Abstract
Spermatogenesis, which is regulated by multiple cell death mechanisms, is an extremely complex process. The significance of cell death during spermatogenesis is a topic of interest because of its potential medical implications. Cuproptosis is a new mechanism of cell death discovered in recent years, and recent studies have preliminarily confirmed that cuproptosis is involved in the process of spermatogenic cell death, but its specific role in the process of spermatogenic cell death is still unclear. In this review, the mechanisms of spermatogenic cell death associated with cuproptosis and the effects of key genes of cuproptosis on spermatogenesis are discussed together with some new perspectives for the study of spermatogenic cell death.
Similar content being viewed by others
Facts
-
1.
Cuproptosis is a novel form of cell death that exists in spermatogenic cells, and many cuproptosis related genes are associated with dysfunction in spermatogenesis.
-
2.
The regulatory mechanism of cuproptosis in spermatogenic cells is still unclear, and there may be a potential connection between cuproptosis and other types of cell death.
-
3.
In the future, in-depth research and understanding of the relationship between cuproptosis and other types of spermatogenic cell death is beneficial for a more thorough understanding of the role of cuproptosis in the mechanisms of spermatogenic cell death.
Introduction
Spermatogenesis is in a dynamic balance between cell proliferation and death. Several different types of cell death, which are commonly present in germ cells during spermatogenesis and maturation, are of great significance in eliminating abnormal germ cells and provide conditions for accurate transmission of genetic information. However, the increase in germ cells death can lead to a decrease in quality and quantity of sperm, causing male infertility. Multiple factors can lead to an increase in the mortality rate of spermatogenic cells. Intrinsic factors mainly include lifestyle factors and obesity [1, 2]. External factors mainly include physical factors (such as high temperature [3]), radiation [4], chemical factors (such as nitrite [5]), environmental pollutants(such as di-(2-ethylhexyl) phthalate [6]), and biological factors (such as Zearalenone [7]). In recent years, research on cell death mechanisms has gradually deepened, and the known ways of germ cells death mainly include ferroptosis, apoptosis, pyroptosis, and autophagy, etc. These cell death modes each have different morphological and biochemical characteristics. However, there are still many unknown fields regarding the mechanism of cell death. Further exploration of the scope of cell death is beneficial for a more thorough understanding of spermatogenic cell death.
In 2022, Tsvetkov et al. [8] reported a new way of cell death based on mitochondrial respiration and the tricarboxylic acid cycle (TCA cycle), which is named cuproptosis. Cuproptosis is separate from existing other types of cell death, the accumulation of Cu2+ in cells is the fundamental condition for cuproptosis. When Cu2+ amasses in cells that rely on mitochondrial respiration, excess Cu2+ within cells can be transported to the mitochondria by ionophores, and ferridoxin 1 (FDX1) reduces Cu2+ to Cu+. Cu+ combined with lipoylated drolipoamide S-acetyltransferase (DLAT), inducing heteropolymerization of DLAT. The increase in insoluble DLAT leads to cytotoxicity and induces cell death [9]. Previous studies have shown that copper exposure can cause testicular damage and death of reproductive cells [10, 11]. Mitochondrial respiration dependent cells are more prone to copper death [8]. Testicular germ cells rely on mitochondrial respiration, and mitochondria play important roles in various stages of sperm development, maturation, motility, and fertilization. Multiple studies have shown that the impairment of spermatogenic function is related to TCA cycle and mitochondrial function [12,13,14], which are key links to cuproptosis. Our previous research also showed that copper induction can lead to cuproptosis in mouse testicular germ cells [15], which suggests that cuproptosis may be one of the important mechanisms leading to germ cell death.
Relationship between cuproptosis and impaired spermatogenic function
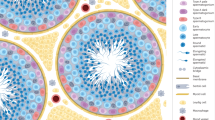
In cuproptosis, FDX1 is a fundamental regulatory factor, and excessive copper ions in cells can induce an increase in the expression level of FDX1, resulting in protein lipoylated. The binding of copper to lipoylated TCA cycle protein leads to lipoylated oligomerization of DLAT, which makes the aggregation of lipoylated proteins and the instability of Fe-S cluster proteins [8], bringing protein toxicity stress response and ultimately cell death. Our previous study [15] has shown that copper homeostasis is disrupted in the testes of copper-overloaded mice, and the expression of FDX1 is significantly upregulated with a significant increase in testicular tissue cell apoptosis rate, suggesting that cuproptosis may be involved in the death of spermatogenic cells. In addition, the decreased ATP levels and mitochondrial dysfunction were observed, further confirming the mitochondrial damage caused by cuproptosis. In further analysis, pathological lesions and disruption of the blood-testis barrier were found in testes of copper overloaded mice, accompanied by a decrease in sperm’s number and vitality. These research evidence suggests a connection between cuproptosis and impaired spermatogenic function (Fig. 1). Next, we described the potential impact of the ways of spermatogenic cell death associated with cuproptosis and cuproptosis key genes on spermatogenic function. Excess Cu2+ within cells can be transported to the mitochondria by ionophores, and FDX1 reduces Cu2+ to Cu+. The binding of copper to lipoylated TCA cycle protein leads to lipoylated oligomerization of DLAT, which promotes the aggregation of lipoylated proteins and the loss of the Fe-S cluster.
Excess Cu2+ within cells can be transported to the mitochondria by ionophores, and ferridoxin 1 (FDX1) reduces Cu2+ to Cu+. The binding of copper to lipoylated TCA cycle protein leads to lipoylated oligomerization of DLAT, which promotes the aggregation of lipoylated proteins and the loss of the Fe-S cluster proteins.
