Abstract
Background
Brain metastases (BM) are common among HER2+ breast cancer (BC) and prognostic stratification is crucial for optimal management. BC-GPA score and subsequent refinements (modified-GPA, updated-GPA) recapitulate prognostic factors. Since none of these indexes includes extracranial disease control, we evaluated its prognostic value in HER2+ BCBM.
Methods
Patients diagnosed with HER2+ BCBM at Istituto Oncologico Veneto-Padova (2002–2021) and Montpellier Cancer Institute (2001–2015) were included as exploratory and validation cohorts, respectively. Extracranial disease control at BM diagnosis (no disease/stable disease/response vs. progressive disease) was evaluated.
Results
In the exploratory cohort of 113 patients (median OS 12.2 months), extracranial control (n = 65, 57.5%) was significantly associated with better OS at univariate (median OS 17.7 vs. 8.7 months, p = 0.005) and multivariate analysis after adjustment for BC-GPA (HR 0.61, 95% CI 0.39–0.94), modified-GPA (HR 0.64, 95% CI 0.42–0.98) and updated-GPA (HR 0.63, 95% CI 0.41–0.98). The prognostic impact of extracranial disease control (n = 66, 56.4%) was then confirmed in the validation cohort (n = 117) at univariate (median OS 20.2 vs. 9.1 months, p < 0.001) and multivariate analysis adjusting for BC-GPA (HR 0.41, 95% CI 0.27–0.61), modified-GPA (HR 0.44, 95% CI 0.29–0.67) and updated-GPA (HR 0.42, 95% CI 0.28–0.63).
Conclusions
Extracranial disease control provides independent prognostic information in HER2+ BCBM beyond commonly used prognostic scores.
Similar content being viewed by others
Background
Breast cancer (BC) is one of the main causes of brain metastases (BM) among solid tumours. About 30–50% of patients with metastatic BC will eventually develop central nervous system (CNS) involvement [1]. In particular, the incidence of BM in metastatic BC patients has progressively increased over the years [2], mainly due to a better control of systemic disease and longer survival. The risk is especially high in patients with Human Epidermal growth factor Receptor 2 (HER2)-positive or triple negative BC [3, 4].
The diagnosis of BM in BC patients is generally considered as associated with worse prognosis compared to other metastatic sites. However, patients with BCBM represent a heterogeneous population and prognosis is variable according to clinical and histopathologic factors [5,6,7,8]. A personalised clinical management with a multimodal integration of local and systemic treatments is therefore recommended [9, 10]. In this complex scenario, an adequate prediction of patient prognosis is needed to support the treatment decision making process. For example, recent guidelines suggest the possibility to delay potentially toxic local therapies such as whole-brain radiotherapy (WBRT) if highly active systemic treatments, such as novel anti-HER2 drugs, are available, with the aim of postponing long-term cognitive side effects in patients for which a long survival is expected [10, 11]. Moreover, anti-HER2 therapies have deeply changed the treatment approach and prognosis of early and advanced HER2-positive BC and the development of anti-HER2 drugs with high intracranial activity has represented one of the major improvements in the treatment of BCBM [12, 13]. As the number of treatment options for patients with HER2-positive BCBM expands and patient prognosis improves, an accurate prognostic prediction is becoming even more relevant, specifically in this subgroup of patients.
Efforts to prognosticate the outcome of patients with BCBM have led to the subsequent development of several different and eventually more precise scores. Some of the first indexes, e.g. the Graded Prognostic Assessment (GPA), were originally developed based on data from cohorts including patients with different primary solid tumours [14]. Subsequently, acknowledging the relevant differences in clinical behaviour and treatment options according to tumour histology, tumour-specific scores have been proposed. In 2012, Sperduto et al. identified, in a cohort of 400 BC patients with BM, Karnofsky Performance Status (KPS), BC subtype (as defined according to hormone receptors and HER2 status) and age as independent prognostic factors and these three variables were included in the breast-specific GPA index [15]. The accuracy of this score was then improved by the addition of number of BM (modified breast-GPA score) as proposed by Subbiah et al. [16] and further updated by the addition of presence/absence of extracranial metastases (updated breast-GPA) [17].
