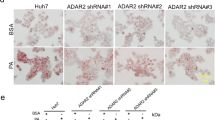
Abstract
Non-alcoholic fatty liver disease (NAFLD) has become a growing public health problem. However, the complicated pathogenesis of NAFLD contributes to the deficiency of effective clinical treatment. Here, we demonstrated that liver-specific loss of Arid2 induced hepatic steatosis and this progression could be exacerbated by HFD. Mechanistic study revealed that ARID2 repressed JAK2-STAT5-PPARγ signaling pathway by promoting the ubiquitination of JAK2, which was mediated by NEDD4L, a novel E3 ligase for JAK2. ChIP assay revealed that ARID2 recruited CARM1 to increase H3R17me2a level at the NEDD4L promoter and activated the transcription of NEDD4L. Moreover, inhibition of Jak2 by Fedratinib in liver-specific Arid2 knockout mice alleviated HFD-induced hepatic steatosis. Downregulation of ARID2 and the reverse correlation between ARID2 and JAK2 were also observed in clinical samples. Therefore, our study has revealed an important role of ARID2 in the development of NAFLD and provided a potential therapeutic strategy for NAFLD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
RNA-seq data supporting the results of this study have been deposited in the NCBI GEO database under accession number GSE216171. All the other data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding author upon reasonable request.
References
Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–65.
Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90.
Adams LA, Lymp JF, Sauver JS, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21.
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
Zhang L, Wang W, Li X, He S, Yao J, Wang X, et al. MicroRNA-155 promotes tumor growth of human hepatocellular carcinoma by targeting ARID2. Int J Oncol. 2016;48:2425–34.
Duan Y, Tian L, Gao Q, Liang L, Zhang W, Yang Y, et al. Chromatin remodeling gene ARID2 targets cyclin D1 and cyclin E1 to suppress hepatoma cell progression. Oncotarget. 2016;7:45863–75.
Oba A, Shimada S, Akiyama Y, Nishikawaji T, Mogushi K, Ito H, et al. ARID2 modulates DNA damage response in human hepatocellular carcinoma cells. J Hepatol. 2017;66:942–51.
Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011;21:396–420.
Muchardt C, Yaniv M. ATP-dependent chromatin remodelling: SWI/SNF and Co. are on the job. J Mol Biol. 1999;293:187–98.
Li S, Liu C, Li N, Hao T, Han T, Hill DE, et al. Genome-wide coactivation analysis of PGC-1alpha identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab. 2008;8:105–17.
Moore A, Wu L, Chuang JC, Sun X, Luo X, Gopal P, et al. Arid1a loss drives nonalcoholic steatohepatitis in mice through epigenetic dysregulation of hepatic lipogenesis and fatty acid oxidation. Hepatology. 2019;69:1931–45.
Lee JB, Yoon SJ, Lee SH, Lee MS, Jung H, Kim TD, et al. Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARgamma. J Endocrinol. 2017;235:223–35.
Murtaza M, Khan G, Aftab MF, Afridi SK, Ghaffar S, Ahmed A, et al. Cucurbitacin E reduces obesity and related metabolic dysfunction in mice by targeting JAK-STAT5 signaling pathway. PloS One. 2017;12:e0178910.
Pal S, Yun R, Datta A, Lacomis L, Erdjument-Bromage H, Kumar J, et al. mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol Cell Biol. 2003;23:7475–87.
Lee YH, Coonrod SA, Kraus WL, Jelinek MA, Stallcup MR. Regulation of coactivator complex assembly and function by protein arginine methylation and demethylimination. Proc Natl Acad Sci USA 2005;102:3611–6.
Bauer UM, Daujat S, Nielsen SJ, Nightingale K, Kouzarides T. Methylation at arginine 17 of histone H3 is linked to gene activation. EMBO Rep. 2002;3:39–44.
Karakashev S, Fukumoto T, Zhao B, Lin J, Wu S, Fatkhutdinov N, et al. EZH2 inhibition sensitizes CARM1-high, homologous recombination proficient ovarian cancers to PARP inhibition. Cancer Cell. 2020;37:157–67.e6.
Liu F, Ma F, Wang Y, Hao L, Zeng H, Jia C, et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat Cell Biol. 2017;19:1358–70.
Kawai M, Namba N, Mushiake S, Etani Y, Nishimura R, Makishima M, et al. Growth hormone stimulates adipogenesis of 3T3-L1 cells through activation of the Stat5A/5B-PPARgamma pathway. J Mol Endocrinol. 2007;38:19–34.
Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3.
Li WX. Canonical and non-canonical JAK–STAT signaling. Trends Cell Biol. 2008;18:545–51.
Han J, Wang Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell. 2018;9:145–51.
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74.
Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–92.
Loesch R, Chenane L, Colnot S. ARID2 Chromatin Remodeler in Hepatocellular Carcinoma. Cells. 2020;9:2152.
Coughlan N, Thillainadesan G, Andrews J, Isovic M, Torchia J. β-Estradiol-dependent activation of the JAK/STAT pathway requires p/CIP and CARM1. Biochimica et Biophysica Acta. 2013;1833:1463–75.