Spermatogenic cell death associated with cuproptosis
As the basic condition for cuproptosis, copper is required for the activity of various metalloenzymes involved in energy and antioxidant metabolism [16]. Copper also contributes to maintain the function of the cellular redox system, thereby protecting spermatogenic cells from oxidative damage [17]. Copper catalyzes the Fenton-like reaction and generates reactive oxygen species (ROS) and hydroxyl radicals via the Fenton and Haber-Weiss reactions. ROS have a biphasic effect, with appropriate levels of ROS having physiological effects on spermatogenesis, while excessive ROS can lead to oxidative damage in the reproductive microenvironment. Copper exposure causes oxidative stress accompanied by increased ROS and MDA in the testis, leading to decreased sperm quality and structural abnormalities in the testis, germ cell apoptosis, and vacuolization of the Sertoli cells [18]. Meanwhile, increased levels of ROS trigger DNA instability and lipid peroxidation in spermatozoa [19]. Moreover, disruptions in copper metabolism during cuproptosis lead to copper accumulation. Excessive copper can trigger oxidative stress, thereby accelerating apoptosis and autophagy in testicular cells [20]. Subsequently, the p53 signaling pathway was activated in apoptosis and autophagy, enhancing FDX1 synthesis and accelerating the occurrence of cuproptosis [21]. Accumulated copper leads to the buildup of ROS and lipid peroxides while simultaneously depleting Glutathione (GSH), promoting both ferroptosis and cuproptosis [22]. In addition, copper exposure increases the expression caspase-1 and pyroptosis-related genes, such as interleukin-1B (IL1B) and NLR family pyrin domain containing 3 (NLRP3), leading to the induction of pyroptosis [23]. These research evidences suggest a potential connection between cuproptosis and other types of cell death (autophagy, apoptosis, pyroptosis, and ferroptosis). Due to the complex mechanisms of cell death, a deeper understanding of the relationship between cuproptosis and other types of spermatogenic cell death is beneficial for a more thorough understanding of the role of cuproptosis in the mechanisms of spermatogenic cell death. Next, we mainly described other types of spermatogenic cell death associated with cuproptosis.
Ferroptosis associated with cuproptosis
Previous studies have shown that the number of cuproptosis regulatory factors is closely related to the number of ferroptosis regulatory factors in different tumor cells, and knocking down ferroptosis key proteins in cells results in significant changes in cuproptosis [24]. Moreover, both ferroptosis and cuproptosis are closely related to mitochondrial metabolism, indicating a significant correlation between ferroptosis and cuproptosis [8, 25]. In the testes, copper sulfate not only induces mitochondrial toxic stress leading to cuproptosis [15], but also drives ferroptosis by affecting the degradation of Glutathione Peroxidase 4 (GPX4) [26], suggesting a close relationship between ferroptosis and cuproptosis in the testes. GSH, as an important intracellular regulator of metabolites, may be a common key link in regulating ferroptosis and cuproptosis [27] (Fig. 2). On the one hand, GSH acts as a substrate for GPX4 to reduce lipid peroxidation and alleviate ferroptosis [28]. On the other hand, GSH controls the concentration of susceptible copper ion pools, reduces the aggregation of lipoylated proteins, and inhibits cuproposis [8]. GSH exhibits inhibitory effects on both ferroptosis and cuproposis, and the inhibition of GSH can induce both ferroptosis [29] and cuproposis [8]. When GSH is inhibited, the elimination of ROS is weakened, and excess Fe2+ produces more ROS through the Fenton reaction, which eventually leads to ferroptosis. At the same time, the damage of mitochondria aggravates the reduction of GSH, which weakens the intracellular Cu+ clearance. The excess Cu+ occurs cuproposis through the aggregation of lipoylated proteins and the loss of Fe-S proteins. In addition, more Fe2+ is released to increase intracellular oxidative stress, forming a ferroptosis-cuproptosis self-cycle [30]. Thus, GSH may be a common key link between cuproptosis and ferroptosis.
GSH plays an important role in ferroptosis and cuproptosis. Excessive Fe2+ produces ROS through the Fenton reaction, which causes lipid peroxidation and ferroptosis. GSH can clear the excess ROS and inhibit ferroptosis. GSH regulates Cu+ concentration and can reduce Cu+ concentration by promoting intracellular Cu+ transport. In addition, GSH binds to Cu+ and can effectively inhibit DLAT aggregation and Fe-S protein loss, thereby inhibiting cuproptosis. Ferroptosis damages mitochondria, further depleting GSH. When GSH is reduced, GXP4 is also reduced and intracellular copper regulation is inhibited, leading to cuproptosis. At the same time, Fe-S cluster proteins are lost, producing more iron ions and aggravating ferroptosis.
Apoptosis associated with cuproptosis
A key feature in copper-induced testicular cuproptosis is copper metabolism disorder, leading to copper overload [15]. Copper overload leads to changes in mitochondrial membrane potential, triggering the release of cytochrome C and the generation of excessive ROS. Ultimately, these changes activate the Caspase-9/3/7 signaling pathway, resulting in testicular apoptosis [31, 32]. Excessive copper ions trigger concurrent cuproptosis and apoptosis, indicating a potential relationship between cuproptosis and apoptosis in the testis (Fig. 3). Copper exposure can induce excessive ROS production to damage DNA, triggering the p53 signaling pathway in apoptosis and cell cycle arrest [33]. P53 may be a key link between apoptosis and cuproptosis. On the one hand, p53 regulates the expression of multiple genes involved in Fe-S cluster biogenesis. One of these genes is FDXR, which encodes a ferritin reductase. FDXR is responsible for transferring electrons from NADPH to FDX1, and then to cytochrome P450. This process is key to achieving Fe-S cluster protein biogenesis [21]. FDX1 is a key protein in cuproptosis, reducing non-toxic Cu2+ to toxic Cu+. Excessive accumulation of Cu+ leads to the aggregation of lipoylated proteins and loss of Fe-S cluster proteins, resulting in protein toxicity and cell death [34]. On the other hand, p53 plays a crucial role in mitochondrial apoptosis. Activation of p53 promotes the expression of pro-apoptotic proteins Bax and Bak, inhibits the expression of anti-apoptotic protein Bcl-2. In addition, p53 activates apoptosis-related factors and induces cell apoptosis [35]. Therefore, there is a significant correlation between cuproptosis and apoptosis, and p53 may play an important bridging role in their association.
