Abstract
Background
Not all patients with advanced pancreatic cancer (PC) profit from 2nd-line chemotherapy. We evaluated predictive factors that are routinely collected during clinical care with the aim to support an informed and shared decision.
Methods
In a prospective study across 35 German sites, 151 patients with PC previously treated with gemcitabine/nab-paclitaxel were enrolled and 146 patients received biweekly nanoliposomal irinotecan/5-fluorouracil/FA. We investigated whether time-to-treatment-failure of 1st-line (TTF1) predicts 2nd-line treatment outcome. Patients were stratified into three equal cohorts based on TTF1. Primary endpoint was TTF2, with secondary endpoints including overall survival (OS) and growth modulation index (GMI).
Results
Median TTF2 was 3.71 months (95% CI 2.50–4.11). Median OS was 7.72 months (95% CI 6.11–9.00). TTF1 did not predict TTF2 or OS (HR 0.93, 95% CI 0.58-1.47. Baseline parameters significantly associated with TTF2 and OS included neutrophil count, CRP levels, and liver metastases, whereas ECOG performance score (PS) was primarily associated with OS and to a lesser extent with TTF2. During treatment, patients with a CA 19-9 reduction of ≥25% had significantly improved TTF2 and OS (p < 0.001).
Conclusion
TTF1 is not predictive of TTF2 or OS. Therefore, 2nd-line treatment should not be withheld irrespective of duration of TTF1. CA 19-9 dynamics can be used to predict further benefit to some extent.
Clinical trial registration
EudraCT: 2016-005147-17; ClinicalTrials.gov: NCT03468335.
Similar content being viewed by others
Background
Ductal pancreatic adenocarcinoma (PC) is the fifth leading cause of cancer-related deaths worldwide, with ~411,600 fatalities each year [1]. At diagnosis, less than 30% of patients qualify for a surgical resection, which is the only chance for cure. Median survival for patients with unresectable disease ranges from 8 to 12 months, with even lower survival for those with metastases. In selected patients, 1st-line systemic chemotherapy is well established. It prolongs median survival up to 11.1 months [2,3,4], and about 40–50% of these patients are still able to continue to 2nd-line therapy [5]. Targeted treatment options are available for a small subset of patients after molecular profiling, with a high potential for future KRAS-directed therapies [6].
For advanced PC, 2nd-line chemotherapy with nanoliposomal irinotecan (Nal-IRI) in combination with 5-fluorouracil (5-FU)/folinic acid (FA) increases median overall survival (OS) from 4.2 months to 6.1 months compared to 5-FU/FA alone [7] after failure of a gemcitabine-based regimen. However, some grade 3/4 toxicities are reported (e.g. 13% diarrhoea and 14% fatigue). A novel formulation of liposomal irinotecan/5-FU/FA showed similar results, with an OS of 7.4 months (95% CI 6.1–8.4) in the PAN-HEROIC-1 trial [8].
The ESMO guidelines therefore endorse Nal-IRI/5-FU/FA as an effective, tolerable 2nd line treatment ‘for fit patients’ [9]. ASCO guidelines recommend 2nd-line treatment ‘for patients with ECOG PS 0-1, a relatively favourable comorbidity profile and patient preference, and a support system for aggressive medical therapy’, though the definition of a ‘relatively favourable comorbidity profile’ is left open [10]. To support such a personalized treatment decision, predictive factors should be easily accessible and simple to assess. Retrospective analyses have identified a list of potential candidates. These include patient-related factors (ECOG PS, body-mass index (BMI), age, C-reactive protein (CRP), leucocyte or neutrophil count, albumin, LDH, smoking status), tumour-related factors (CA 19-9, type and number of metastases, the stage at diagnosis) and treatment-related parameters, such as the duration of 1st-line treatment [11,12,13].
Some combinations of these parameters have been used to establish predictive scores or nomograms with significant discriminatory power [11,12,13]. As an example, Vienot et al. [14] classified patients into three prognostic groups with a median OS of 11.3 (95% CI 9.1–12.9), 3.6 months (95% CI 2.6–4.7) and 1.4 months (95% CI 1.2–1.7), respectively. Their calculation is based on a somewhat complex visual translation of nine individual factors into points. Summing them up results in a probability score for survival. However, despite its external validation, this scoring system did not find its way into clinical practice, probably due to its complexity.
