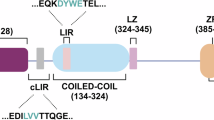
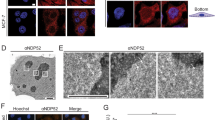
Abstract
The role of mitophagy, a process that allows the removal of damaged mitochondria from cells, remains unknown in multiple sclerosis (MS), a disease that is found associated with dysfunctional mitochondria. Here we have qualitatively and quantitatively studied the main players in PINK1-mediated mitophagy in peripheral blood mononuclear cells (PBMCs) of patients with relapsing–remitting MS. We found the variant c.491G>A (rs550510, p.G140E) of NDP52, one of the major mitophagy receptor genes, associated with a MS cohort. Through the characterization of this variant, we discovered that the residue 140 of human NDP52 is a crucial modulator of NDP52/LC3C binding, promoting the formation of autophagosomes in order to drive efficient mitophagy. In addition, we found that in the PBMC population, NDP52 is mainly expressed in B cells and by ensuring efficient mitophagy, it is able to limit the production of the proinflammatory cytokine TNF-α following cell stimulation. In sum, our results contribute to a better understanding of the role of NDP52 in mitophagy and underline, for the first time, a possible role of NDP52 in MS.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All the software used is freely available. The scripts, input, and outputs files generated during the study will be freely available in a GitHub repository associated with our publication (https://github.com/ELELAB/LC3C_NDP52_GE_mutant). The MD trajectories will be available in Open Science Framework (OSF): https://osf.io/48wzq/.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–31.
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52.
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–58.
Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–27.
Su K, Bourdette D, Forte M. Mitochondrial dysfunction and neurodegeneration in multiple sclerosis. Front Physiol. 2013;4:169.
Chiurchiu V. Novel targets in multiple sclerosis: to oxidative stress and beyond. Curr Top Med Chem. 2014;14:2590–9.
Gonzalo H, Nogueras L, Gil-Sánchez A, Hervás JV, Valcheva P, González-Mingot C, et al. Impairment of mitochondrial redox status in peripheral lymphocytes of multiple sclerosis patients. Front Neurosci. 2019;13:938.
Lou G, Palikaras K, Lautrup S, Scheibye-Knudsen M, Tavernarakis N, Fang EF. Mitophagy and neuroprotection. Trends Mol Med. 2020;26:8–20.
Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–6.
Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, et al. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460:127–39.
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–14.
Padman BS, Nguyen TN, Uoselis L, Skulsuppaisarn M, Nguyen LK, Lazarou M. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat Commun. 2019;10:408.
Patergnani S, Castellazzi M, Bonora M, Marchi S, Casetta I, Pugliatti M, et al. Autophagy and mitophagy elements are increased in body fluids of multiple sclerosis-affected individuals. J Neurol Neurosurg Psychiatry. 2018;89:439–41.
Castellazzi M, Patergnani S, Donadio M, Giorgi C, Bonora M, Fainardi E, et al. Correlation between auto/mitophagic processes and magnetic resonance imaging activity in multiple sclerosis patients. J Neuroinflammation. 2019;16:131.
Inomata M, Niida S, Shibata KI, Into T. Regulation of toll-like receptor signaling by NDP52-mediated selective autophagy is normally inactivated by A20. Cell Mol Life Sci. 2012;69:963–79.
von Muhlinen N, Akutsu M, Ravenhill BJ, Foeglein Á, Bloor S, Rutherford TJ, et al. LC3C, bound selectively by a noncanonical LIR Motif in NDP52, is required for antibacterial autophagy. Mol Cell. 2012;48:329–42.
Skytte Rasmussen M, Mouilleron S, Kumar Shrestha B, Wirth M, Lee R, et al. ATG4B contains a C-terminal LIR motif important for binding and efficient cleavage of mammalian orthologs of yeast Atg8. Autophagy. 2017;13:834–53.
Wirth M, Zhang W, Razi M, Nyoni L, Joshi D, O’Reilly N, et al. Molecular determinants regulating selective binding of autophagy adapters and receptors to ATG8 proteins. Nat Commun. 2019;10:2055.
Cheng X, Wang Y, Gong Y, Li F, Guo Y, Hu S, Liu J, Pan L. Structural basis of FYCO1 and MAP1LC3A interaction reveals a novel binding mode for Atg8-family proteins. Autophagy. 2016;12:1330–9.
Kirkin V, Rogov VV. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell. 2019;76:268–85.
Johansen T, Lamark T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J Mol Biol. 2020;432:80–103.
Sakurai S, Tomita T, Shimizu T, Ohto U. The crystal structure of mouse LC3B in complex with the FYCO1 LIR reveals the importance of the flanking region of the LIR motif. Acta Crystallogr F Struct Biol Commun. 2017;73:130–7.