p53 may be a key link between cuproptosis and apoptosis. p53 regulates the expression of FDXR, which encodes a ferredoxin reductase responsible for transferring electrons from NADPH to FDX1, thus realizing Fe-S cluster biogenesis. FDX1 converts Cu2+ to Cu+ and promotes DlTA protein aggregation and the loss of Fe-S cluster proteins. Copper exposure induces oxidative stress, which is accompanied by elevated levels of ROS, thus inducing cuproptosis. Excess copper leads to the production of excess ROS, which results in p53 activation and subsequent activation of pro-apoptotic BH3 members of the Bcl-2 family (Bax, Bak). Bax and Bak neutralize the antiapoptotic protein Bcl-2, disrupting mitochondrial outer membrane permeabilization (MOMP) so that mitochondrial proteins spread into the cytosol. Cytochrome c (Cytc) binds and activates Apaf-1 and pro-caspase-9 to form apoptosomes. In the apoptosome, caspase-9 is activated by autoproteolytic cleavage, initiating a caspase cascade reaction that leads to programmed apoptosis.
Autophagy associated with cuproptosis
It has been shown that CuSO4 induces autophagy in mouse GC-1 cells and testis through the oxidative stress-dependent AMPK/mTOR pathway by downregulating p-mTOR/mTOR and subsequently upregulating p-AMPKα/AMPKα and p-ULK1/ULK1 [26]. In mouse testicular tissues, CuSO4-mediated induction of both autophagy and cuproptosis [15] reveals a potential connection between autophagy and cuproptosis (Fig. 4). Copper induces oxidative stress by catalyzing ROS generation via Fenton and Haber-Weiss reactions, thereby activating p53 [36]. p53 serves as a redox-sensitive hub that coordinates oxidative stress in apoptosis and autophagy. Thus, it may play an important role in the link between autophagy and cuproptosis. On the one hand, p53 affects the biogenesis of Fe-S cluster proteins by regulating the expression of the FDXR gene, leading to a deficiency of Fe-S clusters and ultimately inducing cuproptosis [21]. On the other hand, p53 promotes autophagy by transactivating its target genes, mainly by activating the p53/AMPK/mTOR signaling pathway. The AMPK/mTOR signaling pathway is an important regulatory pathway for cellular autophagy, and oxidative stress can activate the AMPK/mTOR signaling pathway [37]. Additionally, ATPase Cu-Transporting 7 Beta (ATP7B) is a critical copper transporter whose downregulation has been demonstrated to disrupt copper metabolism, resulting in intracellular copper accumulation and the triggering of cuproptosis [38]. ATP7B deficiency promotes transcription factor EB nuclear translocation through reduced mTOR activity, thereby significantly upregulating autophagy-related gene expression [39]. In summary, cuproptosis and autophagy are closely related, and P53 and ATP7B may be critical in the association between cuproptosis and autophagy.
p53 and ATP7B are the critical link between cuproptosis and autophagy. P53 not only promotes cuproptosis by regulating FDXR gene expression but also promotes autophagy by activating the AMPK/mTOR signaling pathway. Suppression of ATP7B disrupts Cu+ efflux, resulting in progressive copper accumulation that induces cuproptosis and autophagy.
Pyroptosis associated with cuproptosis
Pyroptosis can affect testicular spermatogenesis. Excessive ROS can induce damage to the testes and spermatogenic cells, promoting the expression of caspase-1, interleukin-1β (IL-1β), and NLRP3, thereby triggering pyroptosis [6, 40]. Excessive copper leads to the accumulation of ROS in cells [41], and ROS is considered a key factor in activating pyroptosis [40]. Therefore, copper exposure may induce pyroptosis by generating excessive ROS. Copper accumulation is a hallmark feature of cuproptosis, there may be a potential connection between cuproptosis and pyroptosis (Fig. 5). Activation of nuclear factor erythroid 2-related factor 2 (Nrf2) is a member of the transcription factor family, playing a key role in regulating cellular redox homeostasis by inducing various detoxifying and antioxidant enzymes. Elevated ROS levels trigger Nrf2 signaling pathway activation for ROS elimination and tissue homeostatic maintenance [42]. Nrf2 inhibits the production of NLRP3 inflammasomes and prevents pyroptosis [43]. Meanwhile, Nrf2 binds to the ATP7B promoter, enhancing the expression of ATP7B protein and transferring excess Cu+ from the cell to the extracellular space, thereby reducing cuproptosis [44]. To summarise, there is a potential correlation between cuproptosis and pyroptosis. The Nrf2 may be a central mediator in the association between cuproptosis and pyroptosis.
Nrf2 may be an important hub for cuproptosis and pyroptosis. Copper catalyzes the Fenton-like reaction and generates ROS, leading to cellular pyroptosis. Nucleotide oligomerization structural domain-like receptor protein 3 (NLRP3) binds to an apoptosis-associated speck-like protein (ASC), which recruits the pro-caspase-1 to assemble into the NLRP3 inflammasome. Activated caspase-1 cleaves the GSDMD protein to form GSDMD with an N-terminal sequence, which translocates to bind to the cell membrane to form a membrane pore. This process leads to cell death and the secretion of pro-inflammatory mediators, including interleukin 18 (IL-18) and interleukin-1β (IL-1β). However, excessive ROS activate Nrf2, inhibit the production of inflammasomes, and inhibit pyroptosis. In addition, Nrf2 stimulates the synthesis of ATP7B to alleviate cuproptosis by transporting excess intracellular copper.
The association between cuproptosis key genes and impaired spermatogenic function
Ten key genes were dentified for cuproptosis, including positive regulation factors FDX1, DLAT, lipoyltransferase 1 (LIPT1), pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1), lipoic acid synthetase (LIAS), pyruvate dehydrogenase E1 subunit beta, glutaminase (GLS), dihydrolipoamide dehydrogenase (DLD), cyclin-dependent kinase inhibitor 2A (CDKN2A), and negative regulatory factor metal-regulatory transcription factor-1 (MTF1). Several cuproptosis genes may be involved in spermatogenic function, and the following are the effects of their related genes on spermatogenesis (Table 1).