Moreno T, Monterde B, González-Silva L, Betancor-Fernández I, Revilla C, Agraz-Doblas A, et al. ARID2 deficiency promotes tumor progression and is associated with higher sensitivity to chemotherapy in lung cancer. Oncogene. 2021;40:2923–35.
Fukumoto T, Lin J, Fatkhutdinov N, Liu P, Somasundaram R, Herlyn M, et al. ARID2 deficiency correlates with the response to immune checkpoint blockade in melanoma. J Investigative Dermatol. 2021;141:1564–72.e4.
Bala P, Singh AK, Kavadipula P, Kotapalli V, Sabarinathan R, Bashyam MD. Exome sequencing identifies ARID2 as a novel tumor suppressor in early-onset sporadic rectal cancer. Oncogene. 2021;40:863–74.
Jiang H, Cao H-J, Ma N, Bao W-D, Wang J-J, Chen T-W, et al. Chromatin remodeling factor ARID2 suppresses hepatocellular carcinoma metastasis via DNMT1-Snail axis. Proc Natl Acad Sci. 2020;117:4770–80.
O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl J Med. 2013;368:161–70.
Stepkowski SM, Chen W, Ross JA, Nagy ZS, Kirken RA. STAT3: an important regulator of multiple cytokine functions. Transplantation. 2008;85:1372–7.
Li T, Weng J, Zhang Y, Liang K, Fu G, Li Y, et al. mTOR direct crosstalk with STAT5 promotes de novo lipid synthesis and induces hepatocellular carcinoma. Cell Death Dis. 2019;10:619.
Lee J-B, Yoon S-J, Lee S-H, Lee M-S, Jung H, Kim T-D, et al. Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARγ. J Endocrinol. 2017;235:223–35.
Barclay JL, Nelson CN, Ishikawa M, Murray LA, Kerr LM, McPhee TR, et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181–92.
Raab JR, Resnick S, Magnuson T. Genome-wide transcriptional regulation mediated by biochemically distinct SWI/SNF complexes. PLoS Genet. 2015;11:e1005748.
Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–64.
Liu CS, Yang-Yen HF, Suen CS, Hwang MJ, Yen JJ. Cbl-mediated K63-linked ubiquitination of JAK2 enhances JAK2 phosphorylation and signal transduction. Sci Rep. 2017;7:4613.
Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol Cell Biol. 2002;22:3316–26.
Zhang FK, Ni QZ, Wang K, Cao HJ, Guan DX, Zhang EB, et al. Targeting USP9X-AMPK Axis in ARID1A-deficient hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2022;14:101–27.
Deng Y-Z, Chen P-P, Wang Y, Yin D, Koeffler HP, Li B, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem. 2007;282:36571–81.
Acknowledgements
We thank Zhonghui Weng, Yifan Bu and Lin Qiu from Institutional Center for Shared Technologies and Facilities of SINH, CAS for technical assistance. We appreciate the New World Group for their Charitable Foundation to establish the Institute for Nutritional Sciences, SIBS, CAS-New World Joint laboratory, which have given full support to this study.
Funding
This work was supported by the National Natural Science Foundation of China (81972757 and 82172950), Youth Innovation Promotion Association of Chinese Academy of Sciences grant (2017324) and Sanofi-SIBS 2018 Young Faculty Award to Jing-Jing Li and the National Natural Science Foundation of China (82030084 and 81730083) to Dong Xie.
Author information
Authors and Affiliations
Contributions
HC, JL and DX conceived the project and designed the research studies. HC performed most of the experiments described. HJ, KD, XQ, NM, FZ, YW, QZ and JX provided help with animal and technical assistance in the mouse experiments. QN, SX and BZ assisted with the in vitro assays. BZ provided conceptual advice and helpful discussion. HC analyzed data. HC, JL and DX wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
Experiments about human tissues have been approved by the Biomedical Research Ethics Committee, Shanghai Institute for Biological Science, CAS in 2019 (ER-SIBS-251902). All animal studies were performed under the approval of the Institutional Animal Care and Use Committee of Shanghai Institute of Nutrition and Health, CAS (IACUC, SINH, CAS).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by M Bianchi
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, HJ., Jiang, H., Ding, K. et al. ARID2 mitigates hepatic steatosis via promoting the ubiquitination of JAK2. Cell Death Differ 30, 383–396 (2023). https://doi.org/10.1038/s41418-022-01090-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41418-022-01090-0
This article is cited by
-
Post-translational modifications in the pathophysiological process of metabolic dysfunction‑associated steatotic liver disease
Cell & Bioscience (2025)
-
GSDME promotes MASLD by regulating pyroptosis, Drp1 citrullination-dependent mitochondrial dynamic, and energy balance in intestine and liver
Cell Death & Differentiation (2024)
-
Genomic and transcriptomic profiling of hepatocellular carcinoma reveals a rare molecular subtype
Discover Oncology (2024)