Abstract
Background
Thyroid cancer is diagnosed at relatively young ages compared to other adult cancers, for reasons that remain unclear. Our study aimed to investigate associations of in-utero and newborn characteristics with differentiated thyroid cancer (DTC) incidence in adult women.
Methods
From the U.S. nationwide Sister Study cohort, we included 47,913 cancer-free women at baseline (2003–2009). We assessed associations of participants’ in-utero and newborn characteristics and DTC during follow-up using Cox regression models adjusted for attained age (timescale) and race/ethnicity.
Results
During follow-up (median = 13.1 years), 239 incident DTC cases were identified. Higher DTC incidence was associated with maternal pre-pregnancy or gestational diabetes (hazard ratio [HR] = 2.36, 95%CI = 0.97–5.74, 5 affected cases), gestational hypertension or hypertension-related disorders (HR = 1.99, 95%CI = 1.20–3.32, 16 affected cases), and higher birth weight (HR per kg=1.24, 95%CI = 0.95–1.60). Births occurring at least two weeks before the due date were associated with lower DTC incidence (HR = 0.47, 95%CI = 0.23–0.97, 8 affected cases). In a model simultaneously adjusted for all these factors, all exposures remained associated with DTC incidence. We observed no associations for other in-utero and newborn characteristics.
Conclusions
These findings contribute to a growing body of evidence that in-utero exposures related to maternal metabolic abnormalities may influence thyroid cancer risk later in life.
Similar content being viewed by others
Introduction
Thyroid cancer has emerged as the fifth most common cancer diagnosed among women globally, with most cases classified as differentiated thyroid cancer (DTC) [1]. Yet, the etiology of thyroid cancer remains largely unknown, with obesity and childhood exposure to ionizing radiation among the few established modifiable risk factors [2]. The disease is diagnosed at relatively young ages compared to most other adulthood cancers [2], suggesting a potential etiologic role of early-life exposures [3, 4].
Throughout gestation, the developing thyroid is highly susceptible to endogenous (e.g., maternal iodine deficiency, thyroid dysfunction, diabetes, fluctuations in maternal and placental estrogen levels) [5,6,7,8,9] and exogenous (e.g., cigarette smoking, endocrine-disrupting chemicals [EDCs]) stressors. These factors may disrupt fetal thyroid function [10, 11], expose the fetus to elevated levels of growth factors [12] and sex steroid hormones [13], which have been suggested to contribute to thyroid cancer development in adulthood [3, 4, 14,15,16]. Some epidemiologic studies have suggested positive associations for factors reflecting fetal hormone exposures, including maternal and congenital thyroid disorders [17], higher birth weight [17,18,19], postpartum hemorrhage [17], and hyperemesis gravidarum [20], lending some support to the hypothesis that the gestational period is a susceptible exposure window in thyroid carcinogenesis. However, evidence on the impact of in-utero and birth characteristics on subsequent risk of thyroid cancer remains scarce.
To contribute to the limited research on this topic, we prospectively evaluated associations of a wide range of self-reported in-utero and newborn factors with DTC incidence in the Sister Study, a large U.S. nationwide cohort including more than 50,000 adult women who had a sister with a breast cancer diagnosis but were themselves breast cancer-free at enrollment.
Methods
Study sample
The study design, data collection, and outcome measurements of the Sister Study cohort have been previously described [21]. Briefly, between 2003 and 2009, the Sister Study recruited women aged 35–74 residing in the United States (including Puerto Rico) who had a sister diagnosed with breast cancer but no prior diagnosis themselves. At baseline, participants provided detailed information on early-life, demographic, medical, and lifestyle factors through computer-assisted telephone interviews and written questionnaires. Anthropometric measurements were also collected during in-person examinations. Detailed follow-up questionnaires were sent every 2–3 years, with response rates consistently exceeding 85% [22]. The study is approved annually by the National Institutes of Health Institutional Review Board. All participants provided written informed consent. Data are complete through mid-September 2021 (data release 11.1, https://sisterstudy.niehs.nih.gov/English/cohort-over.htm, https://sisterstudy.niehs.nih.gov/English/enroll-data.htm#SAQS).