FDX1
Mammalian mitochondria contain several ferredoxin proteins, among which FDX1 plays a major role in mitochondrial respiration and energy metabolism, and is a key gene in the regulation of cuproptosis. In cells with knockdown of FDX1, copper, and heme a/a3 levels are decreased, affecting cytochrome c oxidase and NADH production, which in turn reduces mitochondrial respiration [45]. In addition, FDX1-deficient cells produce less ATP, along with abnormalities in fatty acid oxidation, amino acid metabolism, and glucose metabolism, which may lead to abnormal spermatogenesis [46]. FDX1 is present in Leydig cells, Sertoli cells, and spermatogenic cells during testicular development, and there is a significant increase in FDX1 positivity after the occurrence of cuproptosis, along with Sertoli cells, Leydig cells decreased and spermatogenic cell vacuoles degenerated and necrotic [15]. It has been shown that deficiency of FDX1b, the paralogous homolog encoded by FDX1, downregulates the expression of insulin-like peptide 3 (INSL3), insulin-like growth factor 3 (IGF3), and inhibin subunit a (INHA), whereas INSL3 and IGF3 are involved in the differentiation and proliferation of spermatogenesis A spermatogonia, and inha is used for the regulation of FSH, which has a significant role in Sertoli cell proliferation and function, and when their expression is reduced, spermatogonia proliferation and differentiation are abnormal and the function of the Sertoli cells is impaired, which in turn affects spermatogenesis [47, 48]. In conclusion, FDX1 may affect spermatogenesis by influencing metabolism or spermatogonia proliferation and differentiation.
GLS
GLS is an enzyme mainly located in mitochondria that catalyzes the hydrolysis of glutamine to glutamate. GLS is classified into two subtypes GLS1 and GLS2 [49]. GLS1 is associated with cellular senescence [50]. In the recent study, it was reported [51] that GLS2 gene activity is essential for nematode sperm function and maintains sperm function by controlling cellular redox homeostasis. The tumor suppressor gene P53, expressed in mammalian primary spermatocytes, plays an important role in spermatogenesis during meiotic prophase. This role of P53 may be related to its up-regulation of the function of many antioxidant genes, including GLS2, which acts as a target gene for the tumor suppressor P53 and mediates the function of the P53 protein in regulating cellular energy metabolism and antioxidant defense mechanisms [52]. GLS2 regulates cellular energy metabolism by increasing glutamine hydrolysis, which increases mitochondrial respiration rate and ATP production, it also further increases reduced glutathione, an important antioxidant molecule, and ROS scavenger, by increasing glutamate, which reduces the level of ROS and enhances cellular antioxidant defenses, allowing cells to avoid oxidative stress-induced apoptosis [53]. Thus GLS maintains spermatogenesis function by reducing oxidative stress through antioxidants.
PDHA1
PDHA1 is one of the proteins involved in aerobic glycolysis. Cuproptosis-related molecular Cluster 2 in spermatogenic dysfunction had high PDHA1 expression, and a large number of CD4+ T cell infiltrates [54], whereas in a model of autoimmune testicular inflammation characterized by spermatogenic dysfunction established, the spermatogonial tubules are predominantly infiltrated by CD4+ T cell, further suggesting a correlation between cuproptosis and immune infiltration of the testis [55]. PDHA1 overexpression promotes mitochondrial respiration, leading to excessive ROS production and apoptosis [56]. PDHA1 overexpression activates the mitochondrial pathway of apoptosis, and the ratio of pro-apoptotic protein Bax to anti apoptotic protein Bcl-2 increases, leading to the loss of mitochondrial membrane potential and the release of cytochrome C from mitochondria into the cytoplasm, promotes apoptotic body formation, and activates the caspase, and then execute apoptosis [57]. Therefore, PDHA1 causes spermatogenic disorders by promoting CD4+ T cell infiltration and mitochondrial apoptosis pathway.
LIAS
LIAS is a key enzyme in mitochondria, and its catalytic synthesis of lipoic acid plays an important role in mitochondrial energy metabolism and antioxidant defense [58]. Lipoic acid affects energy metabolism by maintaining the activity of the PDH complex in the TCA. Studies have shown that lipoic acid has a positive effect on oxidative stress caused by metals in the body, which can chelate these metal ions, eliminate excess ROS, and facilitate the regeneration of glutathione, vitamin C, and vitamin E [59]. The downregulation of LIAS in spermatogenesis disorders patients leads to a decrease in lipoic acid, which affects the function of the PDH complex, thereby inhibiting TCA circulation and energy supply, which may lead to spermatogenesis arrest (such as meiosis disorder), while lipoic acid deficiency leads to ROS accumulation, damages ROS-sensitive sperm cells, promotes resting memory CD4 T cell infiltration, triggers local inflammatory response, destroys the integrity of the blood-testicular barrier, and aggravates testicular microenvironment damage [54].
DLD
DLD is a mitochondrial enzyme belonging to the pyruvate dehydrogenase complex, which is one of the key genes for cupproposis [60]. The important role of DLD in cell death has been suggested, DLD can produce large amounts of ROS in melanoma cells, leading to apoptosis [61]. Similarly, during spermatogenesis, DLD produces ROS in redox reactions, and a small amount of ROS is involved in sperm acrosome reaction, capacitation, hyperactivation, and sperm-oocyte interaction, but excessive ROS production can cause oxidative stress, leading to sperm DNA damage and promoting spermatogenic cell apoptosis [62].
MTF1
MTF1 encodes a metal transcription factor that activates metallothionein to protect cells from heavy metals such as cadmium, zinc, and copper. MTF1 was positively correlated with TIMP2 (metalloproteinase tissue inhibitor), which has the effect of maintaining sperm membrane integrity and preventing sperm DNA fragmentation [63]. In Cd exposure models, increased MTF1 expression inhibited mitochondrial apoptosis pathways (e.g., decreased Bax/Caspase-3, increased Bcl-2) and maintained sperm viability and DNA integrity [64]. Previous studies have inferred that MTF1 overexpression can reduce double-stranded DNA fragmentation and S phase arrest in spermatogenic cells, while inhibiting MTF1 expression leads to inhibition of spermatogenic cells and support cell proliferation, increased ROS production, and then promotes spermatogenic cell death [65, 66].
