Abstract
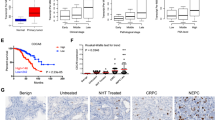
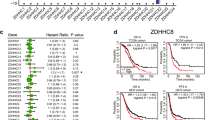
Emerging evidence indicates that transcriptional regulation plays pivotal roles in modulating cellular vulnerability to ferroptosis. However, the intricate mechanisms governing these processes remain poorly understood. In this study, we identify ATOH8, a basic helix-loop-helix (bHLH) transcription factor, as a key player in ferroptosis regulation. ATOH8 is significantly upregulated in tumor cells following treatment with a ferroptosis inducer. Overexpression of ATOH8 increases the susceptibility of tumor cells to ferroptosis, while deletion of ATOH8 promotes ferroptosis evasion. Mechanistically, ATOH8 confers the sensitivity of tumor cells to ferroptosis by suppressing the transcription of stearoyl-CoA desaturase (SCD). Additionally, another bHLH family member, TCF3, is found to functions as a co-factor with ATOH8 by forming a TCF3-ATOH8 transcriptional repressive complex that suppresses SCD transcription. Furthermore, searching for upstream element reveals that EZH2 epigenetically suppresses ATOH8 expression by promoting DNA methylation in the ATOH8 promoter region and increasing the level of H3K27 me3. Importantly, pharmacological inhibition of EZH2 in a combined with a ferroptosis inducer markedly impedes tumor growth both in vitro and in vivo. Collectively, our study elucidates a molecular link between ferroptosis and epigenetic and transcriptional regulation, highlighting the potential of EZH2 and ATOH8 as therapeutic targets for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The ChIP-seq and mRNA-seq data generated in this study have been deposited in the NCBI Gene Expression Omnibus (GEO) database under accession numbers GSE282996 (ChIP-seq) and GSE282997 (mRNA-seq). The lipidomic data are available within the Original Data File. All other data are available from the corresponding authors upon request.
References
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20:1181–92.
Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–91.
Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4.
Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, et al. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019;79:5355–66.
Dai C, Chen X, Li J, Comish P, Kang R, Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020;27:645–56.
Liu J, Song X, Kuang F, Zhang Q, Xie Y, Kang R, et al. NUPR1 is a critical repressor of ferroptosis. Nat Commun. 2021;12:647.
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc(). Cell Death Differ. 2020;27:662–75.
Yang M, Luo H, Yi X, Wei X, Jiang DS. The epigenetic regulatory mechanisms of ferroptosis and its implications for biological processes and diseases. MedComm (2020). 2023;4:e267.
Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z, Wei X, et al. Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med. 2020;160:552–65.
Chen M, Jiang Y, Sun Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun. 2021;550:77–83.
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F, et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41–53.
Divvela SSK, Saberi D, Brand-Saberi B. Atoh8 in Development and Disease. Biology (Basel) 2022;11:136.
Lee J, Roh JL. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023;559:216119.
Zhang W, Dai J, Hou G, Liu H, Zheng S, Wang X, et al. SMURF2 predisposes cancer cell toward ferroptosis in GPX4-independent manners by promoting GSTP1 degradation. Mol Cell. 2023;83:4352–69.e4358.
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Torres-Machorro AL. Homodimeric and Heterodimeric Interactions among Vertebrate Basic Helix-Loop-Helix Transcription Factors. Int J Mol Sci. 2021;22:12855.
Sen U, Coleman C, Sen T. Stearoyl coenzyme A desaturase-1: multitasker in cancer, metabolism, and ferroptosis. Trends Cancer. 2023;9:480–9.
Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839–48.
Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–9.
Patel N, Varghese J, Masaratana P, Latunde-Dada GO, Jacob M, Simpson RJ, et al. The transcription factor ATOH8 is regulated by erythropoietic activity and regulates HAMP transcription and cellular pSMAD1,5,8 levels. Br J Haematol. 2014;164:586–96.
Liu X, Li X, Wang S, Liu Q, Feng X, Wang W, et al. ATOH8 binds SMAD3 to induce cellular senescence and prevent Ras-driven malignant transformation. Proc Natl Acad Sci USA. 2023;120:e2208927120.
Chen C, Wang Z, Ding Y, Wang L, Wang S, Wang H, et al. DNA Methylation: From Cancer Biology to Clinical Perspectives. Front Biosci. 2022;27:326.
Yu Y, MohamedAl-Sharani H, Zhang B. EZH2-mediated SLC7A11 upregulation via miR-125b-5p represses ferroptosis of TSCC. Oral Dis. 2023;29:880–91.
Lai Y, Han X, Xie B, Xu Y, Yang Z, Wang D, et al. EZH2 suppresses ferroptosis in hepatocellular carcinoma and reduces sorafenib sensitivity through epigenetic regulation of TFR2. Cancer Sci. 2024;115:2220–34.
Zhang Y, Tang B, Song J, Yu S, Li Y, Su H, et al. Lnc-PDZD7 contributes to stemness properties and chemosensitivity in hepatocellular carcinoma through EZH2-mediated ATOH8 transcriptional repression. J Exp Clin Cancer Res. 2019;38:92.
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46.
Liang JJ, Peng H, Wang JJ, Liu XH, Ma L, Ni YR, et al. Relationship between the structure and function of the transcriptional regulator E2A. J Biol Res (Thessalon). 2021;28:15.
Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104.
Liu F, Song DY, Huang J, Yang HQ, You D, Ni JD. Long non-coding RNA CIR inhibits chondrogenic differentiation of mesenchymal stem cells by epigenetically suppressing ATOH8 via methyltransferase EZH2. Mol Med. 2021;27:12.
Xiao H, Du X, Tao Z, Jing N, Bao S, Gao WQ, et al. Taurine Inhibits Ferroptosis Mediated by the Crosstalk between Tumor Cells and Tumor-Associated Macrophages in Prostate Cancer. Adv Sci (Weinh). 2024;11:e2303894.
Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–39.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFC1404101 and 2022YFA1302704 to W.Q.G.), the National Natural Science Foundation of China (82072843 to Y.X.F., W2431055 and U23A20441 to W.Q.G. and 82372698 to B.D.), the Science and Technology Commission of Shanghai Municipality (21JC1404100 to W.Q.G. and 19411967400 to B.D.), the Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai to W.Q.G., 111 Project (B21024) and KC Wong foundation to W.Q.G., Summit Plateau Program, Research Physician Program, Shanghai Jiao Tong University School of Medicine to B.D., Shanghai Municipal Health Commission (2019LJ11, 2020CXJQ03) to B.D.
Author information
Authors and Affiliations
Contributions
Experiments were conceived and designed by Y.F., W.Q.G. and B.D. Experiments were performed by H.X., X.D., H.H. and W.G. Data were analyzed by H.X., H.H., Z.T., S.B. and Z.W. The paper was written by H.X., Y.F. and W.Q.G. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, H., Du, X., Hou, H. et al. ATOH8 confers the vulnerability of tumor cells to ferroptosis by repressing SCD expression. Cell Death Differ 32, 1397–1412 (2025). https://doi.org/10.1038/s41418-025-01482-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41418-025-01482-y
This article is cited by
-
Epigenetic and post-translational regulatory networks of ferroptosis in the tumor immune microenvironment
Experimental Hematology & Oncology (2026)
-
Immune cells dying from ferroptosis: mechanisms and therapeutic opportunities
Cell Death & Disease (2025)