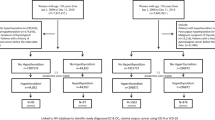
Of the 50,884 participants, we excluded those who had a history of any invasive cancer (n = 2911) or reported any chemotherapy or radiotherapy for cancer (n = 55) before baseline, and those who withdrew from the study (n = 5). After exclusions, there were 47,913 individuals in our study sample (Fig. 1).
Outcome definition
Thyroid cancer diagnoses were identified through self-report and when available, confirmed using medical records or pathology reports. By the end of follow-up in mid-September 2021, 252 cases had been reported, of which 187 were verified through medical records or pathology reports, and one additional case was confirmed via the National Death Index, death certification, or next of kin, resulting in 188 (74.6%) confirmed cases. Agreement between self-report and confirmed thyroid cancer diagnosis was excellent (positive predictive value >90%). For the current analysis, the case definition for DTC (n = 239) included all self-reported cases excluding those with the following histologic types: poorly differentiated thyroid carcinoma (confirmed with pathology reports; n = 5), anaplastic thyroid carcinoma (histology code: 8021; n = 1), medullary thyroid carcinoma (histology codes: 8346, 8347, 8510, n = 5), and indeterminate (histology code: 8265, 9084, n = 2). DTC cases with confirmed histology codes (n = 174) were further classified according to the following histologic types: papillary thyroid carcinomas (histology codes: 8050, 8260, 8340-8344, 8350, 8450-8460, n = 164), follicular thyroid carcinomas (histology codes: 8290, 8330-8335, n = 7), and unspecified carcinomas and neoplasms (histology code: 8000, 8010, n = 3).
Exposure definition
Using the baseline self-administered family history questionnaires, we ascertained information on participants’ perinatal exposures, including maternal, paternal, and birth characteristics. Characteristics of the participants’ mothers before and during pregnancy included pre-pregnancy or gestational diabetes, gestational hypertension or hypertension-related disorders (i.e., pre-eclampsia, eclampsia, and toxemia), morning sickness with vomiting, first-hand or household secondhand smoking during pregnancy, time since last pregnancy, and age at delivery. Information on the participants’ fathers characteristics included first-hand smoking during three months before conception, and age at delivery. Participants’ own birth and postpartum characteristics included birth weight, birth order, singleton versus multiple birth, gestational age at birth, whether they were breastfed, and duration of breastfeeding. For all of these, we considered responses of “definitely” and “probably” as exposed and categorized “probably not” and “definitely not” as unexposed. For birth weight analyses, women were asked to provide their birth weight, which we classified into clinically relevant categories: <2500 g, 2500–3999 g, and ≥4000 g. Women who did not specify their birth weight but reported it being less than 5 pounds (2268 g) were categorized into the <2500 g category. Missing data were classified using missing indicator variables.
Baseline covariates
In-person measurements of weight and height at baseline were used to calculate body mass index (BMI, weight in kilograms divided by the square of height in meters). Information on other characteristics, including age, self-identified race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, Non-Hispanic all other races, Unknown), personal attained educational level, personal history of benign thyroid disease, and household annual income were assessed during the computer-assisted telephone interview. Individuals were considered to have a history of benign thyroid disease if they reported a diagnosis of hyperthyroidism, hypothyroidism, non-toxic goiter/thyroid nodules, or any other thyroid conditions, or if they ever self-reported use of thyroid hormone substitutes, antithyroid, or iodine/iodide substances for medical purposes or supplements for thyroid function.