Laio A, Parrinello M. Escaping free-energy minima. Proc Natl Acad Sci USA. 2002;99:12562–6.
Bonomi M, Barducci A, Parrinello M. Reconstructing the equilibrium boltzmann distribution from well-tempered metadynamics. J Comput Chem. 2009;30:1615–21.
Bussi G, Laio A. Using metadynamics to explore complex free-energy landscapes. Nat Rev Phys. 2020;2:200–12.
Richter B, Sliter DA, Herhaus L, Stolz A, Wang C, Beli P, et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc Natl Acad Sci USA. 2016;113:4039–44.
Moore AS, Holzbaur ELF. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proc Natl Acad Sci USA. 2016;113:3349–58.
Turco E, Fracchiolla D, Martens S. Recruitment and activation of the ULK1/Atg1 kinase complex in selective autophagy. J Mol Biol. 2020;432:123–34.
Karanasios E, Stapleton E, Manifava M, Kaizuka T, Mizushima N, Walker SA, et al. Dynamic association of the ULK1 complex with omegasomes during autophagy induction. J Cell Sci. 2013;126:5224–38.
Sabatino JJ, Pröbstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20:728–45.
Wilhelmus MM, van der Pol SM, Jansen Q, Witte ME, van der Valk P, Rozemuller A, et al. Association of Parkinson disease-related protein PINK1 with Alzheimer disease and multiple sclerosis brain lesions. Free Radic Biol Med. 2011;50:469–76.
Ellinghaus D, Zhang H, Zeissig S, Lipinski S, Till A, Jiang T, et al. Association between variants of PRDM1 and NDP52 and crohn’s disease, based on exome sequencing and functional studies. Gastroenterology. 2013;145:339–47.
Verlhac P, Grégoire IP, Azocar O, Petkova DS, Baguet J, Viret C, Faure M. Autophagy receptor NDP52 regulates pathogen-containing autophagosome maturation. Cell Host Microbe. 2015;17:515–25.
van der Burgh R, Nijhuis L, Pervolaraki K, Compeer EB, Jongeneel LH, van Gijn M, et al. Defects in mitochondrial clearance predispose human monocytes to interleukin-1β hypersecretion. J Biol Chem. 2014;289:5000–12.
McNicholas N, Hutchinson M, McGuigan C, Chataway J. 2017 McDonald diagnostic criteria: a review of the evidence. Mult Scler Relat Disord. 2018;24:48–54.
Cascella R, Strafella C, Ragazzo M, Manzo L, Costanza G, Bowes J, et al. KIF3A and IL-4 are disease-specific biomarkers for psoriatic arthritis susceptibility. Oncotarget. 2017;8:95401–11.
Cascella R, Strafella C, Longo G, Ragazzo M, Manzo L, De Felici C, et al. Uncovering genetic and non-genetic biomarkers specific for exudative age-related macular degeneration: significant association of twelve variants. Oncotarget. 2018;9:7812–21.
Rogov VV, Rozenknop A, Rogova NY, Löhr F, Tikole S, Jaravine V, et al. A universal expression tag for structural and functional studies of proteins. ChemBioChem. 2012;13:959–63.
Williamson MP. Using chemical shift perturbation to characterise ligand binding. Prog Nucl Magn Reson Spectrosc. 2013;73:1–16.
Suzuki H, Tabata K, Morita E, Kawasaki M, Kato R, Dobson RC, et al. Structural basis of the autophagy-related LC3/Atg13 LIR complex: recognition and interaction mechanism. Structure. 2014;22:47–58.
Webb B, Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics. 2016;54:5.6.1–5.6.37.
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539.
Abraham MJ, Murtola T, Schultz R, Pall S, Smith JC, Hess B, et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1:19–25.
Piana S, Lindorff-Larsen K, Shaw DE. How robust are protein folding simulations with respect to force field parameterization? Biophys J. 2011;100:47–9.
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926.
Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J Chem Phys. 2007;126:014101.
Harvey MJ, De Fabritiis G. An implementation of the smooth particle mesh Ewald method on GPU hardware. J Chem Theory Comput. 2009;5:2371–7.
Tribello GA, Bonomi M, Branduardi D, Camilloni C, Bussi G. PLUMED 2: new feathers for an old bird. Comput Phys Commun. 2014;185:604–13.
Tiberti M, et al. PyInteraph: a framework for the analysis of interaction networks in structural ensembles of proteins. J Chem Inf Model. 2014;54:1537–51.