It should however be acknowledged that all these prognostic scores were designed based on data from patients’ cohorts including all BC subtypes (as defined according to hormone receptors and HER2 status). As differences in biology, clinical history and treatment options among BC subtypes increase, further development from breast-specific prognostic scores to BC subtype-specific prognostic scores may be required to account for this evolution. For example, new targeted therapeutic options for patients with HER2-positive BCBM have significantly improved intracranial disease control, thus potentially enhancing the prognostic impact of extracranial disease control (beyond the simple presence or absence of extracranial disease), specifically in this subgroup [18]. Moreover, extracranial disease control also impacts treatment decision in this setting.
This study was designed to assess and validate the potential prognostic impact of extracranial disease control, beyond commonly used prognostic scores, specifically in patients with HER2-positive BCBM.
Methods
Patients
Consecutive patients newly diagnosed with BM from HER2-positive BC at Istituto Oncologico Veneto - Padova (Italy) between 2002 and 2021 were included as exploratory cohort. Patients newly diagnosed with HER2-positive BCBM at Montpellier Cancer Institute - Montpellier (France) between 2001 and 2015 were included as validation cohort. Inclusion criteria were: histologically proven HER2-positive BC according to current ASCO/CAP recommendations [19], age >18 years at time of BC diagnosis, intraparenchymal BM radiologically confirmed using contrast-enhanced cerebral computed tomography scan and/or magnetic resonance imaging of the brain, availability of key prognostic factors needed to calculate GPA prognostic scores (age, KPS, tumour subtype, presence of extracranial disease). Patients with diagnosis of leptomeningeal carcinomatosis alone, in the absence of intraparenchymal BM, were excluded, while patients with intraparenchymal BM who were also diagnosed with leptomeningeal disease at time or after BM diagnosis were included.
Demographic, clinicopathologic and treatment data were retrospectively collected from medical charts in a dedicated database. Oestrogen receptor (ER) and progesterone receptor (PgR) expression were determined by immunohistochemistry; positivity was defined as immunohistochemistry staining in at least 1% of tumour cells. The tumour was considered HER2 positive if scored 3+ by immunohistochemistry or if the HER2 gene was amplified by fluorescence or chromogenic in situ hybridisation (FISH/CISH) for immunohistochemistry 2+ cases.
For each patient, breast-GPA, modified breast-GPA and updated breast-GPA were calculated based on published criteria [15,16,17]. Each score divides patients into four groups: 0–1.0, 1.5–2.0, 2.5–3.0, and 3.5–4.0; a score of 4.0 is associated with best prognosis for all three scores. Radiological extracranial disease control at the time of BM diagnosis (disease control: no evidence/stable disease/partial response/complete response of extracranial disease vs. progression of extracranial disease) was evaluated according to RECIST 1.1 criteria.
This study was reviewed and approved by the involved Institutional Review Boards and Ethics Committees. Where necessary according to local regulation, written informed consent was obtained from participants. The study was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical analysis was performed using IBM SPSS (version 25). Descriptive statistics were performed for patients’ demographics and clinical characteristics. The Chi-squared test (χ2) test was used to study association between variables.
Overall survival from BM diagnosis (OS) was defined as time from BM diagnosis to death from any cause. Patients alive without event at cut-off date of this analysis were censored at date of last follow-up. OS was estimated using the Kaplan–Meier method and reported with its 95% confidence intervals (95% CIs). The log-rank test was used to compare OS between groups. Univariate and multivariate Cox regression modelling for proportional hazards was used to calculate hazard ratios and their 95% CI. Likelihood ratio test χ2 and p values were generated comparing consolidate prognostic scores with and without the addition of extracranial disease control in order to test for the additional prognostic value of extracranial disease control.
All reported p values were two-sided and significance level was set at 5% (p < 0.05).
Results
Patient characteristics in the exploratory cohort
Overall, 113 HER2-positive BC patients diagnosed with BM were identified and included in the exploratory cohort. Main patient and tumour characteristics are summarised in Table 1.