Statistical analyses
Follow-up time was calculated from age at enrollment to the age of the first diagnosis of any invasive cancer, death, loss of follow-up, or mid-September 2021, whichever occurred first. We used Cox proportional hazards models adjusted for race/ethnicity with attained age as the timescale to estimate hazard ratios (HR) for DTC incidence in relation to in-utero and newborn characteristics. For the main analyses, parental ages at delivery, birth weight, birth order, and time since last pregnancy of participants’ mother were considered both categorically and continuously. Missing data were excluded from analyses of continuous variables. There was no evidence of departure from linearity for the association between continuous variables and thyroid cancer incidence when assessing with Martingale residuals. Non-proportional hazards assumptions were assessed through plots of scaled Schoenfeld residuals against attained age, and formal testing included introducing an interaction term between exposures and attained age; no evidence of violation was identified.
We conducted several sensitivity analyses including (1) considering death and the diagnosis of invasive cancer other than thyroid and non-melanoma cancers as competing risks using Fine-Gray models [23], (2) restricting the outcome to pathologically confirmed cases, (3) restricting the outcome to papillary thyroid carcinomas, (4) conducting complete case analysis by excluding individuals with missing data on a model-specific basis, and (5) calculating E-values for both the observed association estimates and the limit of the confidence interval closest to the null. The E-value is defined as the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome, conditional on the measured covariates, to fully explain the observed associations [24, 25]. We also conducted stratified analyses according to known thyroid cancer risk factors and potential effect modifiers, including baseline BMI, benign thyroid disease status, and socioeconomic indicators (i.e., personal attained educational level and household annual income). Tests for multiplicative interactions were conducted by including a cross-product term in the model and evaluating the F-test-based P value. P values were two-sided with an alpha of 0.05.
Data analyses were conducted using SAS 9.4, R version 4.3.1 and R studio version 2023.06.2.
Results
The baseline descriptive statistics are provided in Table 1. The median age at baseline was 55.4 years (interquartile range, IQR, 48.9–62.1). Among 47,913 women included in the study, most participants were non-Hispanic White (n = 39947, 83.4%), and 30.0% (n = 14361) reported having a BMI of 30 or more. Approximately half of the participants had completed a bachelor’s degree or higher (n = 24450, 51.0%) and 33.7% reported a household annual income of $100,000 or more (n = 16133).
During follow-up (median: 13.1 years, IQR 11.5–15), 239 cases of incident DTC were reported. We found a higher DTC incidence among women born to mothers with pre-pregnancy or gestational diabetes (HR = 2.36, 95%CI 0.97–5.74, 5 affected cases), and gestational hypertension or hypertension-related disorders (HR = 1.99, 95%CI 1.20–3.32, 15 affected cases), compared to those born to mothers without these conditions. Conversely, a lower DTC incidence was observed in women who were born at least two weeks before their due date (HR = 0.47, 95%CI 0.23–0.97, 8 affected cases), compared to those born within two weeks or after their due date. Compared to those with a birth weight between 2500 and 3999 g, individuals with a lighter birth weight had a lower DTC incidence (HR = 0.60, 95%CI 0.32–1.14, 10 affected cases), while those with a heavier birth weight had a higher incidence (HR = 1.50, 95%CI 0.96–2.33, 23 affected cases). For every additional kilogram in birth weight, there was a 24% increased incidence of DTC (95%CI 0.95–1.60). We found no associations of thyroid cancer incidence with other maternal, paternal, and newborn characteristics (Table 2).
Because of the potential co-occurrence of gestational complications (e.g., gestational diabetes and hypertension) and associations with preterm delivery and small-for-gestational-age births [26], we evaluated the independent associations of pregnancy complications, gestational length at birth, and birth weight in mutually adjusted models. The HRs were similar, albeit slightly weaker for pre-pregnancy and gestational diabetes and birth weight (Table 3). The association for birth weight did not meaningfully change after excluding participants who were born at least two weeks before the due date or part of a multiple birth (167 cases, HR per kg = 1.16, 95% CI 0.87–1.56). The results were also consistent after further excluding those whose mothers had pre-pregnancy or gestational diabetes and gestational hypertension or hypertension-related disorders (152 cases, HR per kg = 1.13, 95% CI 0.83–1.54) (Supplementary Table 1). No significant interactions were observed for birth weight and DTC risk by baseline BMI, benign thyroid disease, or household annual income, although the association was strongest for women in the lowest category of education (high school education, GED diploma, or less, 18 exposed cases, HR = 2.61 for every additional kilogram, 95% CI 1.22–5.59) (Supplementary Table 2).
