence of immune effector cell-associated neurotoxicity syndrome. Additionally, CAR T-cell products targeting CD19 or CD19/CD22 patients presented higher MRDneg-CR/CRi rates than those targeting CD22 alone. In conclusion, our findings suggest that 4-1BB-based CAR T-cell therapy targeting CD19 offers the best efficacy and safety profile in R/R B-ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are derived from publicly available published articles, which are cited in the manuscript. No individual participant data were generated or analyzed during the current study. All data extracted and analyzed are included in the published articles and supplementary materials referenced herein.
References
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. https://doi.org/10.1056/NEJMoa1709866.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. https://doi.org/10.1016/S0140-6736(21)01222-8.
Roddie C, Dias J, O’Reilly MA, Abbasian M, Cadinanos-Garai A, Vispute K, et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-t therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J Clin Oncol. 2021;39:3352–63. https://doi.org/10.1200/JCO.21.00917.
Leahy AB, Devine KJ, Li Y, Liu H, Myers R, DiNofia A, et al. Impact of high-risk cytogenetics on outcomes for children and young adults receiving CD19-directed CAR T-cell therapy. Blood. 2022;139:2173–85. https://doi.org/10.1182/blood.2021012727.
Lamble AJ, Myers RM, Taraseviciute A, John S, Yates B, Steinberg SM, et al. Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv. 2023;7:575–85. https://doi.org/10.1182/bloodadvances.2022007423.
Molinos-Quintana Á, Alonso-Saladrigues A, Herrero B, Caballero-Velázquez T, Galán-Gómez V, Panesso M, et al. Impact of disease burden and late loss of B cell aplasia on the risk of relapse after CD19 chimeric antigen receptor T Cell (Tisagenlecleucel) infusion in pediatric and young adult patients with relapse/refractory acute lymphoblastic leukemia: role of B-cell monitoring. Front Immunol. 2024;14:1280580. https://doi.org/10.3389/fimmu.2023.1280580.
Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-targeted chimeric antigen receptor (CAR) T cells in CAR-naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. 2021;39:3044–55. https://doi.org/10.1200/JCO.20.03458.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59. https://doi.org/10.1056/NEJMoa1709919.
Ortiz-Maldonado V, Rives S, Español-Rego M, Alonso-Saladrigues A, Montoro M, Magnano L, et al. Factors associated with the clinical outcome of patients with relapsed/refractory CD19 + acute lymphoblastic leukemia treated with ARI-0001 CART19-cell therapy. J Immunother Cancer. 2021;9:e003644 https://doi.org/10.1136/jitc-2021-003644.
Myers RM, Taraseviciute A, Steinberg SM, Lamble AJ, Sheppard J, Yates B, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 2022;40:932–44. https://doi.org/10.1200/JCO.21.01405.
Singh N, Frey NV, Engels B, Barrett DM, Shestova O, Ravikumar P, et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat Med. 2021;27:842–50. https://doi.org/10.1038/s41591-021-01326-5.
Schultz LM, Jeyakumar N, Kramer AM, Sahaf B, Srinagesh H, Shiraz P, et al. CD22 CAR T cells demonstrate high response rates and safety in pediatric and adult B-ALL: Phase 1b results. Leukemia. 2024;38:963–8. https://doi.org/10.1038/s41375-024-02220-y.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38:171–80. https://doi.org/10.1080/02763869.2019.1588072.
Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21:111. https://doi.org/10.1186/s12874-021-01308-8.
Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14:395–411. https://doi.org/10.1002/sim.4780140406.
Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 2010;36. https://doi.org/10.18637/jss.v036.i03.
Van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. https://doi.org/10.1002/sim.1040.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
Elsallab M, Ellithi M, Hempel S, Abdel-Azim H, Abou-el-Enein M. Long-term response to autologous anti-CD19 chimeric antigen receptor T cells in relapsed or refractory B cell acute lymphoblastic leukemia: a systematic review and meta-analysis. Cancer Gene Ther. 2023;30:845–54. https://doi.org/10.1038/s41417-023-00593-3.
Roddie C, Sandhu KS, Tholouli E, Logan AC, Shaughnessy P, Barba P, et al. Obecabtagene autoleucel in adults with B-cell acute lymphoblastic leukemia. N Engl J Med. 2024;391:2219–30. https://doi.org/10.1056/NEJMoa2406526.
Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40:945–55. https://doi.org/10.1200/JCO.20.03585.
