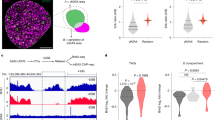
Abstract
Cytoplasmic DNA emerges as a consequence of genomic instability. However, its potential role in disease diagnosis has yet to be fully explored. Here we analyzed DNA remnants in mature red blood cells (rbcDNA) from both healthy individuals and cancer patients. Our study unveiled distinct genomic profiles in rbcDNA from cancer patients with early-stage solid tumors compared to those of healthy donors. Significant changes in read counts at specific genomic regions within rbcDNA were identified in patients, which were termed tumor-associated rbcDNA features. These features demonstrated potential for highly accurate early-stage cancer detection, proposing a novel approach for cancer detection. Moreover, tumor-associated rbcDNA features were observed in tumor mouse models, with some features being conserved between mice and humans. Chronic, but not transient, up-regulation of interleukin-18 is essential for the development of these features by promoting DNA damage in bone marrow hematopoietic cells through the up-regulation of NR4A1. These results underscore the remote regulation of chromosomal stability in hematopoietic cells by solid tumors and propose tumor-associated rbcDNA features as a promising strategy for early cancer detection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
This study did not generate new unique materials. The data described in this manuscript have been deposited in the Genome Sequence Archive (GSA) in the national genomics data center. The assigned accession numbers of the submission are HRA006186 for the human datasets and CRA013839 for the mouse datasets. The supplemental materials can be found in Supplementary information, Tables S1–S18. All data are available in the main text or the supplementary materials.
All analyses were performed using previously published or developed tools, as indicated in Materials and Methods. The pipeline and software versions used for analysis and visualization are available on GitHub at https://github.com/GaoXlab/MNDNA_scripts_forSun.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Crosby, D. et al. Early detection of cancer. Science 375, eaay9040 (2022).
Ma, L. et al. Liquid biopsy in cancer: current status, challenges and future prospects. Signal Transduct. Target. Ther. 9, 336 (2024).
Mattox, A. K. et al. The origin of highly elevated cell-free DNA in healthy individuals and patients with pancreatic, colorectal, lung, or ovarian cancer. Cancer Discov. 13, 2166–2179 (2023).
Martin-Alonso, C. et al. Priming agents transiently reduce the clearance of cell-free DNA to improve liquid biopsies. Science 383, eadf2341 (2024).
Miller, K. N. et al. Cytoplasmic DNA: sources, sensing, and role in aging and disease. Cell 184, 5506–5526 (2021).
He, X. et al. Cytoplasmic DNAs: Sources, sensing, and roles in the development of lung inflammatory diseases and cancer. Front. Immunol. 14, 1117760 (2023).
Jakupciak, J. P. et al. Performance of mitochondrial DNA mutations detecting early stage cancer. BMC Cancer 8, 285 (2008).
Haupts, A. et al. Comparative analysis of nuclear and mitochondrial DNA from tissue and liquid biopsies of colorectal cancer patients. Sci. Rep. 11, 16745 (2021).
van der Pol, Y. et al. The landscape of cell-free mitochondrial DNA in liquid biopsy for cancer detection. Genome Biol. 24, 229 (2023).
Crasta, K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58 (2012).
Di Bona, M. & Bakhoum, S. F. Micronuclei and cancer. Cancer Discov. 14, 214–226 (2024).
Bolognesi, C. et al. Clinical application of micronucleus test in exfoliated buccal cells: A systematic review and metanalysis. Mutat. Res. Rev. Mutat. Res. 766, 20–31 (2015).
Murgia, E., Ballardin, M., Bonassi, S., Rossi, A. M. & Barale, R. Validation of micronuclei frequency in peripheral blood lymphocytes as early cancer risk biomarker in a nested case–control study. Mutat. Res. 639, 27–34 (2008).
Zhang, C.-Z. et al. Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184 (2015).
Utani, K., Kawamoto, J. K. & Shimizu, N. Micronuclei bearing acentric extrachromosomal chromatin are transcriptionally competent and may perturb the cancer cell phenotype. Mol. Cancer Res. 5, 695–704 (2007).
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017).
Anastasiadi, A. T. et al. Exploring unconventional attributes of red blood cells and their potential applications in biomedicine. Protein Cell 15, 315–330 (2024).