The interpretation of the findings did not change when accounting for deaths and other cancer diagnoses as competing risks or when considering only medically confirmed thyroid cancer, papillary thyroid cancer cases, or complete case analysis (Supplementary Table 3). The E-values for the associations of gestational hypertension or hypertension-related disorders and being born at least two weeks before the due date, and birth weight less than 2500 g were 3.40, and 3.65, respectively.
Discussion
Due to a paucity of data, few studies have examined early life exposures in relation to thyroid cancer risk later in life. Using data from a large nationwide U.S. cohort of women, the current study revealed notable associations for several in-utero and newborn factors, including higher birth weight and maternal metabolic complications (e.g., pre-pregnancy and gestational diabetes, gestational hypertension or hypertension-related disorders). Conversely, being born at least two weeks before the due date was associated with a lower incidence of DTC.
The magnitude of the association for birth weight (24% higher DTC risk for each additional kilogram, 95% CI 0.95–1.60) is consistent with results from previous studies suggesting increased risks between 14 and 30% per kilogram [17, 19]. Studies on early life exposures and childhood thyroid cancer incidence (age at diagnosis <20 years) have yielded less consistent results, with two studies reporting positive associations with risks varying between 11 and 12% per 500 grams [18, 27], while one study showed no association [28]. However, considering that the etiology of childhood thyroid cancer may be different from adulthood thyroid cancer, findings from these latter studies may not be directly comparable to ours.
Only one prior study extensively examined the associations of maternal pregnancy complications and DTC risk among adult offspring [17]. In a registry-based study combining data from four Nordic countries (mean age at diagnosis: 27.5 years), Kitahara et al. found increased incidence of thyroid cancer associated with maternal benign thyroid conditions (OR ranging from 3.3 to 67.4), maternal postpartum hemorrhage (132 exposed cases, OR = 1.28, 95%CI 1.06-1.55), pre-pregnancy diabetes (15 exposed cases, OR = 1.69, 95%CI 0.98–2.93), and congenital hypothyroidism (5 exposed cases, OR = 4·55, 95% CI 1.58-13.1). However, no associations were observed for gestational diabetes or pre-pregnancy or gestational hypertension [17].
Birth weight and maternal complications have been suggested to influence BMI in adolescence and adulthood [29,30,31], as well as adult benign thyroid disease [32]. Thus, associations of these perinatal factors with DTC incidence may be mediated by these downstream factors. However, this hypothesis was not supported by our data, as positive associations of perinatal exposures and DTC incidence persisted after stratifying by adulthood exposures. This suggests that biological factors contributing to thyroid cancer development may originate at a very young age.
Birth weight, preterm delivery, and maternal complications are closely associated factors [26]. In the current study, excluding women who were born at least two weeks before the due date or who were part of a multiple birth did not change the results for birth weight, suggesting that the association for birth weight remains independent of preterm birth. The associations between birth weight and pre-pregnancy and gestational diabetes with DTC were somewhat attenuated after adjusting for gestational hypertension or pre-eclampsia, indicating the presence of both shared and distinct underlying mechanisms. Both higher birth weight and gestational diabetes may serve as markers for aspects of the fetal environment, such as increased levels of growth factors [33] or insulin receptor activation leading to IGF-I overproduction [34]. Epidemiologic and molecular studies support a possible mediating role of IGF-I, IGF binding protein, and deregulation of the IGF axis in thyroid carcinogenesis [15, 35]. Furthermore, higher birth weight may indicate elevated levels of fetal thyroid hormones [36], with longitudinal studies suggesting that even within the clinically normal range, low levels of thyroid-stimulating hormone and high levels of thyroid hormones in adults are associated with thyroid cancer incidence [37]. On the other hand, gestational diabetes may contribute to thyroid cancer development through the effects of hyperglycemia and hyperinsulinemia on epigenetic changes in the offspring [38].