CDKN2A
CDKN2A is a gene encoding the P16 protein, which binds to CDK4 and CDK6 and inhibits the formation of kinase-active complexes between CDK4 and cyclin D, thereby regulating the cell cycle and arresting cells in the G phase [67, 68]. CDKN2A mainly acts on the G1 phase and blocks CDK activity, reducing cellular senescence and cell cycle arrest by inhibiting CDKN2A [69]. During spermatogenesis, if CDKN2A is overexpressed, it may arrest the development of spermatogenic cells in the G1 phase or promote cell senescence and finally lead to abnormal spermatogenesis.
SLC31A1/CTR1
Aberrant expression of SLC31A1/CTR1, a transmembrane protein that plays an important role in copper homeostasis and cellular copper uptake, leads to copper accumulation, which in turn triggers cuproposis [70]. Mice with high expression of CTR1 in the testes and specific knockout of CTR1 began to lose germ cells at the 28th day of life and had hypoplasia in the testis, suggesting that CTR1 is essential for spermatogenesis [71]. It has been shown that oxidative stress promotes the transcription of CTR1 by upregulating specific protein 1 (SP1), thereby increasing cellular copper uptake and FDX1 expression and causing cuproposis [72]. thereby, oxidative stress may also lead to abnormal spermatogenic cell death and ultimately to abnormal spermatogenesis by regulating CRT1.
DLAT
DLAT is a key regulatory gene for cuproptosis, and as a core component of pyruvate dehydrogenase complex (PDC), it is involved in mitochondrial respiration and TCA cycle metabolism, affecting ATP production [73]. Spermatogenesis is dependent on mitochondria [74], abnormal expression of DLAT not only causes disruption of the TCA cycle [75] and insufficient energy supply to spermatogenic cells, affecting spermatogenesis, but also aggravates oxidative stress, damaging sperm DNA integrity and inducing sperm apoptosis. DLAT may play an important function in spermatogenic disorders through its role in cell death. Knocked down DLAT can downregulate LC3-II/Beclin-1 and thus inhibit autophagy [76], which can removal abnormal cell structures and misfolded proteins in spermatogenesis, if DLAT is expressed abnormally, it may lead to autophagy defects and affect cellular homeostasis in spermatogenesis [77]. The high expression of DLAT is closely related to cell proliferation, migration, and regulation of immune microenvironment[78]. DLAT positively correlates with Treg and Th2 cell infiltration [79], and its role in immunomodulation may affect the testicular immune microenvironment, which is dependent on an immunosuppressive environment for immune privilege in the testis, and DLAT abnormalities may disrupt this balance, inducing autoimmune testicular inflammation and further impairing spermatogenesis [80].
Others
In other genes for cuproptosis, there may also be an association with spermatogenic disorders. Dihydrolipoamide branched-chain transacylase E2 (DBT) and its nanocomplexes can change mitochondrial membrane stability, reduce the release of cytochrome c, and inhibit apoptosis by adjusting the Bcl-2/Bax ratio [81]. In spermatogenic cells, DBT may regulate apoptosis through this mechanism. ATP7A and ATP7B are highly homologous P-type copper transporters, which regulate copper homeostasis through precise cell localization and transport mechanisms [82], copper ions are required for spermatogenesis, and may cause spermatogenesis disorders when ATP7A and ATP7B function abnormally. Glycine cleavage system protein H (GCSH) can inhibit the activation of the JAK-STAT signaling pathway [83], which is important in the self-renewal function of spermatogonial stem cells, leading to abnormal spermatogenesis.
Conclusions and prospects
Cuproptosis is a novel mechanism of cell death, and the exploration of cuproptosis at the level of spermatogenic cells is helpful for further understanding the mechanisms of spermatogenic cell death. According to the current research evidence, cuproptosis is involved in the death process of spermatogenic cells, but its specific regulatory mechanism still needs further research. Meanwhile, cuproptosis may be interrelated with a variety of other types of spermatogenic death, and the study of the crosstalk between cuproptosis and other types of cell death and their regulatory mechanisms at the level of spermatogenic cells is conducive to further improving the molecular mechanism of spermatogenic cell death, enriching the pathological basis of male infertility, and providing a basis and ideas for the next step of developing new drugs or preparations to protect male fertility.
References
Rotimi DE, Singh SK. Implications of lifestyle factors on male reproductive health. JBRA Assist Reprod. 2024;28:320–30.
Li M, Zhao Q, Wang S, Song Y, Zhai L, Zhao J. Differential impairment mechanism of sperm production via induction of miR-34c-activated apoptosis and spermatogenesis pathway in diet-induced obesity and resistant mice and GC-1 Spg cells. Int J Mol Sci. 2024;25:7451.
Moeinian N, Fathabadi FF, Norouzian M, Abbaszadeh HA, Nazarian H, Afshar A, et al. The effects of vitamin C and vitamin B12 on improving spermatogenesis in mice subjected to long-term scrotal heat stress. Clin Exp Reprod Med. 2024;51:334–43.
Zhang X, Chen X, Wang A, Wang L, He C, Shi Z, et al. Yiqi Jiedu decoction attenuates radiation injury of spermatogenic cells via suppressing IκBα/NF-κB pathway-induced excessive autophagy and apoptosis. J Ethnopharmacol. 2024;318:116903.
Liu H, Du X, Zhang Z, Ge K, Chen X, Losiewicz MD, et al. Co-exposure of microcystin and nitrite enhanced spermatogenic disorders: the role of mtROS-mediated pyroptosis and apoptosis. Environ Int. 2024;188:108771.