Median age at the time of BM diagnosis was 55 years (range 26–84). More than half of the tumours (n = 66, 58.4%) were hormone receptor positive. Most patients (n = 85, 75.2%) had a conserved performance status (KPS ≥ 70) at the time of BM diagnosis. Approximately one third of patients presented a single BM while 44% had more than 3 brain lesions at time of BM diagnosis. Moreover, although the large majority of patients presented extra-CNS lesions (n = 97, 85.8%), in most cases extracranial disease was under control (n = 65, 57.5%) at the time of BM diagnosis. Median time between BM diagnosis and extracranial status assessment was 6.0 days (95% CI 0.0–12.2). Among patients with systemic disease control at BM diagnosis, 30.8% (n = 20) eventually developed an extracranial progression subsequently, with a median time to extracranial progression of 5.2 months (96% CI 3.2–7.2) in this subgroup of patients. Concomitant leptomeningeal metastasis at BM diagnosis were present in 8 (7.1%) patients (3 with extracranial disease control and 5 with extracranial progression).
After BM diagnosis, the majority of patients received active therapy for BCBM with at least one treatment modality, local or systemic, while only 13 patients (11.5%) were treated with best supportive care alone. Most patients (n = 91, 80.5%) received at least one line of systemic treatment, which included an anti-HER2 targeted agent in most cases (n = 79, 69.9%). Comprehensive data regarding treatment received by patients included in the exploratory cohort are reported in Supplementary Table 1.
Prognostic factors for overall survival in the exploratory cohort: univariate and multivariate analysis
With a median follow-up of 53.3 months (95% CI 33.9–72.6), 92 patients (81.4%) had died. Median OS from the time of BM diagnosis was 12.2 months (95% CI 6.2–18.1). Among the 64 patients for whom the cause of death was available, 73.4% (n = 47) died because of intracranial progression (60.7% among patients with extracranial progression at BM diagnosis and 83.3% among patients with extracranial control; p = 0.042).
The association between several known prognostic factors and OS from BM diagnosis was investigated using univariate Cox regression modelling (Table 2). As expected, Karnofsky Performance Status, number of BM and presence of extracranial metastases were all significantly associated with OS from BM diagnosis. All the prognostic score tested (breast-GPA, modified breast-GPA, updated breast-GPA) were significantly associated with OS from BM diagnosis.
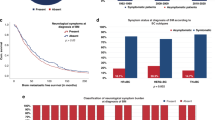
In addition, extra-CNS disease control was also identified as a significant prognostic factor at univariate analysis with a median OS of 17.7 and 8.7 months for patients with or without extracranial disease control, respectively (log-rang p = 0.005; Fig. 1a).
To evaluate whether extra-CNS disease control added independent prognostic information to each of the three prognostic scores (breast-GPA, modified breast-GPA, updated breast-GPA), a multivariate analysis was performed. Each prognostic score was tested separately to account for the significant overlap among the different prognostic scores.
Extra-CNS disease control maintained its independent prognostic value after correction for each of the validated prognostic scores: breast-GPA score (HR 0.61, 95% CI 0.39–0.94, p = 0.025), modified breast-GPA score (HR 0.64, 95% CI 0.42–0.98, p = 0.039) and updated breast-GPA score (HR 0.63, 95% CI 0.41–0.98, p = 0.040). The likelihood ratio test was also used to evaluate for the added prognostic value of extracranial disease control to each GPA score, showing an improvement in terms of prognosis prediction by the inclusion of extracranial disease control (Table 3 and Supplementary Table 4).
Validation cohort: patient characteristics and prognostic factors for overall survival
To validate our results, 117 patients diagnosed with HER2-positive BCBM at the Montpellier Cancer Institute between 2001 and 2015 were included as validation cohort (Table 1). Similarly to the exploratory cohort, median age at BC diagnosis was 50 years (range 22–79). Almost half of the tumours (n = 51, 43.6%) were hormone receptor positive and most patients (n = 88, 75.2%) had a conserved performance status (KPS ≥ 70) at time of BM diagnosis. More than half of cases (n = 66, 56.4%) presented more than 3 brain lesions at time of BM diagnosis. Although the large majority of patients presented extra-CNS lesions (n = 105, 89.7%), in most cases extracranial disease was under control (n = 66, 56.4%) at time of BM diagnosis and median time between BM diagnosis and extracranial disease assessment was 6.0 day (95% CI 1.8–10.2). Among patients with systemic disease control at BM diagnosis, 45.4% (n = 30) eventually developed an extracranial progression and median time to extracranial progression was 5.0 months (96% CI 3.9–6.1). Six (5.1%) patients presented concomitant leptomeningeal metastases (4 among patients with extracranial disease control and 2 among patient with extracranial progression). Most patients (n = 102, 87.9%) in the validation cohort received systemic treatments (which included an anti-HER2 therapy in 83.6% of cases) and radiotherapy (n = 101, 86.3%). Detailed data regarding treatment received by patients included in the validation cohort are presented in Supplementary Table 2.