Maternal metabolic complications may also result from exposure to EDCs [39], which have been implicated in thyroid cancer development in adults [10]. While most EDC exposures do not appear to significantly affect fetal thyroid function [40], certain chemicals—such as polybrominated diphenyl ethers, per- and polyfluoroalkyl substances—can potentially impair thyroid hormone enzyme activity, reduce iodine availability to the fetus, and disrupt fetal thyroid function [13, 41]. In the Sister Study, the rarity of in-utero exposure to endocrine disruptors, such as diethylstilbestrol, and the lack of data on maternal and fetal exposures to EDCs before and during pregnancy [42] in this study precluded a direct evaluation of their effects on thyroid cancer risk in offspring. Given the lack of prior studies on this association, future studies should include detailed assessments of both maternal and fetal EDC exposure and metabolic conditions.
Strengths of this study include the large sample size, long follow-up, and wide range of in-utero and birth characteristics. While detection bias is a significant consideration in epidemiological studies on thyroid cancer incidence [2], it is unlikely to explain the notable associations in our study, as there is no apparent connection between the in-utero and newborn factors examined in our study and access to thyroid or cancer screening in adulthood. Our analysis also included an evaluation of potential factors that could influence the likelihood of incidental detection of thyroid cancer, such as household annual income and educational levels; the strength of the associations did not differ substantially by these factors.
The current study has several limitations. As the Sister Study recruited only women with a family history of breast cancer, the results may not be generalizable to men or to women without a family history of breast cancer. Self-reported early-life exposures may be prone to misclassification bias, potentially with varying degree of validity [43,44,45], although it is unlikely that report of perinatal characteristics varied by future case status; this type of bias is expected to drive associations toward the null. Although the Sister Study provided phone cards to encourage participants to contact their mothers or other relatives, whether participants contacted relatives or consulted records before reporting early-life events is unclear. Despite the large size of this cohort, there were small case numbers corresponding with some exposures of interest, which limited the statistical power of our study and our ability to test for interactions between different in-utero and newborn factors. We lacked information on maternal thyroid status, pre-pregnancy hypertension, pre-pregnancy and pregnancy BMI, and maternal weight gain during pregnancy. High maternal pre-pregnancy BMI and excessive weight gain during pregnancy have been suggested to be predictors for metabolic syndrome and obesity in the offspring [46, 47], thus potentially influencing the study results. We did not account for some well-established (e.g., exposure to ionizing radiation in childhood) or possible risk factors (e.g., iodine deficiency) of thyroid cancer, but these factors were unlikely to have been strong confounders as they are not likely to be associated with other in-utero and newborn exposures. The high E-values in analyses concerning maternal complications indicated that HRs of at least 3 for the unmeasured confounder and DTC incidence would be necessary to fully explain the observed associations.
In conclusion, our findings contribute to a growing body of evidence that in-utero exposures related to maternal metabolic abnormalities may influence thyroid cancer risk later in life, suggesting a potential target in the primary prevention of DTC. Further research using objective exposure information collected from birth and medical records or biological measurements at the time of pregnancy or birth are needed to confirm our findings while minimizing the potential for exposure misclassification and provide additional mechanistic insights.
Data availability
Data used in this article are available as described on the Sister Study website (The Sister Study: Collaborations and Data Requests, nih.gov; https://sisterstudy.niehs.nih.gov/English/cohort-over.htm; https://sisterstudy.niehs.nih.gov/English/enroll-data.htm#SAQS) or by request via the Sister Study tracking and review system (www.sisterstudystars.org; registration required).
Code availability
Computing code is available at: https://github.com/tvtrinh-tran/In-utero_factors_Thyroid_Cancer_Incidence.
References
Ferlay J, E. M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer, https://gco.iarc.fr/today Accessed December 29, 2023, (2020).