Our prospective PREDICT trial aimed to evaluate readily accessible clinical routine parameters which could serve as arguments in the individual decision process before the start of 2nd-line chemotherapy. The primary endpoint tested the impact of 1st-line treatment duration (TTF1) on the duration of 2nd-line therapy (TTF2), because TTF1 has been regarded as an independent surrogate measure for general treatment tolerability and was predictive of the efficacy of subsequent treatment in another trial of PC [11] or in advanced soft tissue sarcoma [15]. Secondary endpoints were the overall survival and the growth modulation index. Additionally, longitudinal monitoring of CA 19-9 levels was performed as a potential indicator of treatment success or futility as early as possible after the start of 2nd-line treatment.
Methods
Patient population
This prospective phase III trial included patients with locally advanced or metastatic pancreatic cancer after failure of 1st-line therapy with gemcitabine and nab-paclitaxel (Gem/nab-Pac). Eligible participants were at least 18 years old, had histologically or cytologically confirmed pancreatic ductal adenocarcinoma, and an ECOG PS of 0–2. Patients were required to have received one line of systemic Gem/nab-Pac-based therapy for advanced disease, irrespective of prior adjuvant therapy, or an adjuvant Gem/nab-Pac-based chemotherapy regimen with documented progression less than six months after termination. Additional inclusion criteria required documentation of prior therapy (duration, maximum toxicity, reason for discontinuation), adequate blood cell count, liver enzymes, and renal function. The PREDICT trial (EudraCT: 2016-005147-17) was approved by the Ethics Committee of the Saarland Medical Association and was performed in accordance with the Declaration of Helsinki and the EU Clinical Trials Directive 2001/20/EC.
Procedures
All patients were planned to receive biweekly treatment of Nal-IRI/5-FU/FA, consisting of Nal-IRI (70 mg/m² body surface area (BSA) 90 min intravenous (IV) infusion) followed by FA (400 mg/m² BSA as 30 min IV infusion) and 5-FU (2400 mg/m² BSA as 46-h IV infusion.). The starting dose of 5-FU was reduced in patients with known partial dihydropyrimidine dehydrogenase deficiency.
Study endpoints
The primary objective was to confirm that a longer Time-To-Treatment-Failure during 1st-line treatment (TTF1) predicts the benefit of 2nd-line treatment using a tertile split of the trial population. The corresponding primary endpoint was TTF2, defined as time from date of signed informed consent (IC) until permanent treatment discontinuation due to progressive disease, unacceptable toxicity, protocol violations, non-compliance, investigator decision, medical conditions or complications, death, withdrawal of informed consent, extended delay of study treatment, or not further specified other reason. TTF2 was censored if discontinuation was due to study termination by sponsor or loss to follow-up.
Secondary endpoints included overall survival (OS), defined as time from date of signed IC to death or if censored the last known date alive. The growth modulation index (GMI) was defined as the ratio of time to progression with the nth-line of therapy (TTPn) to the (n-1)th-line (TTPn-1). A GMI > 1.33 is considered a sign of activity in phase II trials [15, 16].
Statistical analysis
To evaluate the impact of 1st-line treatment, a tertile split was applied to divide patients into three approximately equally sized cohorts based on TTF1 (low, medium, and high). Comparative analyses were performed by contrasting the TTF1 low and TTF1 high cohorts. The working hypothesis expected an increase of TTF2 by 100% in the TTF1 high cohort compared to the TTF1 low cohort. Time-to-event distributions were estimated using Kaplan–Meier techniques. Hypothesis testing was performed using a log-rank test with a one-sided significance level of α = 0.05. Analysis of OS was performed in the same manner.