The validation cohort presented a longer median follow-up of 96.6 months (95% CI 84.4–108.8), at which time 111 patients (94.9%) had died. Median OS in the validation cohort was 12.7 months (95% CI 8.5–17.7), very similar to what observed in the exploratory cohort. Intracranial progression represented the cause of death for 52 of the 91 (57.1%) patients for whom this information was available (43.6% among patients with extracranial progression at BM diagnosis and 67.3% among patients with extracranial control; p = 0.023).
The association between known prognostic factors and OS from BM diagnosis in the validation cohort was investigated using univariate Cox regression modelling (Supplementary Table 3). Karnofsky performance status and all GPA scores were associated with OS, while number of BM and presence of extracranial metastases were not confirmed as significant predictor of OS in the validation cohort. In addition, the prognostic role of extracranial disease control was confirmed with a median OS of 20.2 months and 9.1 months in patients with and without extracranial disease control, respectively (log-rank p < 0.001; Fig. 1b).
Therefore, we tested in multivariate analysis if extra-CNS disease control added independent prognostic information to each of the three prognostic scores (breast-GPA, modified breast-GPA, updated breast-GPA). In the validation cohort, the prognostic impact of extracranial disease control was maintained at multivariate analysis after adjusting for breast-GPA (HR 0.41, 95% CI 0.27–0.61, p < 0.001), modified breast-GPA (HR 0.44, 95% CI 0.29–0.67, p < 0.001) and updated breast-GPA (HR 0.42, 95% CI 0.28–0.63, p < 0.001). The added prognostic value of extracranial disease control was also confirmed applying the likelihood ratio test to the model adding extracranial disease control to each GPA score (Table 3 and Supplementary Table 4). A graphic representation of OS in the validation cohort according to extracranial disease control in each prognostic group of the three GPA scores is shown in Fig. 2.
Discussion
The therapeutic scenario of HER2-positive BC patients diagnosed with BM has radically changed over the last few decades with the introduction of several novel HER2-targeted agents with relevant intracranial activity alongside with improvements in locoregional treatment modalities [18]. In the context of increased chances of intracranial disease control, the prognostic impact of extracranial disease control (beyond the simple presence or absence of extracranial disease) might potentially be enhanced. Therefore, we designed the present study to evaluate the potential prognostic impact of extracranial disease control, beyond commonly used prognostic scores, specifically in patients with HER2-positive BCBM. To that end, we assessed the prognostic impact of extracranial disease control in two separate large cohorts of HER2+ BCBM patients and successfully identified in both cohorts that it adds independent prognostic information to commonly used prognostic indexes.
It’s worth noticing that in both cohorts the majority of patients did not present a progressive extracranial disease at the time of BM diagnosis. This information carries important therapeutic implications: on one hand, current guidelines recommend not to switch systemic treatments in case of isolated brain progression; on the other hand, more aggressive locoregional therapies such as neurosurgery are generally discouraged in case of concomitant progressive systemic disease [9, 11]. Extracranial disease control should therefore be always assessed at the moment of BM diagnosis and should be taken into account in the multidisciplinary management of these patients.
The prognostic impact of extracranial disease control has been previously assessed in cohorts including patients with BM from different solid tumours; however, contradictory results have been reported [20,21,22] and extracranial disease control has not been univocally established as an independent prognostic factor among patients with BM. Moreover, even if the recently updated breast-GPA [17] has been modified to include the presence or absence of extra-CNS lesions, the potential prognostic impact of extracranial disease control or progression was not evaluated.