Rabian F, Beauvais D, Marchand T, Furst S, Huynh A, Brissot E, et al. Efficacy and tolerance of brexucabtagene autoleucel in adults with R/R B-ALL: A GRAALL study from the DESCAR-T registry. Blood Adv. 2024;8:5493–6. https://doi.org/10.1182/bloodadvances.2024013962.
Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol. 2020;7:e816–26. https://doi.org/10.1016/S2352-3026(20)30277-5.
Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunol. 2019;8:e1049. https://doi.org/10.1002/cti2.1049.
Guedan S, Madar A, Casado-Medrano V, Shaw C, Wing A, Liu F, et al. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J Clin Invest. 2020;130:3087–97. https://doi.org/10.1172/JCI133215.
Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–54. https://doi.org/10.1038/s41591-022-01969-y.
Schubert M-L, Schmitt A, Hückelhoven-Krauss A, Neuber B, Kunz A, Waldhoff P, et al. Treatment of adult ALL patients with third-generation CD19-directed CAR T cells: results of a pivotal trial. J Hematol OncolJ Hematol Oncol. 2023;16:79. https://doi.org/10.1186/s13045-023-01470-0.
Shah BD, Cassaday RD, Park JH, Houot R, Oluwole OO, Logan AC, et al. Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3. J Immunother Cancer. 2023;11:e007118. https://doi.org/10.1136/jitc-2023-007118.
Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135:1650–60. https://doi.org/10.1182/blood.2019002936.
Myers RM, Devine K, Li Y, Lawrence S, Leahy AB, Liu H, et al. Reinfusion of CD19 CAR T cells for relapse prevention and treatment in children with acute lymphoblastic leukemia. Blood Adv. 2024;8:2182–92. https://doi.org/10.1182/bloodadvances.2024012885.
An L, Lin Y, Deng B, Yin Z, Zhao D, Ling Z, et al. Humanized CD19 CAR-T cells in relapsed/refractory B-ALL patients who relapsed after or failed murine CD19 CAR-T therapy. BMC Cancer. 2022;22:393. https://doi.org/10.1186/s12885-022-09489-1.
Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293–300. https://doi.org/10.1038/s41586-019-1805-z.
Cao J, Wang G, Cheng H, Wei C, Qi K, Sang W, et al. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2018;93:851–8. https://doi.org/10.1002/ajh.25108.
Boulch M, Cazaux M, Loe-Mie Y, Thibaut R, Corre B, Lemaître F, et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci Immunol. 2021;6:eabd4344. https://doi.org/10.1126/sciimmunol.abd4344.
Jiang H, Li C, Yin P, Guo T, Liu L, Xia L, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am J Hematol. 2019;94:1113–22. https://doi.org/10.1002/ajh.25582.
Gabelli M, Oporto-Espuelas M, Burridge S, Chu J, Farish S, Hedges E, et al. Maintenance therapy for early loss of B-cell aplasia after anti-CD19 CAR T-cell therapy. Blood Adv. 2024;8:1959–63. https://doi.org/10.1182/bloodadvances.2023011168.
Maintenance therapy for early loss of B-cell aplasia after anti-CD19 CAR T-cell therapy | Blood Advances | American Society of Hematology n.d. https://ashpublications.org/bloodadvances/article/8/8/1959/498307/Maintenance-therapy-for-early-loss-of-B-cell (accessed November 12, 2025).
Laetsch TW, Maude SL, Rives S, Hiramatsu H, Bittencourt H, Bader P, et al. Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J Clin Oncol. 2023;41:1664–9. https://doi.org/10.1200/JCO.22.00642.
Kwag D, Yoon J-H, Min GJ, Park S-S, Park S, Lee S-E, et al. Outcome of second allogeneic hematopoietic cell transplantation in adult patients with relapsed B-cell acute lymphoblastic leukemia in the era of new immunotherapeutic agents. Bone Marrow Transpl. 2025;60:1249–57. https://doi.org/10.1038/s41409-025-02639-6.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–23. https://doi.org/10.1182/blood-2014-12-580068.
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, et al. Potent Anti-leukemia Activities of Chimeric Antigen Receptor–Modified T Cells against CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic Leukemia. Clin Cancer Res. 2017;23:3297–306. https://doi.org/10.1158/1078-0432.CCR-16-1799.
Jacoby E, Bielorai B, Avigdor A, Itzhaki O, Hutt D, Nussboim V, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol. 2018;93:1485–92. https://doi.org/10.1002/ajh.25274.
Li S, Zhang J, Wang M, Fu G, Li Y, Pei L, et al. Treatment of acute lymphoblastic leukaemia with the second generation of CD 19 CAR -T containing either CD 28 or 4-1 BB. Br J Haematol. 2018;181:360–71. https://doi.org/10.1111/bjh.15195.
Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. 2020;38:415–22. https://doi.org/10.1200/JCO.19.01892.
Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–8. https://doi.org/10.1182/blood.2019001641.
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133:1652–63. https://doi.org/10.1182/blood-2018-11-883710.
Ghorashian S, Kramer AM, Onuoha S, Wright G, Bartram J, Richardson R, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408–14. https://doi.org/10.1038/s41591-019-0549-5.
Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33:2854–66. https://doi.org/10.1038/s41375-019-0488-7.
Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol. 2019;98:1721–32. https://doi.org/10.1007/s00277-019-03685-z.
Tu S, Huang R, Guo Z, Deng L, Song C, Zhou X, et al. Shortening the ex vivo culture of CD19-specific CAR T-cells retains potent efficacy against acute lymphoblastic leukemia without CAR T-cell-related encephalopathy syndrome or severe cytokine release syndrome. Am J Hematol. 2019;94:E322–5. https://doi.org/10.1002/ajh.25630.
Ma F, Ho J, Du H, Xuan F, Wu X, Wang Q, et al. Evidence of long-lasting anti-CD19 activity of engrafted CD19 chimeric antigen receptor–modified T cells in a phase I study targeting pediatrics with acute lymphoblastic leukemia. Hematol Oncol. 2019;37:601–8. https://doi.org/10.1002/hon.2672.
Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol. 2020;38:1938–50. https://doi.org/10.1200/JCO.19.03279.
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol OncolJ Hematol Oncol. 2020;13:30. https://doi.org/10.1186/s13045-020-00856-8.
Zhang X, Lu X, Yang J, Zhang G, Li J, Song L, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020;4:2325–38. https://doi.org/10.1182/bloodadvances.2020001466.
Wang J, Mou N, Yang Z, Li Q, Jiang Y, Meng J, et al. Efficacy and safety of humanized anti-CD19-CAR-T therapy following intensive lymphodepleting chemotherapy for refractory/relapsed B acute lymphoblastic leukaemia. Br J Haematol. 2020;191:212–22. https://doi.org/10.1111/bjh.16623.
An F, Wang H, Liu Z, Wu F, Zhang J, Tao Q, et al. Influence of patient characteristics on chimeric antigen receptor T cell therapy in B-cell acute lymphoblastic leukemia. Nat Commun. 2020;11:5928. https://doi.org/10.1038/s41467-020-19774-x.
Gu R, Liu F, Zou D, Xu Y, Lu Y, Liu B, et al. Efficacy and safety of CD19 CAR T constructed with a new anti-CD19 chimeric antigen receptor in relapsed or refractory acute lymphoblastic leukemia. J Hematol OncolJ Hematol Oncol. 2020;13:122. https://doi.org/10.1186/s13045-020-00953-8.
Heng G, Jia J, Li S, Fu G, Wang M, Qin D, et al. Sustained therapeutic efficacy of humanized anti-CD19 chimeric antigen receptor T cells in relapsed/refractory acute lymphoblastic leukemia. Clin Cancer Res. 2020;26:1606–15. https://doi.org/10.1158/1078-0432.CCR-19-1339.
Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol. 2021;39:1650–9. https://doi.org/10.1200/JCO.20.02262.
Shah BD, Bishop MR, Oluwole OO, Logan AC, Baer MR, Donnellan WB, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood. 2021;138:11–22. https://doi.org/10.1182/blood.2020009098.
Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. 2021;27:1797–805. https://doi.org/10.1038/s41591-021-01497-1.
Tan Y, Cai H, Li C, Deng B, Song W, Ling Z, et al. A novel full-human CD22-CAR T cell therapy with potent activity against CD22low B-ALL. Blood Cancer J. 2021;11:71. https://doi.org/10.1038/s41408-021-00465-9.
Ortíz-Maldonado V, Rives S, Castellà M, Alonso-Saladrigues A, Benítez-Ribas D, Caballero-Baños M, et al. CART19-BE-01: a multicenter trial of ARI-0001 cell therapy in patients with CD19+ relapsed/refractory malignancies. Mol Ther. 2021;29:636–44. https://doi.org/10.1016/j.ymthe.2020.09.027.
Kadauke S, Myers RM, Li Y, Aplenc R, Baniewicz D, Barrett DM, et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J Clin Oncol. 2021;39:920–30. https://doi.org/10.1200/JCO.20.02477.
