Abstract
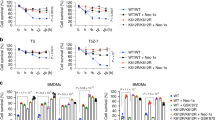
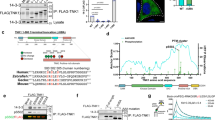
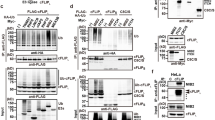
ABIN1 is a polyubiquitin-binding protein known to regulate NF-κB activation and cell death signaling. Mutations in Abin1 can cause severe immune diseases in human, such as psoriasis, systemic lupus erythematosus, and systemic sclerosis. Here, we generated mice that disrupted the ubiquitin-binding domain of ABIN1 (Abin1UBD/UBD) died during later embryogenesis owing to TNFR1-mediated cell death, similar to Abin1−/− mice. Abin1UBD/UBD cells were rendered sensitive to TNF-α-induced apoptosis and necroptosis as the inhibition of ABIN1UBD and A20 recruitment to the TNF-RSC complex leads to attenuated RIPK1 deubiquitination. Accordingly, the embryonic lethality of Abin1UBD/UBD mice was rescued via crossing with RIPK1 kinase-dead mice (Ripk1K45A/K45A) or the co-deletion of Ripk3 and one allele of Fadd, but not by the loss of Ripk3 or Mlkl alone. Unexpectedly, Abin1UBD/UBD mice with the co-deletion of Ripk3 and both Fadd alleles died at E14.5. This death was caused by spontaneous RIPK1 ubiquitination-dependent multiple inflammatory cytokines over production and could be rescued by the co-deletion of Ripk1 or Tnfr1 combined with Ifnar. Collectively, these data demonstrate the importance of the ABIN1 UBD domain, which mediates the ABIN1-A20 axis, at limiting RIPK1 activation-dependent cell death during embryonic development. Furthermore, our findings reveal a previously unappreciated ubiquitin pathway that regulates RIPK1 ubiquitination by FADD/Casp8 to suppress spontaneous IKKε/TBK1 activation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The authors declare that all data supporting the findings of this study are present in the paper and/or the Supplementary Materials. Additional data supporting the present study are available from the corresponding author upon reasonable request.
References
Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41:1228–33.
Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–7.
He CF, Liu YS, Cheng YL, Gao JP, Pan TM, Han JW, et al. TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 are associated with clinical features of systemic lupus erythematosus in a Chinese Han population. Lupus. 2010;19:1181–6.
Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, et al. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J Cell Biol. 1999;145:1471–82.
Callahan JA, Hammer GE, Agelides A, Duong BH, Oshima S, North J, et al. Cutting edge: ABIN-1 protects against psoriasis by restricting MyD88 signals in dendritic cells. J Immunol. 2013;191:535–9.
Caster DJ, Korte EA, Nanda SK, McLeish KR, Oliver RK, G’Sell RT, et al. ABIN1 dysfunction as a genetic basis for lupus nephritis. J Am Soc Nephrology. 2013;24:1743–54.
Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, et al. ABIN-1 binds to NEMO/IKKgamma and co-operates with A20 in inhibiting NF-kappaB. J Biol Chem. 2006;281:18482–8.
Gao L, Coope H, Grant S, Ma A, Ley SC, Harhaj EW. ABIN1 protein cooperates with TAX1BP1 and A20 proteins to inhibit antiviral signaling. J Biol Chem. 2011;286:36592–602.
Nanda SK, Venigalla RK, Ordureau A, Patterson-Kane JC, Powell DW, Toth R, et al. Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J Exp Med. 2011;208:1215–28.
Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, et al. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature. 2009;457:906–9.
Wagner S, Carpentier I, Rogov V, Kreike M, Ikeda F, Löhr F, et al. Ubiquitin binding mediates the NF-kappaB inhibitory potential of ABIN proteins. Oncogene. 2008;27:3739–45.
Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell. 2011;44:559–71.
Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C, Zhang J, et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528:370–5.
Lu TT, Onizawa M, Hammer GE, Turer EE, Yin Q, Damko E, et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity. 2013;38:896–905.
Dziedzic SA, Su Z, Jean Barrett V, Najafov A, Mookhtiar AK, Amin P, et al. ABIN-1 regulates RIPK1 activation by linking Met1 ubiquitylation with Lys63 deubiquitylation in TNF-RSC. Nat Cell Biol. 2018;20:58–68.
Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–27.
Su Z, Dziedzic SA, Hu D, Barrett VJ, Broekema N, Li W, et al. ABIN-1 heterozygosity sensitizes to innate immune response in both RIPK1-dependent and RIPK1-independent manner. Cell Death Differ. 2019;26:1077–88.
Maelfait J, Roose K, Bogaert P, Sze M, Saelens X, Pasparakis M, et al. A20 (Tnfaip3) deficiency in myeloid cells protects against influenza A virus infection. PLoS Pathog. 2012;8:e1002570.
Parvatiyar K, Barber GN, Harhaj EW. TAX1BP1 and A20 inhibit antiviral signaling by targeting TBK1-IKKi kinases. J Biol Chem. 2010;285:14999–5009.
Zhou J, Wu R, High AA, Slaughter CA, Finkelstein D, Rehg JE, et al. A20-binding inhibitor of NF-κB (ABIN1) controls Toll-like receptor-mediated CCAAT/enhancer-binding protein β activation and protects from inflammatory disease. Proc Natl Acad Sci USA. 2011;108:E998–1006.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9.
Li W, Li B, Giacalone NJ, Torossian A, Sun Y, Niu K, et al. BV6, an IAP antagonist, activates apoptosis and enhances radiosensitization of non-small cell lung carcinoma in vitro. J Thorac Oncol. 2011;6:1801–9.
Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–33.