Kitahara CM, Schneider AB. Epidemiology of thyroid cancer. Cancer Epidemiol, Biomark Prev. 2022;31:1284–97.
Thornburg KL, Shannon J, Thuillier P, Turker MS. In utero life and epigenetic predisposition for disease. Adv Genet. 2010;71:57–78.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N. Engl J Med. 2008;359:61–73.
Centers for Disease Control and Prevention. Pregnancy Complications, https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-complications.html#diabetes Accessed January 2, 2023.
Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–8.
Gutiérrez-Vega S, Armella A, Mennickent D, Loyola M, Covarrubias A, Ortega-Contreras B, et al. High levels of maternal total tri-iodothyronine, and low levels of fetal free L-thyroxine and total tri-iodothyronine, are associated with altered deiodinase expression and activity in placenta with gestational diabetes mellitus. PloS one. 2020;15:e0242743.
Costa MA. The endocrine function of human placenta: an overview. Reprod Biomed Online. 2016;32:14–43.
Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100.
Alsen M, Sinclair C, Cooke P, Ziadkhanpour K, Genden E, van Gerwen M. Endocrine disrupting chemicals and thyroid cancer: an overview. Toxics. 2021;9:14.
Stagnaro-Green A, Pearce E. Thyroid disorders in pregnancy. Nat Rev Endocrinol. 2012;8:650–8.
Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. 2009;215:60–8.
Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health. 2016;15:113.
Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res. 2015;8:8.
Schmidt JA, Allen NE, Almquist M, Franceschi S, Rinaldi S, Tipper SJ. et al.Insulin-like growth factor-i and risk of differentiated thyroid carcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2014;23:976–85.
Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr-Relat Cancer. 2014;21:T273–283.
Kitahara CM, Slettebø Daltveit D, Ekbom A, Engeland A, Gissler M, Glimelius I, et al. Maternal health, in-utero, and perinatal exposures and risk of thyroid cancer in offspring: a Nordic population-based nested case-control study. The Lancet Diab Endocrinol. 2021;9:94–105.
Deziel NC, Zhang Y, Wang R, Wiemels JL, Morimoto L, Clark CJ, et al. Birth characteristics and risk of pediatric thyroid cancer: a population-based record-linkage study in California. Thyroid:J Am Thyroid Assoc. 2021;31:596–606.
Aarestrup J, Kitahara CM, Baker JL. Birthweight and risk of thyroid cancer and its histological types: a large cohort study. Cancer Epidemiol. 2019;62:101564.
Vandraas KF, Vikanes ÅV, Støer NC, Troisi R, Stephansson O, Sørensen, HT, et al. Hyperemesis gravidarum and risk of cancer in offspring, a Scandinavian registry-based nested case-control study. BMC Cancer. 2015;15:398.
Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, et al. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect. 2017;125:127003.
Cohort participation status & response rates, https://sisterstudy.niehs.nih.gov/English/response-rates.htm Accessed January 2, 2023.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74.
Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and r package for computing E-values. Epidemiology. 2018;29:e45–e47.
Wilson, DA, Mateus, J, Ash, E, Turan, TN, Hunt, KJ, Malek, AM. The association of hypertensive disorders of pregnancy with infant mortality, preterm delivery, and small for gestational age. Healthcare (Basel). 2024;12:597.
O’Neill KA, Murphy MF, Bunch KJ, Puumala SE, Carozza SE, Chow EJ, et al. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol. 2015;44:153–68.
Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, McLaughlin CC, et al. Birth characteristics and childhood carcinomas. Br J cancer. 2011;105:1396–401.
Zhao Y, Wang SF, Mu M, Sheng J. Birth weight and overweight/obesity in adults: a meta-analysis. Eur J Pediatr. 2012;171:1737–46.
Qiao Y, Ma J, Wang Y, Li W, Katzmarzyk PT, Chaput JP, et al. Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl. 2015;5:S74–79.
Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–6.