Previous studies already suggested the prognostic role of extracranial disease control in patients with HER2-positive BCBM. In 2009, Park et al. reported extracranial disease control as a significant prognostic factor at multivariate analysis in 77 patients diagnosed with HER2+ BCBM after the introduction of anti-HER2 targeted therapies. However, this study did not address the added value of extracranial disease control as compared to validated prognostic scores and the findings were not validated in an independent cohort [23]. More recently, Noteware et al. reported data regarding the potential impact of extracranial disease status in a cohort of 153 patients with HER2-positive BCBM treated with CNS radiation. In this cohort, OS from first BM diagnosis was significantly worse for patients with progressive extracranial disease as compared to patients with stable/responding extracranial disease or no extracranial disease (log-rank p = 0.008) [24]. Still, additional value of extracranial disease control as compared to prognostic scores was not reported, neither validated in an independent cohort.
These findings highlight the importance of an accurate selection of homogeneous groups of patients with BM in order to better capture different clinical behaviours and treatment availabilities and therefore to identify more specific prognostic factors. In this context, even BC-specific scores may not be sufficiently precise to adequately describe prognosis in all the different BC subgroups. Indeed, according to the breast-GPA and the updated-GPA, no patients with HER2-positive BCBM can be classified in the worst prognosis category. Nevertheless, a wide range of outcomes has been described also among patients with HER2-positive BCBM [15, 17]. The accurate prognostic assessment of BC patients diagnosed with BM is not trivial: in this challenging clinical scenario, where different treatment modalities are usually integrated, prognostic scores can aid physicians in treatment choice. In particular, as HER2 positivity is considered a positive prognostic factor for BC patients with BM and, in some cases with expected good prognosis, delay of local treatment is now being proposed by guidelines, an adequate prediction of outcome for these patients is even more crucial for treatment planification [3].
Our findings demonstrate in two independent patient cohorts that extracranial disease control carries significant independent prognostic information, beyond commonly used prognostic score. This observation supports the integration of this feature in future prognostic scores specifically designed for HER2-positive BC patients with BM. Indeed, the impact of extracranial control on patients’ outcome could become even more relevant in the next few years as the number of anti-HER2 targeted agents continues to increase. Recently approved drugs, such as trastuzumab deruxtecan and tucatinib, demonstrated a remarkable intracranial disease response rate [25,26,27] while several new strategies are being tested in dedicated clinical trials (NCT04539938, NCT04512261, NCT04639271). Moreover, the introduction in clinical practise of new drugs able to change the history of HER2-positive disease highlights the need of continuously reassessing prognostic factors in contemporary cohorts.
Data availability
Data are available upon reasonable request to the corresponding author.
References
Lin NU, Gaspar LE, Soffietti R. Breast cancer in the central nervous system: multidisciplinary considerations and management. Am Soc Clin Oncol Educ Book. 2017;37:45–56.
Smedby KE, Brandt L, Bäcklund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101:1919–24.
Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121:991–1000.
Darlix A, Griguolo G, Thezenas S, Kantelhardt E, Thomssen C, Dieci MV, et al. Hormone receptors status: a strong determinant of the kinetics of brain metastases occurrence compared with HER2 status in breast cancer. J Neurooncol. 2018;138:369–82.
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3:1069–77.
Witzel I, Laakmann E, Weide R, Neunhöffer T, Park-Simon TJ, Schmidt M, et al. Treatment and outcomes of patients in the Brain Metastases in Breast Cancer Network Registry. Eur J Cancer. 2018;102:1–9.
Griguolo G, Dieci MV, Giarratano T, Giorgi CA, Orvieto E, Ghiotto C, et al. Beyond breast specific-Graded Prognostic Assessment in patients with brain metastases from breast cancer: treatment impact on outcome. J Neurooncol. 2017;131:369–76.
Griguolo G, Jacot W, Kantelhardt E, Dieci MV, Bourgier C, Thomssen C, et al. External validation of Modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases: a multicentric European experience. Breast 2018;37:36–41.
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40:492–516.
le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32:1332–47.
Ramakrishna N, Anders CK, Lin NU, Morikawa A, Temin S, Chandarlapaty S, et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40:2636–55.
Guarneri V, Barbieri E, Dieci MV, Piacentini F, Conte P. Anti-HER2 neoadjuvant and adjuvant therapies in HER2 positive breast cancer. Cancer Treat Rev. 2010;36:S62–6.
Miglietta F, Bottosso M, Griguolo G, Dieci MV, Guarneri V. Major advancements in metastatic breast cancer treatment: when expanding options means prolonging survival. ESMO Open. 2022;7:100409.
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4.
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–7.
