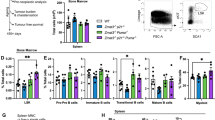
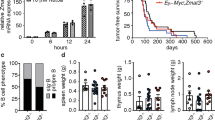
Abstract
TP53, the most frequently mutated gene in human cancer, encodes a transcriptional activator that induces myriad downstream target genes. Despite the importance of p53 in tumor suppression, the specific p53 target genes important for tumor suppression remain unclear. Recent studies have identified the p53-inducible gene Zmat3 as a critical effector of tumor suppression, but many questions remain regarding its p53-dependence, activity across contexts, and mechanism of tumor suppression alone and in cooperation with other p53-inducible genes. To address these questions, we used Tuba-seqUltra somatic genome editing and tumor barcoding in a mouse lung adenocarcinoma model, combinatorial in vivo CRISPR/Cas9 screens, meta-analyses of gene expression and Cancer Dependency Map data, and integrative RNA-sequencing and shotgun proteomic analyses. We established Zmat3 as a core component of p53-mediated tumor suppression and identified Cdkn1a as the most potent cooperating p53-induced gene in tumor suppression. We discovered that ZMAT3/CDKN1A serve as near-universal effectors of p53-mediated tumor suppression that regulate cell division, migration, and extracellular matrix organization. Accordingly, combined Zmat3-Cdkn1a inactivation dramatically enhanced cell proliferation and migration compared to controls, akin to p53 inactivation. Together, our findings place ZMAT3 and CDKN1A as hubs of a p53-induced gene program that opposes tumorigenesis across various cellular and genetic contexts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA sequencing data are available from the GEO under the accession number GSE289471 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE289471). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [61] partner repository with the dataset identifier PXD047240 and 10.6019/PXD047240. For DepMap analyses, somatic mutations, copy number status, CRISPR gene effect scores, gene expression, and cell line metadata files were downloaded from the DepMap Public 23Q4 Primary Files release. Previously published data on p53-dependent gene regulation were retrieved from www.TargetGeneReg.org [31], which contains 15 mouse and 57 human transcription profiling datasets. Additionally, genome-wide data on p53 binding were available from 9 curated ChIP datasets derived from mouse cells [33] and 28 ChIP datasets from human cells [62]. All other data supporting the findings of this study are in the article, supplemental information, or are available from the corresponding author upon reasonable request. Source data are provided in this paper.
Code availability
All code required to download, process, and visualize DepMap analyses is provided at https://github.com/brooksbenard/tp53_p21_zmat3/tree/main.
References
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333.
Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–14.
Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, et al. Distinct p53 transcriptional programs dictate acute DNA damage responses and tumor suppression. Cell. 2011;145:571–83.
Jiang D, Brady CA, Johnson TM, Lee EY, Park EJ, Scott MP, et al. Full p53 transcriptional activation potential is dispensable for tumor suppression in diverse lineages. Proc Natl Acad Sci USA. 2011;108:17123–8.
Mello SS, Valente LJ, Raj N, Seoane JA, Flowers BM, McClendon J, et al. A p53 super-tumor suppressor reveals a tumor suppressive p53-Ptpn14-yap axis in pancreatic cancer. Cancer Cell. 2017;32:460–473.e6.
Ho T, Tan BX, Lane D. How the other half lives: what p53 does when it is not being a transcription factor. Int J Mol Sci. 2020;21:13.
Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Trends Cell Biol. 2021;31:298–310.
Indeglia A, Murphy ME. Elucidating the chain of command: our current understanding of critical target genes for p53-mediated tumor suppression. Crit Rev Biochem Mol Biol. 2024;59:128–38.
Wang M, Attardi LD. A balancing act: p53 activity from tumor suppression to pathology and therapeutic implications. Annu Rev Pathol Mech Dis. 2022;17:205–26.
Hassin O, Oren M. Drugging p53 in cancer: one protein, many targets. Nat Rev Drug Discov. 2023;22:127–44.
Janic A, Valente LJ, Wakefield MJ, Di Stefano L, Milla L, Wilcox S, et al. DNA repair processes are critical mediators of p53-dependent tumor suppression. Nat Med. 2018;24:947–53.
Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JP, et al. p53 represses the mevalonate pathway to mediate tumor suppression. Cell. 2019;176:564–580.e19.
Suzuki S, Venkatesh D, Kanda H, Nakayama A, Hosokawa H, Lee E, et al. GLS2 is a tumor suppressor and a regulator of ferroptosis in hepatocellular carcinoma. Cancer Res. 2022;82:3209–22.
Indeglia A, Leung JC, Miller SA, Leu JIJ, Dougherty JF, Clarke NL, et al. An African-specific variant of TP53 reveals PADI4 as a regulator of p53-mediated tumor suppression. Cancer Discov. 2023;13:1696–719.
Bieging-Rolett KT, Kaiser AM, Morgens DW, Boutelle AM, Seoane JA, Van Nostrand EL, et al. Zmat3 is a key splicing regulator in the p53 tumor suppression program. Mol Cell. 2020;80:452–469.e9.
Varmeh-Ziaie S, Okan I, Wang Y, Magnusson KP, Warthoe P, Strauss M, et al. Wig-1, a new p53-induced gene encoding a zinc finger protein. Oncogene. 1997;15:2699–704.
Israeli D, Tessler E, Haupt Y, Elkeles A, Wilder S, Amson R, et al. A novel p53-inducible gene, PAG608, encodes a nuclear zinc finger protein whose overexpression promotes apoptosis. EMBO J. 1997;16:4384–92.
Muys BR, Anastasakis DG, Claypool D, Pongor L, Li XL, Grammatikakis I, et al. The p53-induced RNA-binding protein ZMAT3 is a splicing regulator that inhibits the splicing of oncogenic CD44 variants in colorectal carcinoma. Genes Dev. 2020;35:102–16.
Rogers ZN, McFarland CD, Winters IP, Naranjo S, Chuang CH, Petrov D, et al. A quantitative and multiplexed approach to uncover the fitness landscape of tumor suppression in vivo. Nat Methods. 2017;14:737.
Cai H, Chew SK, Li C, Tsai MK, Andrejka L, Murray CW, et al. A functional taxonomy of tumor suppression in oncogenic KRAS-driven lung cancer. Cancer Discov. 2021;11:1754–73.
Tang YJ, Xu H, Hughes NW, Kim SH, Ruiz P, Shuldiner EG, et al. Functional mapping of epigenetic regulators uncovers coordinated tumor suppression by the HBO1 and MLL1 complexes [Internet]. bioRxiv; 2024 [cited 22 Aug 2024]. p. 2024.08.19.607671. Available from: https://www.biorxiv.org/content/10.1101/2024.08.19.607671v1.
Rogers ZN, McFarland CD, Winters IP, Seoane JA, Brady JJ, Yoon S, et al. Mapping the in vivo fitness landscape of lung adenocarcinoma tumor suppression in mice. Nat Genet. 2018;50:483–6.
Kaiser AM, Gatto A, Hanson KJ, Zhao RL, Raj N, Ozawa MG, et al. p53 governs an AT1 differentiation programme in lung cancer suppression. Nature. 2023;619:851–9.
Blair LM, Juan JM, Sebastian L, Tran VB, Nie W, Wall GD, et al. Oncogenic context shapes the fitness landscape of tumor suppression. Nat Commun. 2023;14:6422.
Lowe SW, Jacks T, Housman DE, Ruley HE. Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proc Natl Acad Sci USA. 1994;91:2026–30.
Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000;14:704–18.
Valente LJ, Tarangelo A, Li AM, Naciri M, Raj N, Boutelle AM, et al. p53 deficiency triggers dysregulation of diverse cellular processes in physiological oxygen. J Cell Biol. 2020 [cited 15 Sep 2020];219. Available from: https://rupress.org/jcb/article/219/11/e201908212/152074/p53-deficiency-triggers-dysregulation-of-diverse.
Morgens DW, Deans RM, Li A, Bassik MC. Systematic comparison of CRISPR-Cas9 and RNAi screens for essential genes. Nat Biotechnol. 2016;34:634–6.