Hong Y, Zhou X, Li Q, Chen J, Wei Y, Long C, et al. X-box binding protein 1 caused an imbalance in pyroptosis and mitophagy in immature rats with di-(2-ethylhexyl) phthalate-induced testis toxicity. Genes Dis. 2024;11:935–51.
Li Y, Zhu Z, Cui H, Ding K, Zhao Y, Ma X. et al. Effect of zearalenone-induced ferroptosis on mice spermatogenesis. Animals (Basel). 2022;12:3026.
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle protein. Science. 2022;375:1254–61.
Wang Y, Zhang L, Zhou F. Cuproptosis: a new form of programmed cell death. Cell Mol Immunol. 2022;19:867–8.
Ouyang Y, Lou Y, Zhu Y, Wang Y, Zhu S, Jing L, et al. Molecular regulatory mechanism of nano-Se against copper-induced spermatogenesis disorder. Biol Trace Elem Res. 2025;203:249–60.
Zhao D, Wu L, Fang X, Wang L, Liu Q, Jiang P, et al. Copper exposure induces inflammation and PANoptosis through the TLR4/NF-kappaB signaling pathway, leading to testicular damage and impaired spermatogenesis in Wilson disease. Chem Biol Interact. 2024;396:111060.
Kuang W, Zhang J, Lan Z, Krishna Deepak RNV, Liu C, et al. SLC22A14 is a mitochondrial riboflavin transporter required for sperm oxidative phosphorylation and male fertility. Cell Rep. 2021;35:109025.
Kang W, Harada Y, Yamatoya K, Kawano N, Kanai S, Miyamoto Y, et al. Extra-mitochondrial citrate synthase initiates calcium oscillation and suppresses age-dependent sperm dysfunction. Lab Invest. 2020;100:583–95.
Cui Y, Han JY, Ren J, Chen HM, Xu BL, Song NN, et al. Untargeted LC-MS-based metabonomics revealed that aristolochic acid I induces testicular toxicity by inhibiting amino acids metabolism, glucose metabolism, β-oxidation of fatty acids and the TCA cycle in male mice. Toxicol Appl Pharm. 2019;373:26–38.
Zhang JY, Yu XJ, Li JJ, Xiao Y, Li GS, Yang F, et al. Cuproptosis mediates copper-induced testicular spermatogenic cell death. Asian J Androl. 2024;26:295–301.
Afridi HI, Kazi TG, Talpur FN, Baig JA, Chanihoon GQ. Essential trace and toxic elemental concentrations in biological samples of male adult referent and Eunuch subjects. Clin Chim Acta. 2022;529:96–103.
Mirnamniha M, Faroughi F, Tahmasbpour E, Ebrahimi P, Harchegani AB. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev Environ Health. 2019;34:339–48.
Zhao D, Zhang X, Li X, Ru S, Wang Y, Yin J, et al. Oxidative damage induced by copper in testis of the red swamp crayfish Procambarus clarkii and its underlying mechanisms. Aquat Toxicol. 2019;207:120–31.
Kolasa A, Marchlewicz M, Adler G, Ciechanowicz A, Głabowski W, Wiszniewska B. Expression of E-SOD, GPX5 mRNAs and immunoexpression of Cu/ZnSOD in epididymal epithelial cells of finasteridetreated rats. Andrologia. 2008;40:303–11.
Chen H, Wang Y, Luo J, Kang M, Hou J, Tang R, et al. Autophagy and apoptosis mediated nano-copper-induced testicular damage. Ecotoxicol Environ Saf. 2022;229:113039.
Xiong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30:876–84.
Zhu Y, Niu X, Ding C, Lin Y, Fang W, Yan L, et al. Carrier-free self-assembly nano-sonosensitizers for sonodynamic-amplified cuproptosis-ferroptosis in glioblastoma therapy. Adv Sci. 2024;11:e2402516.
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J, Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;19:2175–95.
Shen Y, Li D, Liang Q, Yang M, Pan Y, Li H. Cross-talk between cuproptosis and ferroptosis regulators defines the tumor microenvironment for the prediction of prognosis and therapies in lung adenocarcinoma. Front Immunol. 2023;13:1029092.
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–.e3.
Guo H, Ouyang Y, Yin H, Cui H, Deng H, Liu H, et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol. 2022;49:102227.
Liu N, Chen M. Crosstalk between ferroptosis and cuproptosis: from mechanism to potential clinical application. Biomed Pharmacother. 2024;171:116115.
Zhang Y, Swanda RV, Nie L, Liu X, Wang C, Lee H, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12:1589.
Luo Y, Yan P, Li X, Hou J, Wang Y, Zhou S. pH-Sensitive polymeric vesicles for GOx/BSO delivery and synergetic starvation-ferroptosis therapy of tumor. Biomacromolecules. 2021;22:4383–94.
Huang L, Zhu J, Wu G, Xiong W, Feng J, Yan C, et al. A strategy of “adding fuel to the flames” enables a self-accelerating cycle of ferroptosis-cuproptosis for potent antitumor therapy. Biomaterials. 2024;311:122701.
Guo H, Ouyang Y, Wang J, Cui H, Deng H, Zhong X, et al. Cu-induced spermatogenesis disease is related to oxidative stress-mediated germ cell apoptosis and DNA damage. J Hazard Mater. 2021;416:125903.
Li Y, Chen H, Liao J, Chen K, Javed MT, Qiao N, et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ Sci Pollut Res Int. 2021;28:55140–53.
Wang H, Luo Y, Ran R, Li X, Ling H, Wen F, et al. IDO1 modulates the sensitivity of epithelial ovarian cancer cells to cisplatin through ROS/p53-dependent apoptosis. Int J Mol Sci. 2022;23:12002.
Hou HY, Chu X, Duan MD, Zhang YJ, Chen HL, Sun Y, et al. Achieving apoptosis/cuproptosis co-activated synergistic anti-tumor therapy by charges transport engineering. Nano Today. 2025;62:102689.
Demirci Z, Islek Z, Siginc HI, Sahin F, Ucisik MH, Bolat ZB. Curcumin-loaded emulsome nanoparticles induces apoptosis through p53 signaling pathway in pancreatic cancer cell line PANC-1. Toxicol Vitr. 2025;102:105958.