To further explore predictive factors for TTF2 and OS, post-hoc analyses were performed for categorized baseline parameters and biomarkers, including CA 19-9 levels, C-reactive Protein (CRP), albumin, leucocyte and neutrophil count, LDH, presence of liver metastases, BMI, ECOG, Sex, and Age. Furthermore, the predictive value of a CA 19-9 response on subsequent TTF2 and OS after the time of measurement was evaluated for different response cutoff values (decrease from baseline ≥25% and ≥50%) and timeframes (1–5 months). Baseline variables significantly associated with both endpoints at the 5% level were then entered simultaneously into multivariate Cox proportional hazard models to examine their independent contributions to the prediction of TTF2 and OS. The leucocyte count was eliminated from the analysis because neutrophils are more specific and because both parameters are highly redundant.
Results are presented as mean +/- standard deviation for continuous variables, relative frequencies for categorical variables, and median time to event, including 95% confidence interval (CI) for time-to-event variables. The association of baseline characteristics with OS and TTF2 was assessed using log-rank tests.
Results
Between March 2018 and July 2021, a total of 151 patients were enrolled at 35 German sites. Of these, 146 (96.7%) received at least one dose of study medication and were included in the analysis. Overall, 143 2nd-line treatment failure events were observed. Three patients were censored due to loss to follow-up or study termination (CONSORT diagram: supplementary Fig. 1). Median treatment duration of 2nd-line treatment was 3.71 months (95% CI 2.50–4.11 months), with 28.3% of the patients still on treatment after six months. Median OS of 2nd-line therapy was 7.72 months (95% CI 6.11–9.00 months), with 60.5% (95% CI 51.8–68.2%) of the patients alive at six months. Median TTF1 at study inclusion had been 6.5 months. For the analysis, patients were stratified into the TTF1 high (n = 50, 1st-line treatment duration ≥7 months), TTF1 middle (n = 50, 4–7 months) and TTF1 low (n = 49, ≤4 months) cohorts, which were comparable in terms of the other baseline characteristics (Table 1).
Impact of TTF1 on time to treatment failure of 2nd-line therapy
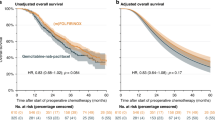
Analysis of TTF2 revealed no statistically significant differences between the TTF1 high and TTF1 low cohort by log-rank test (p = 0.790). The median TTF2 was 3.22 months (95% CI 1.77–4.01) in the TTF1 high and 2.83 months (95% CI 2.10–4.86) in the TTF1 low cohorts, respectively (Fig. 1a, supplementary Table 1). The probability (95% CI) for patients to continue treatment after six months was 30.0% (95% CI 18.1–42.9) and 29.3% (95% CI 17.3–42.5), respectively. The main reasons to discontinue 2nd-line treatment were progressive disease (51.1% vs. 57.4% in the TTF1 high vs. TTF1 low cohort, respectively), followed by death (8.9% vs. 6.4%), ‘unrelated medical condition or complication’ (6.7% vs. 8.5%) and ‘investigator’s decision’ (11.1% vs. 4.3%).
Overall survival
At study closure, we observed 114 events, and 32 patients were censored because they were still alive (10 in the TTF1 high and 15 in the TTF1 low cohorts. The median OS for the entire population was 7.75 months (95% CI 6.11–9.17). In the two TTF1 cohorts, median OS was 6.41 months (95% CI 4.76–9.99 months) for TTF1 high and 8.08 months (95% CI 4.73–10.02 months) for TTF1 low (Fig. 1b, supplementary Table 2). The survival probability for patients after six months was 59.7% (95% CI 44.2–72.1) in TTF1 high and 58.2% (95% CI 42.5–71.0) in TTF1 low. While median OS was numerically shorter (about 1.5 months) in TTF1 high as compared to TTF1, this difference was statistically not significant (log-rank test; p = 0.746) and the survival probability after six months was similar.
Growth modulation index
The ‘growth modulation index’ (GMI), calculated as the ratio of TTP2/TTP1, was analysed for 77 patients with a documented tumour progression on both the 1st-line and 2nd line treatment. Patients who died without documented progression were not considered. Mean (±SD) GMI was 1.02 ± 1.14 overall (n = 77), 0.44 ± 0.31 for the TTF1 high cohort (n = 23) and 1.75 ± 1.60 for TTF1 low cohort (n = 25). A Mann–Whitney U test showed a statistically significant difference between the two cohorts (p < 0.001).