Howell, W. H. The life-history of the formed elements of the blood, especially the red blood corpuscles. J. Morphol. 4, 57–116 (1890).
Hu, M.-M. & Shu, H.-B. Innate immune response to cytoplasmic DNA: Mechanisms and diseases. Annu. Rev. Immunol. 38, 79–98 (2020).
Wang, Y. et al. Cytoplasmic DNA sensing by KU complex in aged CD4+ T cell potentiates T cell activation and aging-related autoimmune inflammation. Immunity 54, 632–647.e9 (2021).
Balmus, G. et al. A high-throughput in vivo micronucleus assay for genome instability screening in mice. Nat. Protoc. 10, 205–215 (2015).
Suzuki, Y. et al. The micronucleus test and erythropoiesis: Effects of cyclic adenosine monophosphate (cAMP) on micronucleus formation. Mutat. Res. 655, 47–51 (2008).
Hayashi, M. The micronucleus test-most widely used in vivo genotoxicity test. Genes Environ. 38, 18 (2016).
Pruitt, S. C., Qin, M., Wang, J., Kunnev, D. & Freeland, A. A signature of genomic instability resulting from deficient replication licensing. PLoS Genet. 13, e1006547 (2017).
Catalina, P. et al. Sequencing micronuclei reveals the landscape of chromosomal instability. bioRxiv https://doi.org/10.1101/2021.1110.1128.466311 (2021).
Elia, H. et al. Human hematopoietic stem/progenitor cells display reactive oxygen species-dependent long-term hematopoietic defects after exposure to low doses of ionizing radiations. Haematologica 105, 2044–2055 (2020).
Plackoska, V., Shaban, D. & Nijnik, A. Hematologic dysfunction in cancer: Mechanisms, effects on antitumor immunity, and roles in disease progression. Front. Immunol. 13, 1041010 (2022).
Li, N., Chen, H. & Wang, J. DNA damage and repair in the hematopoietic system. Acta Biochim. Biophys. Sin. 54, 847–857 (2022).
Beerman, I., Seita, J., Inlay, M. A., Weissman, I. L. & Rossi, D. J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell 15, 37–50 (2014).
Spisz, T. S. et al. Automated sizing of DNA fragments in atomic force microscope images. Med. Biol. Eng. Comput. 36, 667–672 (1998).
Katsman, E. et al. Detecting cell-of-origin and cancer-specific methylation features of cell-free DNA from Nanopore sequencing. Genome Biol. 23, 158 (2022).
Zhang, Z.-W. et al. Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB Life 63, 560–565 (2011).
Wei, L. et al. Circulating tumor DNA measurement provides reliable mutation detection in mice with human lung cancer xenografts. Lab. Invest. 98, 935–946 (2018).
Mammel, A. E., Huang, H. Z., Gunn, A. L., Choo, E. & Hatch, E. M. Chromosome length and gene density contribute to micronuclear membrane stability. Life Sci. Alliance 5, e202101210 (2022).
Ernst, J. & Kellis, M. Chromatin-state discovery and genome annotation with ChromHMM. Nat. Protoc. 12, 2478–2492 (2017).
Mauri, G. et al. Liquid biopsies to monitor and direct cancer treatment in colorectal cancer. Br. J. Cancer 127, 394–407 (2022).
Chen, T. & Guestrin, C. XGBoost: A scalable tree boosting system. in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 785–794 https://doi.org/10.1145/2939672.2939785 (Association for Computing Machinery, 2016).
Kuipers, E. J. et al. Colorectal cancer. Nat. Rev. Dis. Prim. 1, 15065 (2015).
Nigam, M. et al. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 164, 115015 (2023).
Nicholson, B. D. et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): a large-scale, observational cohort study. Lancet Oncol. 24, 733–743 (2023).
Cristiano, S. et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 570, 385–389 (2019).
Moser, A. R., Pitot, H. C. & Dove, W. F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Kettunen, H. L., Kettunen, A. S. L. & Rautonen, N. E. Intestinal immune responses in wild-type and ApcMin/+ mouse, a model for colon cancer. Cancer Res. 63, 5136–5142 (2003).
Vallelian, F. et al. Heme-stress activated NRF2 skews fate trajectories of bone marrow cells from dendritic cells towards red pulp-like macrophages in hemolytic anemia. Cell Death Differ. 29, 1450–1465 (2022).