Li R, Ma Y, He A, Pu Y, Wan X, Sun H, et al. Fasting enhances the efficacy of Sorafenib in breast cancer via mitophagy mediated ROS-driven p53 pathway. Free Radic Biol Med. 2025;229:350–63.
Zhu J, Ao H, Liu M, Cao K, Ma J. UBE2T promotes autophagy via the p53/AMPK/mTOR signaling pathway in lung adenocarcinoma. J Transl Med. 2021;19:374.
Lutsenko S, Roy S, Tsvetkov P. Mammalian copper homeostasis: physiological roles and molecular mechanisms. Physiol Rev. 2025;105:441–91.
Pan C, Ji Z, Wang Q, Zhang Z, Wang Z, Li C, et al. Cuproptosis: mechanisms, biological significance, and advances in disease treatment-A systematic review. CNS Neurosci Ther. 2024;30:e70039.
Hong Y, Zhou Y, Shen L, Wei Y, Long C, Fu Y, et al. Exposure to DEHP induces testis toxicity and injury through the ROS/mTOR/NLRP3 signaling pathway in immature rats. Ecotoxicol Environ Saf. 2021;227:112889.
Meena R, Sahoo SS, Sunil A, Manna D. Cuproptosis: a copper-mediated programmed cell death. Chem Asian J. 2025;20:e202400934.
Huang J, Yue Z, Yu H, Yang Z, Wang Y, Guo B. TAZ ameliorates the microglia-mediated inflammatory response via the Nrf2-ROS-NF-κB pathway. Mol Ther Nucleic Acids. 2022;28:435–49.
Zhang C, Zhao M, Wang B, Su Z, Guo B, Qin L, et al. The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson’s disease. Redox Biol. 2021;47:102134.
Du M, Fu J, Zhang J, Zhu Z, Huang X, Tan W, et al. CircSpna2 attenuates cuproptosis by mediating ubiquitin ligase Keap1 to regulate the Nrf2-Atp7b signalling axis in depression after traumatic brain injury in a mouse model. Clin Transl Med. 2024;14:e70100.
Zulkifli M, Okonkwo AU, Gohil VM. FDX1 is required for the biogenesis of mitochondrial cytochrome c oxidase in mammalian cells. J Mol Biol. 2023;435:168317.
Zhang Z, Ma Y, Guo X, Du Y, Zhu Q, Wang X, et al. FDX1 can impact the prognosis and mediate the metabolism of lung adenocarcinoma. Front Pharm. 2021;12:749134.
Oakes JA, Li N, Wistow BRC, Griffin A, Barnard L, Storbeck KH, et al. Ferredoxin 1b deficiency leads to testis disorganization, impaired spermatogenesis, and feminization in zebrafish. Endocrinology. 2019;160:2401–16.
Assis LH, Crespo D, Morais RD, França LR, Bogerd J, Schulz RW. INSL3 stimulates spermatogonial differentiation in testis of adult zebrafish (Danio rerio). Cell Tissue Res. 2015;363:579–88.
Masisi BK, El Ansari R, Alfarsi L, Rakha EA, Green AR, Craze ML. The role of glutaminase in cancer. Histopathology. 2020;76:498–508.
Johmura Y, Yamanaka T, Omori S, Wang TW, Sugiura Y, Matsumoto M, et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science. 2021;371:265–70.
Liang Q, Yang H, Zhang Z, Zheng J, Qin Z. Loss of mammalian glutaminase orthologs impairs sperm function in Caenorhabditis elegans. iScience. 2023;26:106206.
Matés JM, Segura JA, Martín-Rufián M, Campos-Sandoval JA, Alonso FJ, Márquez J. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr Mol Med. 2013;13:514–34.
Chen K, Wu L, Liu Q, Tan F, Wang L, Zhao D, et al. Glutathione improves testicular spermatogenesis through inhibiting oxidative stress, mitochondrial damage, and apoptosis induced by copper deposition in mice with Wilson disease. Biomed Pharmacother. 2023;158:114107.
Zhao M, Yu W, Liu S, Deng Y, Zhao ZW, Guo J, et al. Identification and immuno-infiltration analysis of cuproptosis regulators in human spermatogenic dysfunction. Front Genet. 2023;14:1115669.
Nagahori K, Hirai S, Hatayama N, Kuramasu M, Omotehara T, Kawata S, et al. Heat shock protein A4L is a potent autoantigen for testicular autoimmunity in mice. J Reprod Immunol. 2021;145:103318.
Wang CH, Lu WL, Chiang SL, Tsai TH, Liu SC, Hsieh CH, et al. T cells mediate kidney tubular injury via impaired PDHA1 and Autophagy in type 1 diabetes. J Clin Endocrinol Metab. 2022;107:2556–70.
Sun J, Li J, Guo Z, Sun L, Juan C, Zhou Y, et al. Overexpression of pyruvate dehydrogenase E1α subunit inhibits Warburg effect and induces cell apoptosis through mitochondria-mediated pathway in hepatocellular carcinoma. Oncol Res. 2019;27:407–14.
Cai Y, He Q, Liu W, Liang Q, Peng B, Li J, et al. Comprehensive analysis of the potential cuproptosis-related biomarker LIAS that regulates prognosis and immunotherapy of pan-cancers. Front Oncol. 2022;12:952129.
Dieter F, Esselun C, Eckert GP. Redox active α-Lipoic acid differentially improves mitochondrial dysfunction in a cellular model of alzheimer and its control cells. Int J Mol Sci. 2022;23:9186.
Lin J, Wang G, Cheng S, Hu Y, Li H, Feng W, et al. Pan-cancer analysis of the cuproptosis-related gene DLD. Mediators Inflamm. 2023;2023:5533444.
Dayan A, Fleminger G, Ashur-Fabian O. Targeting the Achilles’ heel of cancer cells via integrin-mediated delivery of ROS-generating dihydrolipoamide dehydrogenase. Oncogene. 2019;38:5050–61.
