Abstract
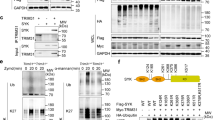
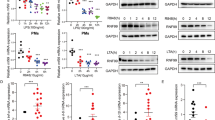
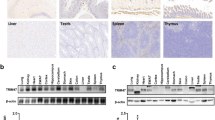
Protein ubiquitination plays an important role in the regulation of TGF-β-activated kinase 1 (TAK1)-mediated NF-κB activation. It is well established that TAK1 activation is tightly regulated with its binding partners, TAK1-binding proteins (TAB1-3). However, the tight regulation of TAK1 activation remains elusive. Here, using Trim26-knockout mice and Trim26-transgenic mice, we found that TRIM26 acts as a positive regulator of TAK1 activation by ubiquitinating its binding partner TAB1. Knockout of Trim26 inhibited TAK1 activation and downstream kinases activation, thus decreasing the induction of proinflammatory cytokines following LPS, TNF-α, and IL-1β stimulation. Mechanistically, TRIM26 catalyzes the K11-linked polyubiquitination of TAB1 at Lys294, Lys319, and Lys335 to enhance the activation of TAK1 and subsequent NF-κB and MAPK signaling. Consequently, Trim26 deficiency protects mice from LPS-induced septic shock in vivo. Moreover, Trim26 deficiency attenuates the severity of dextran sodium sulfate (DSS)-induced colitis. Thus, these finding provides a novel insight into how TAK1 activation is regulated through TRIM26-mediated ubiquitination of TAB1 and reveals the new function of TRIM26 in the regulation of the inflammatory innate immune response.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The data within the article and its Supplementary Information files that support this study are available from the authors upon request.
References
Majer O, Liu B, Barton GM. Nucleic acid-sensing TLRs: trafficking and regulation. Curr Opin Immunol. 2017;44:26–33.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–84.
Bonham Kevin S, Orzalli Megan H, Hayashi K, Wolf Amaya I, Glanemann C, Weninger W, et al. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell. 2014;156:705–16.
Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5.
Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–66.
Lin S-C, Lo Y-C, Wu H. Helical assembly in the MyD88–IRAK4–IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–90.
Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–88.
Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14:546–58.
Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S. TLRs, future potential therapeutic targets for RA. Autoimmun Rev. 2017;16:103–13.
Burgueno JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:263–78.
Joosten LA, Abdollahi-Roodsaz S, Dinarello CA, O’Neill L, Netea MG. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol. 2016;12:344–57.
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61.
Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708.
Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34:307–16.
Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, et al. TABi: An Activator Of the TAK1 MAPKKK in TGF-β signal transduction. Science. 1996;272:1179–82.
Kanayama A, Seth RB, Sun L, Ea C-K, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–48.
Wang C, Deng L, Hong M, Akkaraju GR, Inoue J-I, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51.
Li Q, Yan J, Mao AP, Li C, Ran Y, Shu HB, et al. Tripartite motif 8 (TRIM8) modulates TNF- and IL-1-triggered NF-B activation by targeting TAK1 for K63-linked polyubiquitination. Proc Natl Acad Sci USA. 2011;108:19341–6.
Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, et al. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor α- and Interleukin-1β-induced IKK/NF-κB and JNK/AP-1 activation. J Biol Chem. 2010;285:5347–60.
Dou Y, Xing J, Kong G, Wang G, Lou X, Xiao X, et al. Identification of the E3 ligase TRIM29 as a critical checkpoint regulator of NK cell functions. J Immunol. 2019;203:873–80.
Chen SY, Zhang HP, Li J, Shi JH, Tang HW, Zhang Y, et al. Tripartite motif‐containing 27 attenuates liver ischemia/reperfusion injury by suppressing TAK1 via TAB2/3 degradation. Hepatology. 2020;73:738–58.
Charlaftis N, Suddason T, Wu X, Anwar S, Karin M, Gallagher E. The MEKK1 PHD ubiquitinates TAB1 to activate MAPKs in response to cytokines. EMBO J. 2014;33:2581–96.
van Huizen M, Kikkert M. The role of atypical ubiquitin chains in the regulation of the antiviral innate immune response. Front. Cell Dev. Biol. 2020;7:1–8.
Ran Y, Zhang J, Liu LL, Pan ZY, Nie Y, Zhang HY, et al. Autoubiquitination of TRIM26 links TBK1 to NEMO in RLR-mediated innate antiviral immune response. J Mol Cell Biol. 2016;8:31–43.
Edmonds MJ, Carter RJ, Nickson CM, Williams SC, Parsons JL. Ubiquitylation-dependent regulation of NEIL1 by Mule and TRIM26 is required for the cellular DNA damage response. Nucleic Acids Res. 2017;45:726–38.
Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochemical Sci. 2017;42:297–311.
Tao J, Luo M, Sun H, Zhao H-M, Sun Q-S, Huang Z-M. Overexpression of tripartite motif containing 26 inhibits non-small cell lung cancer cell growth by suppressing PI3K/AKT signaling. Kaohsiung J Med Sci. 2020;36:417–22.
Meyer M, Gaudieri S, Rhodes DA, Trowsdale J. Cluster of TRIM genes in the humanMHC class I region sharing the B30.2 domain. Tissue Antigens. 2002;61:63–71.
Wang P, Zhao W, Zhao K, Zhang L, Gao C. TRIM26 negatively regulates interferon-beta production and antiviral response through polyubiquitination and degradation of nuclear IRF3. PLoS Pathog. 2015;11:e1004726.
Luo L, Lucas RM, Liu L, Stow JL. Signalling, sorting and scaffolding adaptors for Toll-like receptors. J Cell Sci. 2019;133:1–10.