Acknowledgements
We wish to thank Dr Christian Behrends for kindly providing us the construct encoding HA-LC3C; Prof. Honglin Luo for the gift of 3x-Flag-NDP52 plasmid; Dr Anna Kabanova for granting the execution of some revision experiments in her laboratory; Krenare Bruqi for her technical assistance and D.Hodder for his proofreading. We are grateful to Meike Crecelius, Natalia Rogova, and Viktoria Morasch for their help with cloning of NDP52 fragments for ITC and NMR experiments, protein/peptide sample preparation, and ITC data collection.
Funding
This work was supported by grants: ROCHE (Roche per la ricerca 2017) and 5XMILLE Italian Ministry of Health (2017) to FS. The study was partially supported by the Italian Ministry of Health (Progetto di ricerca Finalizzata RF-2018-12366111) and by the Italian Foundation for Multiple Sclerosis (Fism Progetto Speciale 2018/S/5) to LB. The work of NW, VD, and VVR was supported by the DFG-funded Collaborative Research Centre on Selective Autophagy (SFB 1177 “Molecular and Functional Characterization of Selective Autophagy”), Germany. VD and VVR also received funding from Structural Genomic Consortium (SGC). The SGC is a registered charity (no: 1097737) that receives funds from AbbVie, Bayer AG, Boehringer Ingelheim, Canada Foundation for Innovation, Eshelman Institute for Innovation, Genentech, Genome Canada through Ontario Genomics Institute [OGI-196], EU/EFPIA/OICR/McGill/KTH/Diamond, Innovative Medicines Initiative 2 Joint Undertaking [EUbOPEN grant 875510], Janssen, Merck KGaA (aka EMD in Canada and USA), Merck & Co (aka MSD outside Canada and USA), Pfizer, São Paulo Research Foundation-FAPESP, Takeda and Wellcome [106169/ZZ14/Z]. The work of VC was supported by Italian Foundation of Multiple Sclerosis (FISM grant 2017/R/08) and by Ministry of Health, Progetto Giovani Ricercatori (GR-2016-02362380). The work of MK, MT, ML, FL, VD, and EP is supported by Danmarks Grundforskningsfond (DNRF125) and a Carlsberg Foundation Distinguished Fellowship (CF18-0314) to EP’s group. Moreover, the project has been supported by a Netaji Subhash ICAR international fellowship, Government of India to MK to work in EP group. The calculations described in this paper were performed using the DeiC National Life Science Supercomputer Computerome at DTU (Denmark), and a DeiC-Pilot Grant on Abacus (Denmark).
Author information
Authors and Affiliations
Contributions
FS designed the project. ADR and FS wrote the paper. ADR performed analysis in MS patients (Fig. 1), on B cells (Fig. 5a–d) and performed the cloning of NDP52GE. TM investigated the role of NDP52 variants in the TLR signaling (Supplementary Fig. 1) and helped FS in performing Immunofluorescences of Figs. 2, 3, and 5e–g. FS characterized the binding between LC3C and NDP52 variants, performed the mitophagy analysis and Fig. 5e–g. MC helped FS in the characterization of LC3C-NDP52 interaction. PDA participated in the amplification of NDP52-LIR motif from MS patients and HD donors. DFA and LB provided PBMCs of MS patients and the LCL cell line. VC provided PBMCs of HD and MS patients and helped in preparing some cDNAs (Fig. 1). DFA performed FACS analysis (TMRM) and sorted monocytes, B and T cells from healthy patients (Fig. 5a). GC, RC, and VC performed the genetical study. MK, MT, ML, FL, VD, and EP performed Fig. 3e, f. The ITC and NMR experiments were designed by VD and VVR, and performed by NW and FL (Fig. 3 and Supplementary Fig. 2). ADR, EP, VVR, EG, and FS analyzed data. All authors commented on the final draft of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Refer to Human samples paragraph in the Materials and methods section.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by H. Zhang
Rights and permissions
About this article
Cite this article
Di Rita, A., Angelini, D.F., Maiorino, T. et al. Characterization of a natural variant of human NDP52 and its functional consequences on mitophagy. Cell Death Differ 28, 2499–2516 (2021). https://doi.org/10.1038/s41418-021-00766-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-021-00766-3
This article is cited by
-
Autophagy-NLRP3 Inflammasome Crosstalk in Microglia: A Therapeutic Target for Multiple Sclerosis
Inflammation (2026)
-
A variant of the autophagic receptor NDP52 counteracts phospho-TAU accumulation and emerges as a protective factor for Alzheimer’s disease
Cell Death & Disease (2025)
-
NDP52 and its emerging role in pathogenesis
Cell Death & Disease (2025)
-
Advances in research on mitochondrial dysfunction in neurodegenerative diseases
Journal of Neurology (2025)
-
The mitophagy pathway and its implications in human diseases
Signal Transduction and Targeted Therapy (2023)