Drainas AP, Lambuta RA, Ivanova I, Serçin Ö, Sarropoulos I, Smith ML, et al. Genome-wide screens implicate loss of cullin ring ligase 3 in persistent proliferation and genome instability in TP53-deficient cells. Cell Rep. 2020;31:107465.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25.
Fischer M, Schwarz R, Riege K, DeCaprio JA, Hoffmann S. TargetGeneReg 2.0: a comprehensive web-atlas for p53, p63, and cell cycle-dependent gene regulation. NAR Cancer. 2022;4:zcac009.
Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017;36:3943.
Fischer M. Conservation and divergence of the p53 gene regulatory network between mice and humans. Oncogene. 2019;38:4095–109.
Sinha S, Ayushman M, Tong X, Yang F. Dynamically crosslinked poly(ethylene-glycol) hydrogels reveal a critical role of viscoelasticity in modulating glioblastoma fates and drug responses in 3D. Adv Healthc Mater. 2023;12:2202147.
Andrysik Z, Galbraith MD, Guarnieri AL, Zaccara S, Sullivan KD, Pandey A, et al. Identification of a core TP53 transcriptional program with highly distributed tumor suppressive activity. Genome Res. 2017;27:1645–57.
Brennan MS, Brinkmann K, Romero Sola G, Healey G, Gibson L, Gangoda L, et al. Combined absence of TRP53 target genes ZMAT3, PUMA and p21 cause a high incidence of cancer in mice. Cell Death Differ. 2023;31:159–69.
Valente LJ, Gray DHD, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013;3:1339–45.
Moyer SM, Wasylishen AR, Qi Y, Fowlkes N, Su X, Lozano G. p53 drives a transcriptional program that elicits a non-cell-autonomous response and alters cell state in vivo. Proc Natl Acad Sci USA. 2020;117:23663–73.
Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16.
Kasteri J, Das D, Zhong X, Persaud L, Francis A, Muharam H, et al. Translation control by p53. Cancers. 2018;10:133.
Tiu GC, Kerr CH, Forester CM, Krishnarao PS, Rosenblatt HD, Raj N, et al. A p53-dependent translational program directs tissue-selective phenotypes in a model of ribosomopathies. Dev Cell. 2021;56:2089–2102.e11.
Akeno N, Miller AL, Ma X, Wikenheiser-Brokamp KA. p53 suppresses carcinoma progression by inhibiting mTOR pathway activation. Oncogene. 2015;34:589–99.
Kon N, Ou Y, Wang SJ, Li H, Rustgi AK, Gu W. mTOR inhibition acts as an unexpected checkpoint in p53-mediated tumor suppression. Genes Dev. 2021;35:59–64.
Coronel L, Häckes D, Schwab K, Riege K, Hoffmann S, Fischer M. p53-mediated AKT and mTOR inhibition requires RFX7 and DDIT4 and depends on nutrient abundance. Oncogene. 2022;41:1063–9.
Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–60.
Chen S, Thorne RF, Zhang XD, Wu M, Liu L. Non-coding RNAs, guardians of the p53 galaxy. Semin Cancer Biol. 2021;75:72–83.
McIlwain DR, Pan Q, Reilly PT, Elia AJ, McCracken S, Wakeham AC, et al. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc Natl Acad Sci. 2010;107:12186–91.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22.
DiPersio CM, Longmate WM. Roles for integrin α3β1 in development and disease. In: Gullberg D, Eble JA, editors. Integrins in health and disease: key effectors of cell-matrix and cell-cell interactions [Internet]. Cham: Springer International Publishing; 2023 [cited 28 Aug 2024]. p. 27–95. Available from: https://doi.org/10.1007/978-3-031-23781-2_2.
Schubert M, Lindgreen S, Orlando L. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Res Notes. 2016;9:88.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
Kampmann M, Horlbeck MA, Chen Y, Tsai JC, Bassik MC, Gilbert LA, et al. Next-generation libraries for robust RNA interference-based genome-wide screens. Proc Natl Acad Sci USA. 2015;112:E3384–91.
Morgens DW, Wainberg M, Boyle EA, Ursu O, Araya CL, Tsui CK, et al. Genome-scale measurement of off-target activity using Cas9 toxicity in high-throughput screens. Nat Commun. 2017;8:15178.