Correlations of baseline parameters with TTF2 and OS
Further analysis examined the association of various baseline parameters with TTF2 and OS using log-rank tests (Fig. 2, supplementary Table 3). Several parameters were found to be significantly correlated with treatment outcome: elevated CA 19-9 levels (<100 vs. ≥100μ/ml: TTF2 5.95 vs. 2.86 months, p = 0.005; OS 9.26 vs. 7.13 months, p = 0.037), ECOG PS (0 vs ≥1: TTF2 4.76 vs. 2.43 months, p = 0.029; OS 10.28 vs. 6.08 months, p < 0.001), leucocyte counts (normal vs. outside normal: TTF2 4.07 vs. 2.14 months, p < 0.001; OS 8.84 vs. 4.40 months, p < 0.001), neutrophil counts (normal vs. outside normal: TTF2 3.45 vs. 4.01 vs. 2.07 months, p < 0.001; OS 8.61 vs. 8.18 vs. 3.61 months, p < 0.001), presence of liver metastases at progression from 1st-line treatment (yes vs. no: TTF2 2.79 vs. 4.40 months, p = 0.047; OS 6.67 vs. 10.12 months, p = 0.042), and CRP levels (≤10 vs. >10 mg/l: TTF2 5.22 vs. 2.63 months, p = 0.003; OS 9.33 vs. 4.40 months, p < 0.001). Albumin levels were significantly associated with OS (normal vs. outside normal: OS 8.97 vs 6.08 months, p = 0.030) with a trend for TTF2 (normal vs. outside normal: TTF2 4.07 vs. 2.43 months, p = 0.182). In contrast, BMI (<22 vs. ≥22 kg/m2: TTF2 3.22 vs. 3.81 months, p = 0.414; OS 6.24 vs. 8.61 months, p = 0.381), sex (female vs. male: TTF2 3.84 vs. 2.99 months, p = 0.595; OS 7.98 vs. 7.56 months, p = 0.400), age (18–64 vs. 65–74 vs. ≥75 years: TTF2 2.84 vs. 4.86 vs. 2.30 months, p = 0.100; OS 8.18 vs. 6.67 vs. 8.08 months, p = 0.916), and LDH (normal vs. outside normal: TTF2 3.93 vs. 2.86 months, p = 0.207; OS 8.61 vs. 6.67 months, p = 0.059) showed no significant correlation with either TTF2 or OS.
Additional multivariate modelling included the parameters significantly associated with TTF2 and OS (Table 2) in the univariate analysis. CRP levels and neutrophil or leucocyte count where associated with both TTF2 and OS with p-values of 0.02 or less. ECOG PS remained significantly associated with OS but not with TTF2, whereas elevated CA 19-9 levels at baseline lost their discriminatory power for TTF2 as well as for OS.
Correlation of CA 19-9 response with TTF and OS
Longitudinal CA 19-9 measurements were performed routinely. Out of 146 patients, 114 (78.1%) had elevated CA 19-9 baseline values. Among these, 52 (46.0%) patients experienced a moderate CA 19-9 response of ≥25% and 32 (28.1%) patients experienced a pronounced response of ≥50% at any time point during 2nd-line treatment (Table 3). A decrease of ≥25% or ≥50% in CA 19-9 levels at any time was significantly associated with longer TTF2 and increased OS (log-rank p < 0.001). However, given that patients with longer treatment duration have a higher chance of achieving a CA 19-9 response, additional analyses were performed at specific time points for subsequent TTF2 (supplementary Table 4) and OS (Table 4). Of those with elevated CA 19-9 baseline values still alive at the respective time-point, ten (9.1%) patients achieved a ≥25% decrease of CA 19-9 after one month, 35 (35.7%) after two months, and 47 (52.2%) after three months. Three patients (2.7%) achieved a ≥50% decrease of CA 19-9 after one month, 15 (15.3%) after two months, and 27 (30.0%) after three months. Starting with a survival time of at least three months, a CA 19-9 response of ≥25% was highly significantly associated with increased OS (7.91 [CI 95% 5.84–10.70] vs. 3.11 [CI 95% 2.29–5.18] p < 0.001), with similar results after four and five months. A CA 19-9 response of ≥50% was significantly associated with both longer TTF2 (4.06 [CI 95% 1.76–6.63] vs. 2.09 [CI 95% 1.37–2.45] p = 0.001) and increased OS (8.30 [CI 95% 6.23–13.79] vs. 4.72 [CI 95% 3.11–5.84] p < 0.001). These associations remain significant after four and five months.