Monahan B, Farland LV, Shadyab AH, Hankinson SE, Manson JE, Spracklen CN. Birthweight and subsequent risk for thyroid and autoimmune conditions in postmenopausal women. J Dev Orig Health Dis. 2022;13:463–70.
Vatten LJ, Nilsen ST, Odegård RA, Romundstad PR, Austgulen R. Insulin-like growth factor I and leptin in umbilical cord plasma and infant birth size at term. Pediatrics. 2002;109:1131–5.
Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr-Relat cancer. 2012;19:F27–45.
Manzella, L, Massimino, M, Stella, S, Tirrò, E, Pennisi, MS, Martorana, F et al. Activation of the IGF axis in thyroid cancer: implications for tumorigenesis and treatment. Int J Mol Sci. 2019;20:3258.
Shields BM, Knight BA, Hill A, Hattersley AT, Vaidya B. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab. 2011;96:E934–8.
Kim TH, Lee MY, Jin SM, Lee SH. The association between serum concentration of thyroid hormones and thyroid cancer: a cohort study. Endocr-Relat cancer. 2022;29:635–44.
Ma RC, Tutino GE, Lillycrop KA, Hanson MA, Tam WH. Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog Biophys Mol Biol. 2015;118:55–68.
Rolfo A, Nuzzo AM, De Amicis R, Moretti L, Bertoli S, Leone A. Fetal-maternal exposure to endocrine disruptors: correlation with diet intake and pregnancy outcomes. Nutrients. 2020;12:1744.
Sun M, Cao X, Wu Y, Shen L, Wei G. Prenatal exposure to endocrine-disrupting chemicals and thyroid function in neonates: A systematic review and meta-analysis. Ecotoxicol Environ Saf. 2022;231:113215.
Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, et al. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: a mixtures approach. Environ Int. 2020;139:105728.
Das AM, Das BC. Exposure to endocrine-disrupting chemicals in utero and thyroid cancer risk in offspring. The Lancet Diab Endocrinol. 2021;9:255.
Tehranifar P, Liao Y, Flom JD, Terry MB. Validity of self-reported birth weight by adult women: sociodemographic influences and implications for life-course studies. Am J Epidemiol. 2009;170:910–7.
Allen DS, Ellison GT, dos Santos Silva I, De Stavola BL, Fentiman IS. Determinants of the availability and accuracy of self-reported birth weight in middle-aged and elderly women. Am J Epidemiol. 2002;155:379–84.
Sanderson M, Williams MA, White E, Daling JR, Holt VL, Malone KE, et al. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147:136–40.
Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation. 2012;125:1381–9.
Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95:5365–9.
Funding
This research was funded by the Intramural Research Program of the National Cancer Institute, and the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (Z1AES044005 to DPS). Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
TVTT contributed to the analysis, interpretation, drafting of the article, and approval of the final version; CMK contributed to the concept, interpretation, critical review of the manuscript, and approval of the final version; KMO, RT, and DPS contributed to the interpretation, critical review of the manuscript, and approval of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study is approved annually by the National Institutes of Health Institutional Review Board (NIH 02-E-N271). All participants provided written informed consent. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41416_2025_3004_MOESM1_ESM.docx
Supplementary table 1: Association between birth weight and differentiated thyroid cancer incidence after excluding (1) participants who were born 2 or more weeks before the due date or those who were
41416_2025_3004_MOESM2_ESM.docx
Supplementary Table 2: Association between birth weight (per kg, continuous) and differentiated thyroid cancer incidence in the Sister Study cohort by baseline BMI, benign thyroid disease, personal ed
41416_2025_3004_MOESM3_ESM.docx
Supplementary table 3: Association between in-utero and newborn factors and differentiated thyroid cancer incidence in the Sister Study participants: sensitivity analyses
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tran, TVT., O’Brien, K.M., Troisi, R. et al. In-utero and newborn factors and thyroid cancer incidence in adult women in the Sister Study cohort. Br J Cancer 132, 1056–1063 (2025). https://doi.org/10.1038/s41416-025-03004-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03004-6