Hinz M, Scheidereit C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014;15:46–61.
Yang Y, Zhou C, Wang Y, Liu W, Liu C, Wang L, et al. The E3 ubiquitin ligase RNF 114 and TAB1 degradation are required for maternal-to-zygotic transition. EMBO Rep. 2017;18:205–16.
Theivanthiran B, Kathania M, Zeng M, Anguiano E, Basrur V, Vandergriff T, et al. The E3 ubiquitin ligase Itch inhibits p38α signaling and skin inflammation through the ubiquitylation of Tab1. Sci Signal. 2015;8:ra22.
Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunological Rev. 2012;246:95–106.
Song H, Liu B, Huai W, Yu Z, Wang W, Zhao J, et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat Commun. 2016;7:1–11.
Wu H, Liu H, Zhao X, Zheng Y, Liu B, Zhang L, et al. IKIP Negatively Regulates NF-kappaB Activation and Inflammation through Inhibition of IKKalpha/beta Phosphorylation. J Immunol. 2020;204:418–27.
Hirata Y, Takahashi M, Morishita T, Noguchi T, Matsuzawa A. Post-translational modifications of the TAK1-TAB complex. Int J Mol Sci. 2017;18:1–17.
Lei CQ, Wu X, Zhong X, Jiang L, Zhong B, Shu HB. USP19 inhibits TNF-alpha- and IL-1beta-triggered NF-kappaB activation by deubiquitinating TAK1. J Immunol. 2019;203:259–68.
Zhou Q, Cheng C, Wei Y, Yang J, Zhou W, Song Q, et al. USP15 potentiates NF-kappaB activation by differentially stabilizing TAB2 and TAB3. FEBS J. 2020;287:3165–83.
Hu MM, Yang Q, Zhang J, Liu SM, Zhang Y, Lin H, et al. TRIM38 inhibits TNF- and IL-1-triggered NF- B activation by mediating lysosome-dependent degradation of TAB2/3. Proc Natl Acad Sci USA. 2014;111:1509–14.
Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010;29:4198–209.
Zheng Y, Liu Q, Wu Y, Ma L, Zhang Z, Liu T, et al. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. 2018;37:1–18.
Kist M, Komuves LG, Goncharov T, Dugger DL, Yu C, Roose-Girma M, et al. Impaired RIPK1 ubiquitination sensitizes mice to TNF toxicity and inflammatory cell death. Cell Death Differ. 2021;28:985–1000.
Qin Y, Zhou MT, Hu MM, Hu YH, Zhang J, Guo L, et al. RNF26 temporally regulates virus-triggered type I interferon induction by two distinct mechanisms. PLoS Pathog. 2014;10:e1004358.
Wang L, Wu J, Li J, Yang H, Tang T, Liang H, et al. Host-mediated ubiquitination of a mycobacterial protein suppresses immunity. Nature. 2020;577:682–8.
Song G, Liu B, Li Z, Wu H, Wang P, Zhao K, et al. E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1. Nat Immunol. 2016;17:1342–51.
Acknowledgements
We thank Dr. Hui Xiao (Institut Pasteur of Shanghai, CAS, Shanghai, China) for the Flag-TAB2 expression plasmid; Dr. Chen Wang (School of Life Science and Technology, China Pharmaceutical University, China) for the HA-K11R expression plasmid; and Dr. Michael Karin (University of California at San Diego, San Diego, CA) for the expression plasmids of TRAF6, IKKβ, and TAK1; Dr. Zhengfan Jiang (Peking University) for the Flag-ITCH expression plasmid; Dr. Ran Huo (Nanjing Medical University) for the Flag-RNF114 expression plasmid; Dr. Chao Xu (Shandong Provincial Hospital Affiliated to Shandong University) for the Flag-MEKK1 expression plasmid.
Funding
This work was supported by the Natural Science Foundation of China (81930039, 31730026, 81525012 to CG and 31900680 to BL). This work was also supported by the Postdoctoral Science Foundation of China (BX201700146 to BL) and Shandong Provincial Natural Science Foundation (ZR2018BC021 to BL).
Author information
Authors and Affiliations
Contributions
CG conceived and designed the study. JZ and BC performed most of the experiments with help from ZS. LZ, YZ, CM, and FY contributed to the discussion and provided reagents. CG and BL supervised the study. JZ and BL analyzed the data. CG, BL, and JZ wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All mouse experiments were carried out according to the Guidelines of the China Animal Welfare Legislation, as approved by the Ethics Committee of Scientific Research of Shandong University Qilu Hospital, Jinan, Shandong Province, China (Permit number: KYLL-2017(KS)-361).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by: M. Piacentini
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, J., Cai, B., Shao, Z. et al. TRIM26 positively regulates the inflammatory immune response through K11-linked ubiquitination of TAB1. Cell Death Differ 28, 3077–3091 (2021). https://doi.org/10.1038/s41418-021-00803-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-021-00803-1
This article is cited by
-
TRIM26-mediated regulation of TRAF6 ubiquitination enhances host immune response during Toxoplasma gondii infection
Cellular and Molecular Life Sciences (2026)
-
TRIM26 deficiency promotes liver fibrosis progression by mediating macrophage polarization via the EZH2-STAT1 axis
Hepatology International (2026)
-
The emerging role of E3 ubiquitin ligases and deubiquitinases in metabolic dysfunction-associated steatotic liver disease
Journal of Translational Medicine (2025)
-
RNF167 mediates atypical ubiquitylation and degradation of RLRs via two distinct proteolytic pathways
Nature Communications (2025)
-
The ubiquitin ligase Pellino1 targets STAT3 to regulate macrophage-mediated inflammation and tumor development
Nature Communications (2025)