Discussion
Individual outcome of 2nd-line chemotherapy in patients with advanced pancreatic cancer varies widely. While many patients seem to profit, others may be harmed from medical, financial and social side effects. Given the lack of strong evidence to guide the decision to start 2nd-line therapy, readily accessible predictive markers are needed.
In our prospective trial of 2nd-line systemic chemotherapy, we identified patient-related factors such as normal leucocyte or neutrophil count or CRP levels as highly significant predictors for longer TTF2 and for an increase in overall survival, and a good ECOG PS as predictor for longer OS but not for TTF2. In addition, tumour-related parameters such as near-to-normal CA 19-9 levels and lack of liver metastases led to a somewhat better outcome. These readily accessible criteria could help to decide before starting 2nd-line treatment. In contrast, age, sex, body mass index, albumin and LDH levels had no significant impact.
Our study hypothesis—which postulated that time to treatment failure of 1st-line systemic chemotherapy predicts longer TTF2 or OS—was not supported. Even though we separated the patients into three cohorts for better discrimination, the outcome was similar.
The growth modulation index (GMI), which is calculated with as the ratio of TTP2/TTP1 based on measurable progression differentiated both groups with a ratio of 0.44 ± 0.31 for the TTF1 high and 1.75 ± 1.60 for the TTF1 low cohorts, suggesting better 2nd line efficacy in the TTF1 low cohort similar to experiences in soft tissue sarcoma where the GMI is supposed to correlate with OS [15]. However, closer analysis revealed that the duration of 2nd-line treatment and overall survival was similar in both cohorts. The difference in GMI, therefore, is uniquely driven by TTF1 rather than by treatment efficacy, indicating that GMI is not a reliable tool to evaluate the efficacy of 2nd-line chemotherapy in pancreatic cancer.
We confirm prospectively that 2nd-line treatment with Nal-IRI/5-FU/FA can achieve a median overall survival time of 7.72 months (CI 95% 6.11–9.00). This is longer than the OS observed in the pivotal phase III NAPOLI-1 trial (6.1 months) [7] for the same regimen and it is at least in the same range than in other randomized trials, namely those using a novel formulation of liposomal irinotecan in combination with 5-FU/FA (the PAN-HEROIC-1 trial) [8] (OS 7.4 months) or the oxaliplatin-based OFF regimen [17] (OS 5.9 months). Patient selection might account for this discrepancy. However, our patient population was older (68.2 years in our trial vs. 63 years, 62 years and 60 years in the other trials, respectively) and had a comparable performance status, which does not explain the differences in outcome. Because we did not require measurable disease for inclusion, the differences in outcome may be explained by lower burden of liver metastases. In addition, supportive therapy and side effect management may have improved over the years, which is supported by the time on treatment of 3.71 months, which is less than half as long as the OS time of 7.72 months. Nevertheless, survival outcome varies largely, with 25% of the patients in our trial living shorter than 3.78 months and 25% longer than 11.70 months. Thus, not all patients seem to profit alike, and especially those with a very short predicted TTF2/OS might be advised to forgo further chemotherapy and its side effects.