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523.
Kong AT, Leprevost FV, Avtonomov DM, Mellacheruvu D, Nesvizhskii AI. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat Methods. 2017;14:513–20.
FragPipe [Internet]. [cited 9 Sep 2023]. FragPipe. Available from: https://fragpipe.nesvilab.org/.
da Veiga Leprevost F, Haynes SE, Avtonomov DM, Chang HY, Shanmugam AK, Mellacheruvu D, et al. Philosopher: a versatile toolkit for shotgun proteomics data analysis. Nat Methods. 2020;17:869–70.
Schilling B, Rardin MJ, MacLean BX, Zawadzka AM, Frewen BE, Cusack MP, et al. Platform-independent and label-free quantitation of proteomic data using MS1 extracted ion chromatograms in skyline. Mol Cell Proteom. 2012;11:202–14.
Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30:2524–6.
Zhang X, Smits AH, van Tilburg GB, Ovaa H, Huber W, Vermeulen M. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat Protoc. 2018;13:530–50.
Perez-Riverol Y, Bai J, Bandla C, García-Seisdedos D, Hewapathirana S, Kamatchinathan S, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–52.
Riege K, Kretzmer H, Sahm A, McDade SS, Hoffmann S, Fischer M. Dissecting the DNA binding landscape and gene regulatory network of p63 and p53. Lal A, Murphy ME, Bourdon JC, editors. eLife. 2020;9:e63266.
Acknowledgements
We thank Julien Sage for critical reading of the manuscript and Sydney Lu, Scott Dixon, and Camila Bolle for important discussions. We thank Mingxin Gu for her assistance with the in vivo screens. We thank Nitin Raj, Sofia Ferreira, and Kathryn Hanson for advice on RNA-sequencing. We thank Richard Frock for the use of his transilluminator and SpeedVac and Laura Andrejka for assistance with Tuba-seqUltra. We apologize to those whose work we could not cite due to spatial constraints.
Funding
This work was supported by Tobacco-Related Disease Research Program (TRDRP) fellowship T31DT1713, NIH T32CA009302 to AMB, NIH-R01CA234349 to DAP and MMW, and NIH R35CA197591 and TRDRP grant 28IP-0037 to LDA.
Author information
Authors and Affiliations
Contributions
AMB and LDA designed experiments and interpreted the results. AMB performed experiments and analyzed data. ARM and EMM conducted experiments. DY conducted casTLE analysis on sgRNA screens. HX, MW, YJT, and SSL performed Tuba-seqUltra pipeline. JD and RC processed and analyzed shotgun proteomics samples. LJV designed the sgRNA library. BAB performed analyses of DepMap data. SS performed and analyzed 3D migration experiments. MF conducted meta-analysis of p53-dependent gene expression and ChIP-seq binding. FY helped interpret 3D migration experiments. RM helped interpret DepMap data. PKJ helped interpret shotgun proteomics data. DAP and MMW helped interpret Tuba-seqUltra data. MCB helped interpret sgRNA screen results. AMB and LDA wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
RM is on the Advisory Boards of Kodikaz Therapeutic Solutions, Orbital Therapeutics, Pheast Therapeutics, 858 Therapeutics, Prelude Therapeutics, Mubadala Capital, and Aculeus Therapeutics. RM is a co-founder and equity holder of Pheast Therapeutics, MyeloGene, and Orbital Therapeutics. The other authors declare no competing interest.
Ethics
All animal experiments were performed in accordance with the Stanford University Administrative Panel on Laboratory Animal Care (protocol number 10382) guidelines and regulations. Mice (Mus musculus) were maintained at Stanford University’s Comparative Medicine Pavilion and Research Animal Facility according to practices prescribed by the National Institutes of Health and the Institutional Animal Care and Use Committee (IACUC). The Association for Assessment and Accreditation of Laboratory Animal Care provides additional accreditation to Stanford University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boutelle, A.M., Mabene, A.R., Yao, D. et al. Integrative multiomic approaches reveal ZMAT3 and p21 as conserved hubs in the p53 tumor suppression network. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01513-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-025-01513-8