Subbiah IM, Lei X, Weinberg JS, Sulman EP, Chavez-Macgregor M, Tripathy D, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33:2239–45.
Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Beyond an updated Graded Prognostic Assessment (Breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107:334–43.
Garcia-Alvarez A, Papakonstantinou A, Oliveira M. Brain metastases in HER2-positive breast cancer: current and novel treatment strategies. Cancers. 2021;13:2927.
Wolff AC, Elizabeth Hale Hammond M, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Lorenzoni J, Devriendt D, Massager N, David P, Ruíz S, Vanderlinden B, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–24.
Hazuka MB, Burleson WD, Stroud DN, Leonard CE, Lillehei KO, Kinzie JJ. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J Clin Oncol. 1993;11:369–73.
Ahn HK, Lee S, Park YH, Sohn JH, Jo JC, Ahn JH, et al. Prediction of outcomes for patients with brain parenchymal metastases from breast cancer (BC): a new BC-specific prognostic model and a nomogram. Neuro Oncol. 2012;14:1105–13.
Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, et al. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100:894–900.
Noteware L, Broadwater G, Dalal N, Alder L, Herndon JE, Floyd SR, et al. Brain metastasis as first and only metastatic relapse site portends poor outcomes in patients with advanced HER2+ breast cancer. J Clin Oncol. 2022;40:1045. https://doi.org/10.1200/JCO.2022.40.16_suppl.1045.
Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. 165MO Trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients (pts) with active brain metastases: primary outcome analysis from the TUXEDO-1 trial. Ann Oncol. 2022;33:S198.
Jerusalem GHM, Park YH, Yamashita T, Hurvitz SA, Modi S, Andre F, et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J Clin Oncol. 2021;39:526. https://doi.org/10.1200/JCO.2021.39.15_suppl.526.
Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38:2610–9.
Funding
The authors acknowledge grants from: Fondazione AIRC under 5 per mille 2019 - ID. 22759 program- G.L.VG; Veneto Institute of Oncology IOV-IRCCS to MVD and VG; Veneto Institute of Oncology IOV-IRCCS “5x1000 anno di riferimento 2015 - Genomica dei tumori e immunoterapia nell'era dei big data, fase 2” to VG; DOR funding from the University of Padova to GG, MVD, VG; 2019 Conquer Cancer Foundation of ASCO/Shanken Family Foundation Young Investigator Award to GG.
Author information
Authors and Affiliations
Contributions
Study design: GG, MVD and AD. Acquisition of clinical data: MB, GG, LS, MCG, VA, FM, GV, CB, FG, WJ, VG, AD and MVD. Data analysis and interpretation: MB, GG, FG, AD and MVD. Manuscript drafting: MB and GG. Manuscript revision: LS, MCG, VA, FM, CB, FG, WJ, VG, AD and MVD. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
GG reports fees from EliLilly, Novartis and Gilead; FM reports fees from Novartis and Roche; FG reports fees from Gilead and Lilly; VG reports fees from Amgen, Exact Sciences, Gilead, GSK, EliLilly, Merck Serono, MSD, Novartis, Pfizer, Sanofi; MVD reports fees from AstraZeneca, Daiichi Sankyo, EliLilly, Exact Sciences, Gilead, MSD, Novartis, Pfizer, Seagen. WJ reports grants, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Eisai, personal fees and non-financial support from Novartis, personal fees and non-financial support from Roche, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Eli Lilly, personal fees from MSD, personal fees from BMS, personal fees and non-financial support from Chugai, personal fees from Seagen, grants and personal fees from Daiichi Sankyo, outside the submitted work. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the ethics committee of Istituto Oncologico Veneto and Montpellier Regional Cancer Institute and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from participants, where necessary according to local regulation.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bottosso, M., Griguolo, G., Sinoquet, L. et al. Prognostic impact of extracranial disease control in HER2+ breast cancer-related brain metastases. Br J Cancer 128, 1286–1293 (2023). https://doi.org/10.1038/s41416-023-02153-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41416-023-02153-w
This article is cited by
-
Temporal evolution of breast cancer brain metastases treatments and outcomes
npj Breast Cancer (2025)
-
Predictive role of intracranial PD-L1 expression in a real-world cohort of NSCLC patients treated with immune checkpoint inhibition following brain metastasis resection
Journal of Neuro-Oncology (2024)