Rashki Ghaleno L, Alizadeh A, Drevet JR, Shahverdi A, Valojerdi MR. Oxidation of Sperm DNA and Male Infertility. Antioxidants. 2021;10:97.
Mayasula VK, Arunachalam A, Sellappan S, Guvvala PR, Ghosh J. Organic zinc and copper supplementation-associated changes in gene expression and protein profiles in buck spermatozoa. Biol Trace Elem Res. 2022;200:1626–39.
Liu J, Wang E, Cheng Z, Gao Y, Chen C, Jia R, et al. Zinc alleviates cadmium-induced reproductive toxicity via regulating ion homeostasis, metallothionein expression, and inhibiting mitochondria-mediated apoptosis in the freshwater crab Sinopotamon henanense. Ecotoxicol Environ Saf. 2023;262:115188.
Yang Y, Cai Y, Guo J, Dai K, Liu L, Chen Z, et al. Knockdown of KDM5B leads to DNA damage and cell cycle arrest in granulosa cells via MTF1. Curr Issues Mol Biol. 2023;45:3219–37.
Song L, Zeng R, Yang K, Liu W, Xu Z, Kang F. The biological significance of cuproptosis-key gene MTF1 in pan-cancer and its inhibitory effects on ROS-mediated cell death of liver hepatocellular carcinoma. Discov Oncol. 2023;14:113.
Chen Z, Guo Y, Zhao D, Zou Q, Yu F, Zhang L, et al. Comprehensive analysis revealed that CDKN2A is a biomarker for immune infiltrates in multiple cancers. Front Cell Dev Biol. 2021;9:808208.
Xing X, Cai W, Shi H, Wang Y, Li M, Jiao J, et al. The prognostic value of CDKN2A hypermethylation in colorectal cancer: a meta-analysis. Br J Cancer. 2013;108:2542–8.
Choi SA, Moon YJ, Koh EJ, Phi JH, Lee JY, Kim KH, et al. Cyclin-dependent kinase inhibitor 2A is a key regulator of cell cycle arrest and senescence in endothelial colony-forming cells in moyamoya disease. J Korean Neurosurg Soc. 2023;66:642–51.
Fu DG, He JZ, Mu QC, Huo YF, Zhang NM, Zhang L, et al. Inhibition of CTR1 expression improves hypoxia/reoxygenation-induced myoblast injury by blocking cuproptosis. Biochem Biophys Res Commun. 2024;735:150804.
Ghaffari R, Di Bona KR, Riley CL, Richburg JH. Copper transporter 1 (CTR1) expression by mouse testicular germ cells, but not Sertoli cells, is essential for functional spermatogenesis. PLoS ONE. 2019;14:e0215522.
Chen X, Li K, Xiao Y, Wu W, Lin H, Qing X, et al. SP1/CTR1-mediated oxidative stress-induced cuproptosis in intervertebral disc degeneration. Biofactors. 2024;50:1009–23.
Gao P, Li H, Qiao Y, Nie J, Cheng S, Tang G, et al. A cuproptosis-related gene DLAT as a novel prognostic marker and its relevance to immune infiltration in low-grade gliomas. Heliyon. 2024;10:e32270.
Zhang Z, Miao J, Wang Y. Mitochondrial regulation in spermatogenesis. Reproduction. 2022;163:R55–R69.
Li Z, Zhou H, Zhai X, Gao L, Yang M, An B, et al. MELK promotes HCC carcinogenesis through modulating cuproptosis-related gene DLAT-mediated mitochondrial function. Cell Death Dis. 2023;14:733.
Yang Q, Zeng S, Liu W. Roles of cuproptosis-related gene DLAT in various cancers: a bioinformatic analysis and preliminary verification on pro-survival autophagy. PeerJ. 2023;11:e15019.
Rotimi DE, Singh SK. Interaction between apoptosis and autophagy in testicular function. Andrologia. 2022;54:e14602.
Liu D, Wang X, Qian F, Ye D, Deng X, Fang L. DLAT promotes triple-negative breast cancerprogression via YAP1 activation. Cancer Biol Ther. 2024;25:2421578.
Peng Y, Shi R, Yang S, Zhu J. Cuproptosis-related gene DLAT is a biomarker of the prognosis and immune microenvironment of gastric cancer and affects the invasion and migration of cells. Cancer Med. 2024;13:e70012.
Li SY, Kumar S, Gu X, DeFalco T. Testicular immunity. Mol Asp Med. 2024;100:101323.
Shaban NZ, Yehia SA, Awad D, Shaban SY, Saleh SR. A titanium (IV)-dithiophenolate complex and its chitosan nanocomposite: their roles towards rat liver injuries in vivo and against human liver cancer cell lines. Int J Mol Sci. 2021;22:11219.
Ruturaj, Mishra M, Saha S, Maji S, Rodriguez-Boulan E, Schreiner R. Regulation of the apico-basolateral trafficking polarity of the homologous copper-ATPases ATP7A and ATP7B. J Cell Sci. 2024;137:jcs261258.
He R, Li Y, Jiao P, Huang Y, Dong S, MO L, et al. Cuproptosis-related genes score and its hub gene GCSH: a novel predictor for cholangiocarcinomas prognosis based on RNA seq and experimental analyses. J Cancer. 2024;15:1551–67.
Funding
This work was supported by the Sichuan Science and Technology Program [grant number 2024NSFSC1680], Sichuan Provincial Administration of Traditional Chinese Medicine [grant number 2023MS595], and National Natural Science Foundation of China [grant number 82274325].
Author information
Authors and Affiliations
Contributions
JL and XY designed and conceived the review. JL, NL, and HW wrote the initial draft and prepared the figures and table. BL, XT, YG, FY, GL, and LD collected relevant information. XY and RY revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Li, N., Wang, H. et al. Cuproptosis and cuproptosis-related cell death and genes: mechanistic links to spermatogenic cell death. Cell Death Discov. 11, 274 (2025). https://doi.org/10.1038/s41420-025-02553-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-025-02553-2