The most relevant predictive factors for treatment success are patient-related, such as ECOG PS, CRP levels and neutrophil/leucocyte counts at the onset of 2nd-line treatment. Patients with ECOG 0 as compared to patients with ECOG ≥ 1 had nearly twice the TTF2 (increase of 96% from 2.43 months to 4.76 months) and a 69% increase in median OS (10.28 months as compared to 6.08 months). This confirms data from most retrospective analyses of other trials, such as in CONCO-003, where a Karnofsky Index of 90–100% led to a hazard ratio for OS of 0.62 (p = 0.002) [11]. Similarly, in a recent trial with Gem/nab-Pac, patients with an ECOG PS of 2 as compared to ≤1 had a HR for OS of 1.769 [18]. Results for leucocyte/neutrophil count are less consistent across studies [19], but our analysis confirms its predictive value. In contrast, BMI, albumin levels and patient age were not predictive in our analysis, which follows the highly variable results of other trials, where some define these parameters as significant in multivariate analyses and predictive scores [20] and others don’t [11, 21]. Even though these analyses do not suggest an association with the type of drugs, we cannot exclude it. This is relevant because Nal-IRI/5FU/FA in combination with oxaliplatin has recently shown superior efficacy in 1st line treatment with comparable toxicity to Gem/nab-Pac [3, 6], which may lead to a change in 2nd line treatment options.
Any elevation of CA 19-9 levels above baseline correlates with shorter TTF2 and worse overall survival (Table 2, Fig. 2) with steady effects up to 1000 μ/ml (data not shown). At the same time, we could show for the first time that longitudinal assessment of CA 19-9 can help to guide treatment decisions in the second-line setting. A treatment-induced decrease in CA 19-9 levels of ≥25% was associated with a TTF2 of 5.06 months as compared to 1.91 months in patients without a response (Table 3). Similarly, OS in responding patients was significantly longer. During treatment, CA 19-9 levels start to decrease at about two months after inclusion into the trial. Maximum discrimination is reached at three months and stays stable thereafter (Table 3). Similar analyses for up to eight months follow-up have been performed in 1st-line treatment in the MPACT [22] and SIEGE [23] trials using systemic Gem/nab-Pac. In SIEGE, a decrease of CA 19-9 of 50% in week 4 was significantly associated with an objective response by imaging in week 8 but did not correlate with overall survival, whereas in MPACT any CA 19-9 decrease at week 8 was considered as an early marker for chemotherapy efficacy and predicted survival. These results are comparable to ours and confirm that response evaluation by CA 19-9 measurements after three or more months is a key determinant of benefit and can be used to discuss with the patient if chemotherapy is worthwhile and should be continued. This is especially relevant in the 2nd-line setting, where classical response assessment by imaging ist less often perfomed because of the focus on patient comfort and overall survival. In contrast, elevation of CA 19-9 before treatment irrespective of its magnitude should not exclude patients from treatment, because its negative effect on prognosis is counterbalanced by the highly significant benefit for responders.
In summary, readily accessible patient-related parameters can effectively guide treatment decisions of 2nd-line chemotherapy in advanced pancreatic cancer. We confirm prospectively that even a moderately decreased performance status, a leucocyte/neutrophil count outside the normal range or slightly increased CRP levels are strong predictors for limited treatment efficacy. In contrast, a short TTF1 is not a negative predictor for TTF2 or OS. Also, elevated CA 19-9 levels at the start of treatment—despite their prognostic value - are not suitable to guide a treatment decision, because this effect is largely compensated by any treatment-related response. Instead, CA 19-9 response after ≥3 months provides a simple and reliable tool to assess ongoing treatment efficacy, helping to decide whether chemotherapy should be continued or can be stopped.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
References
Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Wainberg ZA, Melisi D, Macarulla T, Pazo Cid R, Chandana SR, De La Fouchardière C, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402:1272–81.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Abrams T, Meyer G, Meyerhardt J, Wolpin B, Schrag D, Fuchs C. Patterns of chemotherapy use in a U.S.-based cohort of patients with metastatic pancreatic cancer. Oncologist. 2017;22:925–33.
Stoop TF, Javed AA, Oba A, Koerkamp BG, Seufferlein T, Wilmik JW, et al. Pancreatuc cancer. Lancet. 2025;405:1182–202.
Wang-Gillam A, Li C-P, Bodoky G, Dean A, Shan Y-S, Jameson G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–57.
Cui J, Qin S, Zhou Y, Zhang S, Sun X, Zhang M, et al. Irinotecan hydrochloride liposome HR070803 in combination with 5-fluorouracil and leucovorin in locally advanced or metastatic pancreatic ductal adenocarcinoma following prior gemcitabine-based therapy (PAN-HEROIC-1): a phase 3 trial. Signal Transduct Target Ther. 2024;9:248.