Zhang X, Zhang H, Xu C, Li X, Li M, Wu X, et al. Ubiquitination of RIPK1 suppresses programmed cell death by regulating RIPK1 kinase activation during embryogenesis. Nat Commun. 2019;10:4158.
Tang Y, Tu H, Zhang J, Zhao X, Wang Y, Qin J, et al. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat Commun. 2019;10:4157.
Kist M, Kőműves LG, Goncharov T, Dugger DL, Yu C, Roose-Girma M, et al. Impaired RIPK1 ubiquitination sensitizes mice to TNF toxicity and inflammatory cell death. Cell Death Differ. 2021;28:985–1000.
Hutti JE, Turk BE, Asara JM, Ma A, Cantley LC, Abbott DW. IkappaB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-kappaB pathway. Mol Cell Biol. 2007;27:7451–61.
Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–9.
Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–60.
Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, Martin-McNulty B, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–76.
Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–85.
McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, et al. A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood. 2011;117:4600–8.
Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49:397–412.
Clapes T, Lefkopoulos S, Trompouki E. Stress and non-stress roles of inflammatory signals during HSC emergence and maintenance. Front Immunol. 2016;7:487.
Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017;130:1693–8.
Kang TB, Jeong JS, Yang SH, Kovalenko A, Wallach D. Caspase-8 deficiency in mouse embryos triggers chronic RIPK1-dependent activation of inflammatory genes, independently of RIPK3. Cell Death Differ. 2018;25:1107–17.
Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–88.
Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202.
Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–51.
Liu Z, Chan FK. Regulatory mechanisms of RIPK1 in cell death and inflammation. Semin Cell Dev Biol. 2021;109:70–5.
Malynn BA, Ma A. A20: a multifunctional tool for regulating immunity and preventing disease. Cell Immunol. 2019;340:103914.
Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014;35:22–31.
Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–10.
Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2016;48:67–73.
Kattah MG, Shao L, Rosli YY, Shimizu H, Whang MI, Advincula R, et al. A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival. J Exp Med. 2018;215:1839–52.
Nanda SK, Lopez-Pelaez M, Arthur JS, Marchesi F, Cohen P. Suppression of IRAK1 or IRAK4 catalytic activity, but not type 1 IFN signaling, prevents lupus nephritis in mice expressing a ubiquitin binding-defective mutant of ABIN1. J Immunol. 2016;197:4266–73.
Nanda SK, Petrova T, Marchesi F, Gierlinski M, Razsolkov M, Lee KL, et al. Distinct signals and immune cells drive liver pathology and glomerulonephritis in ABIN1[D485N] mice. Life Sci Alliance. 2019;2:e201900533.
Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, Peltzer N, et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife. 2014;3:e03464.
Peltzer N, Darding M, Montinaro A, Draber P, Draberova H, Kupka S, et al. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature. 2018;557:112–7.
Heger K, Wickliffe KE, Ndoja A, Zhang J, Murthy A, Dugger DL, et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature. 2018;559:120–4.
Liu Y, Fan C, Zhang Y, Yu X, Wu X, Zhang X, et al. RIP1 kinase activity-dependent roles in embryonic development of Fadd-deficient mice. Cell Death Differ. 2017;24:1459–69.
Acknowledgements
We thank Dr. Xiaodong Wang (National Institute of Biological Sciences, Beijing, China) for providing Ripk3−/− mice, Dr. Jianke Zhang (Thomas Jefferson University, Philadelphia, PA, USA) for providing Fadd+/− mice, Dr. Feng Shao (National Institute of Biological Sciences, Beijing, China) for providing Tnfr1−/− mice and Dr. Qibin Leng (Institute Pasteur of Shanghai, Shanghai, China) for providing Ifnar−/− mice. We also thank Zhonghui Weng (Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences) for animal studies.
Funding
This work was supported by grants from the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA26040306), the National Natural Science Foundation of China (31970688,31771537,82001684,81871101) and the National Key Research and Development Program of China (2018YFC1200201). ZCW was supported by “Chenguang Program” of Shanghai Education Development Foundation (19CG18) and Shanghai Rising Star Program of Science and Technology Commission of Shanghai Municipality (20QA1405600). We also thank support from Shanghai Frontiers Science Center of Cellular Homeostasis and Human Diseases, and GuangCi Professorship Program of Ruijin Hospital Shanghai Jiao Tong University School of Medicine.
Author information
Authors and Affiliations
Contributions
ML and HBZ designed the study; ML performed all experiments and analyzed data with assistance from JLL, MYX, XML, HWZ, XXW, LXW, YJO, XHW, XMZ, HL, QL, FL, WWR, JSD, XZW, and ZCW; YBL analyzed A20−/− Ripk3-/-Fadd-/- mice; CXX and QZ helped to analyze Abin1UBD/UBD mice; JBL, YL, YWZ, AKL, and QFL provided resources and intellectual input. ML and HBZ assembled figure panels and wrote the paper. HBZ supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, University of Chinese Academy of Sciences.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by J. Silke
Rights and permissions
About this article
Cite this article
Li, M., Liu, Y., Xu, C. et al. Ubiquitin-binding domain in ABIN1 is critical for regulating cell death and inflammation during development. Cell Death Differ 29, 2034–2045 (2022). https://doi.org/10.1038/s41418-022-00994-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-00994-1
This article is cited by
-
Necroptosis in cancer: insight from epigenetic, post-transcriptional and post-translational modifications
Journal of Hematology & Oncology (2025)
-
Whole exome sequencing identified six novel genes for depressive symptoms
Molecular Psychiatry (2025)
-
ABIN1 is a negative regulator of effector functions in cytotoxic T cells
EMBO Reports (2024)
-
The regulation of necroptosis by ubiquitylation
Apoptosis (2022)