Gong W-J, Qiu Y, Li M-H, Chen L-Y, Li Y-Y, Yu J-Q, et al. Investigation of the risk factors to predict cytokine release syndrome in relapsed or refractory B-cell acute lymphoblastic leukemia patients receiving IL-6 knocking down anti-CD19 chimeric antigen receptor T-cell therapy. Front Immunol. 2022;13:922212. https://doi.org/10.3389/fimmu.2022.922212.
Talleur AC, Qudeimat A, Métais J-Y, Langfitt D, Mamcarz E, Crawford JC, et al. Preferential expansion of CD8+ CD19-CAR T cells postinfusion and the role of disease burden on outcome in pediatric B-ALL. Blood Adv. 2022;6:5737–49. https://doi.org/10.1182/bloodadvances.2021006293.
Shalabi H, Qin H, Su A, Yates B, Wolters PL, Steinberg SM, et al. CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood. 2022;140:451–63. https://doi.org/10.1182/blood.2022015795.
Ceppi F, Wilson AL, Annesley C, Kimmerly GR, Summers C, Brand A, et al. Modified manufacturing process modulates CD19CAR T-cell engraftment fitness and leukemia-free survival in pediatric and young adult subjects. Cancer Immunol Res. 2022;10:856–70. https://doi.org/10.1158/2326-6066.CIR-21-0501.
Li M, Xue S-L, Tang X, Xu J, Chen S, Han Y, et al. The differential effects of tumor burdens on predicting the net benefits of ssCART-19 cell treatment on r/r B-ALL patients. Sci Rep. 2022;12:378. https://doi.org/10.1038/s41598-021-04296-3.
Wang J, Zhang X, Zhou Z, Liu Y, Yu L, Jia L, et al. A novel adoptive synthetic TCR and antigen receptor (STAR) T-Cell therapy for B-Cell acute lymphoblastic leukemia. Am J Hematol. 2022;97:992–1004. https://doi.org/10.1002/ajh.26586.
Author information
Authors and Affiliations
Contributions
VN, GI, SC, GV, and PB contributed to the conception and original idea of the study. VN, GI, SC, and PB were responsible for data collection. VN and GV performed the data analysis and developed the analytical software. VN, GI, GV, and PB drafted the initial version of the manuscript. CC, MS, SF, and FB critically reviewed and edited the manuscript. All authors reviewed the final version of the manuscript and approved it for submission.
Corresponding author
Ethics declarations
Competing interests
VN declares no conflicts of interest. GI reports consultancy and honoraria from Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, AbbVie, Janssen, Sandoz, Miltenyi, and AstraZeneca. SC declares no conflicts of interest. CC has received consultancy or advisory fees from Regeneron, BMS, and Takeda; and honoraria from Takeda and Novartis. MS-S has received honoraria for presentations from Kite and support for attending meetings from Takeda. SF declares no conflicts of interest. FB has received honoraria from Roche, Novartis, AstraZeneca, Lilly, BMS GmbH & Co KG, Merck, Johnson & Johnson/Janssen, BeiGene, Advantage Pharmaceuticals, ASCEND Therapeutics, and AbbVie; has served on advisory boards or as a consultant for AstraZeneca, Roche/Genentech, Janssen-Cilag, Lilly, AbbVie, Kite (a Gilead company), BeiGene, and Novartis; has participated in speakers’ bureaus for AbbVie, Janssen, Roche, AstraZeneca, Merck, Bristol Myers Squibb/Celgene, Kite/Gilead, and Johnson & Johnson/Janssen; has received research funding from Janssen and AstraZeneca; and has received travel support from AstraZeneca, BeiGene, Johnson & Johnson/Janssen, and AbbVie. GV has received speaker fees from Pfizer, MSD, GSK, and Pierre Fabre; consultancy fees from Reveal Genomics; and has served in an advisory role with AstraZeneca, all outside the submitted work. PB has received honoraria from Allogene, Amgen, BMS, Kite/Gilead, Janssen, Jazz Pharmaceuticals, Miltenyi, Novartis, and Nektar.
Ethics approval and consent to participate
This study is a systematic review and meta-analysis based exclusively on data from previously published studies. No new data were collected, and no individual patient-level data were accessed or analyzed. Therefore, ethical approval and informed consent were not required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Navarro, V., Iacoboni, G., Camarillas, S. et al. CAR T-cell therapy in patients with acute lymphoblastic leukemia: a systematic review and meta-analysis. Bone Marrow Transplant (2026). https://doi.org/10.1038/s41409-026-02803-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41409-026-02803-6