Conroy T, Pfeiffer P, Vilgrain V, Lamarca A, Seufferlein T, O’Reilly EM, et al. ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:987–1002.
Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38:3217–30.
Sinn M, Dalken L, Striefler JK, Bischoff S, Schweitzer N, Pelzer U, et al. Second-line treatment in pancreatic cancer patients: who profits?-Results from the CONKO Study Group. Pancreas. 2016;45:601–5.
Chen LT, Macarulla T, Blanc JF, Mirakhur B, Jong FA, Belanger B, et al. Nomogram for predicting survival in patients treated with liposomal irinotecan plus fluorouracil and leucovorin in metastatic pancreatic cancer. Cancers. 2019;11:1068.
Keyl J, Kasper S, Wiesweg M, Götze J, Schönrock M, Sinn M, et al. Multimodal survival prediction in advanced pancreatic cancer using machine learning. ESMO Open. 2022;7:100555.
Vienot A, Beinse G, Louvet C, de Mestier L, Meurisse A, Fein F, et al. Overall Survival Prediction and Usefulness of Second-Line Chemotherapy in Advanced Pancreatic Adenocarcinoma. J Natl Cancer Inst. 2017;109 https://doi.org/10.1093/jnci/djx037.
Penel N, Demetri G, Blay JY, Cousin S, Maki RG, Chawla S, et al. Growth modulation index as metric of clinical benefit assessment among advanced soft tissue sarcoma patients receiving trabectedin as a salvage therapy. Ann Oncol. 2013;24:537–42.
Krebs MG, Blay J-Y, Le Tourneau C, Hong D, Veronese L, Antoniou M, et al. Intrapatient comparisons of efficacy in a single-arm trial of entrectinib in tumour-agnostic indications. ESMO Open. 2021;6:100072.
Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423–9.
Giordano G, Milella M, Landriscina M, Bergamo F, Tirino G, Santaniello A, et al. Prognostic analysis and outcomes of metastatic pancreatic cancer patients receiving nab-paclitaxel plus gemcitabine as second or later-line treatment. Cancer Med. 2024;13:e7345.
Chen LT, Siveke JT, Wang-Gillam A, Li CP, Bodoky G, Dean AP, et al. Survival with nal-IRI (liposomal irinotecan) plus 5-fluorouracil and leucovorin versus 5-fluorouracil and leucovorin in per-protocol and non-per-protocol populations of NAPOLI-1: Expanded analysis of a global phase 3 trial. Eur J Cancer. 2018;105:71–78.
Gränsmark E, Bågenholm Bylin N, Blomstrand H, Fredrikson M, Åvall-Lundqvist E, Elander NO. Real world evidence on second-line palliative chemotherapy in advanced pancreatic cancer. Front Oncol. 2020;10:1176.
Lee JE, Lee HS, Chung MJ, Park JY, Park SW, Song SY. Analysis of clinical predictive factors affecting the outcome of second-line chemotherapy for gemcitabine-refractory advanced pancreatic cancer. Gut Liver. 2020;14:135–43.
Chiorean EG, Von Hoff DD, Reni M, Arena FP, Infante JR, Bathini VG, et al. CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol. 2016;27:654–60.
Dayimu A, Di Lisio L, Anand S, Roca-Carreras I, Qian W, Al-Mohammad A, et al. Clinical and biological markers predictive of treatment response associated with metastatic pancreatic adenocarcinoma. Br J Cancer. 2023;128:1672–80.