Da Costa, L., Leblanc, T. & Mohandas, N. Diamond-Blackfan anemia. Blood 136, 1262–1273 (2020).
Morgado-Palacin, L. et al. Partial loss of Rpl11 in adult mice recapitulates Diamond-Blackfan anemia and promotes lymphomagenesis. Cell Rep. 13, 712–722 (2015).
Doty, R. T. et al. Single-cell analysis of erythropoiesis in Rpl11 haploinsufficient mice reveals insight into the pathogenesis of Diamond-Blackfan anemia. Exp. Hematol. 97, 66–78.e6 (2021).
Hao, X. et al. Osteoprogenitor-GMP crosstalk underpins solid tumor-induced systemic immunosuppression and persists after tumor removal. Cell Stem Cell 30, 648–664.e8 (2023).
Gerber-Ferder, Y. et al. Breast cancer remotely imposes a myeloid bias on haematopoietic stem cells by reprogramming the bone marrow niche. Nat. Cell Biol. 25, 1736–1745 (2023).
Zhou, T. et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614 (2020).
Tarallo, V. et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149, 847–859 (2012).
Ojala, J. O. & Sutinen, E. M. The role of interleukin-18, oxidative stress and metabolic syndrome in Alzheimer’s disease. J. Clin. Med. 6, 55 (2017).
Ihim, S. A. et al. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 13, 919973 (2022).
Yasuda, K., Nakanishi, K. & Tsutsui, H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 20, 649 (2019).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15.25.11–15.25.14 (2014).
Banerjee, S. & Bond, J. S. Prointerleukin-18 is activated by Meprin β in vitro and in vivo in intestinal inflammation. J. Biol. Chem. 283, 31371–31377 (2008).
Ji, P., Murata-Hori, M. & Lodish, H. F. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 21, 409–415 (2011).
Klapp, V. et al. The DNA damage response and inflammation in cancer. Cancer Discov. 13, 1521–1545 (2023).
Howard, J. E., Smith, J. N. P., Fredman, G. & MacNamara, K. C. IL-18R-mediated HSC quiescence and MLKL-dependent cell death limit hematopoiesis during infection-induced shock. Stem Cell Rep. 16, 2887–2899 (2021).
Shao, L. et al. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl. Cancer Res. 2, 397–411 (2013).
Kotsantis, P. et al. Increased global transcription activity as a mechanism of replication stress in cancer. Nat. Commun. 7, 13087 (2016).
Kim, N. & Jinks-Robertson, S. Transcription as a source of genome instability. Nat. Rev. Genet. 13, 204–214 (2012).
Safe, S. & Karki, K. The Paradoxical roles of orphan nuclear receptor 4A (NR4A) in cancer. Mol. Cancer Res. 19, 180–191 (2021).
Wenzl, K., Troppan, K., Neumeister, P. & Deutsch, J. A. A. The nuclear orphan receptor NR4A1 and NR4A3 as tumor suppressors in hematologic neoplasms. Curr. Drug Targets 16, 38–46 (2015).
de Léséleuc, L. & Denis, F. Nur77 forms novel nuclear structures upon DNA damage that cause transcriptional arrest. Exp. Cell Res. 312, 1507–1513 (2006).
Zhao, B. -x. et al. Orphan receptor TR3 enhances p53 transactivation and represses DNA double-strand break repair in hepatoma cells under ionizing radiation. Mol. Endocrinol. 25, 1337–1350 (2011).
Guo, H. et al. NR4A1 regulates expression of immediate early genes, suppressing replication stress in cancer. Mol. Cell 81, 4041–4058.e15 (2021).
Marinello, J. et al. Topoisomerase I poison-triggered immune gene activation is markedly reduced in human small-cell lung cancers by impairment of the cGAS/STING pathway. Br. J. Cancer 127, 1214–1225 (2022).
De Magis, A. et al. DNA damage and genome instability by G-quadruplex ligands are mediated by R loops in human cancer cells. Proc. Natl. Acad. Sci. USA 116, 816–825 (2019).
Zou, Z., Ohta, T. & Oki, S. ChIP-Atlas 3.0: a data-mining suite to explore chromosome architecture together with large-scale regulome data. Nucleic Acids Res. 52, W45–W53 (2024).
Liu, X. et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 567, 525–529 (2019).
Lee, S.-O. et al. Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol. Endocrinol. 28, 1729–1739 (2014).