Acknowledgements
The authors would like to thank all patients who participated in the PREDICT trial. We would also like to acknowledge the contributions from all investigators and their teams and at the investigational sites: Caritasklinikum Saarbrücken St. Theresia; Carl-von-Basedow-Klinikum, Merseburg; Diakonie Klinikum Schwäbisch Hall; Donau-Isar-Klinikum Deggendorf; DRK Kliniken Berlin Köpenick; Gemeinschaftspraxis Hämatologie-Onkologie, Dresden; Gemeinschaftspraxis für Hämatologie und Onkologie, Ravensburg; Gemeinschaftspraxis für Hämatologie und Onkologie, Westerstede; Hämatologisch-Onkologische Gemeinschaftspraxis, Bad Soden; Helios Klinikum, Bad Saarow; Kliniken Nordoberpfalz, Klinikum Weiden; Klinikum Bremen-Nord; Klinikum der Stadt Ludwigshafen; Klinikum Landshut; Klinikum Nürnberg, Paracelsus Medizinische Privatuniversität; Klinikum St. Marien, Amberg; Klinikum Südstadt, Rostock; Leopoldina Krankenhaus, Schweinfurt; Medizinisches Versorgungszentrum für Blut- und Krebserkrankungen, Potsdam; München Klinik, Neuperlach; MVZ Klinikum, Coburg; MVZ West, Würselen; Onkologie unter Ems MVM, Leer; Onkologisches Ambulanzzentrum, Hannover; Onkopraxis Probstheida, Leipzig; Pi.Tri-Studien, Offenburg; Pius-Hospital, Oldenburg; Schwarzwald-Baar-Klinikum, Villingen-Schwenningen; St. Elisabeth-Krankenhaus, Köln; St. Josef-Hospital, Bochum; St. Josefs-Hospital, Wiesbaden; Universitätsmedizin Mannheim; Universitätsklinikum Marburg; Universitätsklinikum Köln; Universitätsklinikum Ulm. AI-based language editing of the final manuscript was performed to align the revisions of the different authors.
Funding
The PREDICT study was funded by Servier Deutschland GmbH. The funder did not have an influence on study design, data collection, analysis and evaluation of the data or writing of the report.
Author information
Authors and Affiliations
Contributions
MPL conceived the study, conducted the trial as principle investigator and wrote the manuscript. SR organized the study conduct, interpreted data and wrote the manuscript. HB performed statistical analyses and revised the manuscript. GB, SH, TL and MS organized the monitoring, supported the statistical analysis and revised the manuscript. NA, CB, TD, TE, LF, TG, AG, LJ, MK, MK, RP, DR, NS, AS, GS, DW included patients, participated in data collection and analysis, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
ML: Financial Interests, Personal, Research Funding: Servier. CB: Financial Interests, Personal, Advisory Board: Boehringer Ingelheim, Amgen, Bayer. TD: Financial Interests, Personal, Advisory Board: Novartis, Iomedico; Financial Interests, Personal, Invited Speaker: Novartis, Lilly. AG: Financial Interests, Personal, Royalties: Janssen; Financial Interests, Personal, Advisory Board: Janssen, Incyte, Novartis, Shire. MK: Financial Interests, Personal, Advisory Board: AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Janssen, Gilead, Novartis, Pfizer, Roche; Financial Interests, Advisory Board: Takeda. JS: Stock Ownership in Biontech SE. GS: Financial Interests, Personal, Advisory Board: BMS, Novartis, Roche, MSD, Janssen; Financial Interests, Personal, Invited Speaker: Janssen; Financial Interests, Institutional, Local PI: BMS, MSD, AstraZeneca; Non-Financial Interests, Principal Investigator: BMS, MSD, AIO GmbH, IKFZ, AstraZeneca, GLA; Non-Financial Interests, Member: AIO, DGHO, EORTC, DKG. DW: Financial Interests, Personal, Other, Travel: AstraZeneca, Bristol Myers Squibb, Eisai, Roche; Financial Interests, Personal, Speaker’s Bureau: AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Falk, Incyte. All remaining authors have declared no conflicts of interest.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. An independent ethics committee (Ethics Committee of the Medical Association of Saarland) reviewed the study and any amendments. Written informed consent was obtained from each patient before any study specific medical procedure was performed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lutz, M.P., Ansorge, N., Barmashenko, G. et al. Predictive criteria for overall survival and treatment duration of 2nd-line chemotherapy in patients with advanced pancreatic adenocarcinoma (AIO-PAK-0216). Br J Cancer 134, 85–91 (2026). https://doi.org/10.1038/s41416-025-03188-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03188-x