Shaukat, A. & Levin, T. R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 19, 521–531 (2022).
Chung Daniel, C. et al. A Cell-free DNA blood-based test for colorectal cancer screening. N. Engl. J. Med. 390, 973–983 (2024).
Klein, E. A. et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 32, 1167–1177 (2021).
Kerachian, M. A., Azghandi, M., Mozaffari-Jovin, S. & Thierry, A. R. Guidelines for pre-analytical conditions for assessing the methylation of circulating cell-free DNA. Clin. Epigenetics 13, 193 (2021).
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 16, 717–727 (2014).
Magod, P. et al. Exploring the longitudinal glioma microenvironment landscape uncovers reprogrammed pro-tumorigenic neutrophils in the bone marrow. Cell Rep. 36, 109480 (2021).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Noetzli, L. J., French, S. L. & Machlus, K. R. New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arterioscler. Thromb. Vasc. Biol. 39, 1288–1300 (2019).
Kar, S. P. et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat. Genet. 54, 1155–1166 (2022).
Soto, M., García-Santisteban, I., Krenning, L., Medema, R. H. & Raaijmakers, J. A. Chromosomes trapped in micronuclei are liable to segregation errors. J. Cell Sci. 131, jcs214742 (2018).
Utani, K. -i., Kohno, Y., Okamoto, A. & Shimizu, N. Emergence of micronuclei and their effects on the fate of cells under replication stress. PLoS One 5, e10089 (2010).
Wu, S. et al. BRAF inhibitors enhance erythropoiesis and treat anemia through paradoxical activation of MAPK signaling. Signal Transduct. Target. Ther. 9, 338 (2024).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Vasimuddin, M., Misra, S., Li, H. & Aluru, S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). 314–324 https://doi.org/10.1109/IPDPS.2019.00041 (2019).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience 10, giab008 (2021).
Scheinin, I. et al. DNA copy number analysis of fresh and formalin-fixed specimens by shallow whole-genome sequencing with identification and exclusion of problematic regions in the genome assembly. Genome Res. 24, 2022–2032 (2014).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Rex, D. A. B. et al. A comprehensive pathway map of IL-18-mediated signalling. J. Cell Commun. Signal. 14, 173 (2020).
Shen, W.-K. et al. AnimalTFDB 4.0: a comprehensive animal transcription factor database updated with variation and expression annotations. Nucleic Acids Res. 51, D39–D45 (2023).
Jiang, Y. Z. et al. GATA binding protein 2 mediated ankyrin repeat domain containing 26 high expression in myeloid-derived cell lines. World J. Stem Cells 16, 538–550 (2024).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Acknowledgements
We appreciate the technical support from the Biomedical Research Core Facilities, Westlake High-Performance Computing Center, and Laboratory Animal Resources Center of Westlake University. We thank Dr. Jian Yang (Westlake University) for advice on data analysis, Dr. Hui Lin (Zhejiang University), Xiaoxiao Fan (Zhejiang University) and Xiaojie Huang (Zhejiang University) for advice on blood sample collection. Schematic figures were generated with BioRender (https://app.biorender.com/). This project was supported in part by grants from the National Natural Science Foundation of China (81973993), Zhejiang Provincial Natural Science Foundation of China (LR20C070001), Hangzhou Science and Technology Major Project (2018HZKJSA10095), and Key Research and Development Program of Zhejiang Province (2024C03170).
Author information
Authors and Affiliations
Contributions
H.S. and X.Y. contributed to methodology, investigation, visualization, project administration, original draft preparation, and manuscript review and editing. Y.J., X.K., Y.H., H.L., Y.C., and Y.X. participated in the investigation, including clinical sample collection and analysis. Y.L. and J.G. contributed to methodology. P.W., J.L., and K.D. provided supervision. X.G. conceptualized the study, contributed to methodology, investigation and visualization, acquired funding, administered the project, supervised the research, and contributed to original draft preparation, review, and editing. All authors discussed the results and provided comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following financial or non-financial competing interest: X.G. is a shareholder of Timing Biotech Co., Ltd. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, H., Yao, X., Jiao, Y. et al. DNA remnants in red blood cells enable early detection of cancer. Cell Res 35, 568–587 (2025). https://doi.org/10.1038/s41422-025-01122-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01122-7