In a study published in Nature, Chung et al. show that psychedelics can reduce fear behavior by regulating interactions between the immune system and the central nervous system.
Stress-associated psychiatric disorders, including major depressive disorder (MDD) and post-traumatic stress disorder, cause profound debilitation and continue to have worldwide prevalence.1 A growing body of research has solidified the interplay between the peripheral immune system and the central nervous system (CNS) in these disorders, revealing an increased production of proinflammatory immune cells, their mobilization from reserves into circulation, and increased proinflammatory cytokines in circulation.2,3,4,5 Given that one-third of adults diagnosed with stress-associated psychiatric disorders do not achieve remission with currently available treatments,6 it is imperative to carefully dissect the mechanisms of neuroimmune crosstalk to identify new, targeted therapeutic strategies.
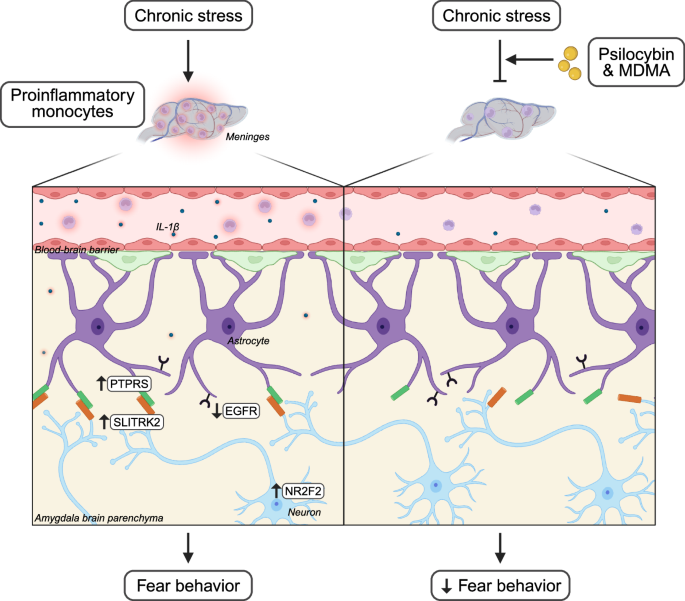
In a compelling study, Chung et al.7 identify a direct peripheral immune-to-CNS communication pathway modulated by psychedelics that can be leveraged to develop novel pharmaceuticals. Stress, which can amplify fear and anxiety, promotes alterations in circulating monocytes that traffic to neurovascular and meningeal compartments in the brain.4 Chung et al. add to this knowledge by demonstrating that chronic restraint stress in rodents promotes fear behavior, accompanied by an increase in various immune cells, including monocytes, in the meninges. They further delineate this proinflammatory state by noting an increase in several proinflammatory cytokines, such as interleukin-1β (IL-1β), in circulation following stress.
To identify the source of this stress-associated meningeal monocyte population, the authors utilized transgenic mice expressing a photoconvertible fluorescent protein to trace cells originating in the spleen. Surprisingly, they discovered that meningeally-recruited monocytes do not primarily originate from the spleen but may also come from other reserves. The authors were curious whether cells in the meninges could communicate directly with the CNS. By detecting biotinylated IL-1β within the brain parenchyma following intra-cisterna magna injections, they further demonstrated that meningeally-recruited cells may release factors, such as IL-1β, directly into the CNS.
Given these intriguing findings, Chung et al. employed a meticulous combinatorial approach to identify the direct communication pathway between meningeally-recruited monocytes, astrocytes, and neurons in the basolateral amygdala (BLA). First, they used transgenic mice expressing the fluorescent protein tdTomato in astrocytes to isolate cells for single-cell RNA sequencing after stress exposure. This analysis identified an astrocyte subpopulation that significantly expanded following stress, marked by reduced epidermal growth factor receptor (EGFR) expression, previously linked to anti-inflammatory signaling in inflammatory disease.8
After the authors determined that astrocytes within the BLA express low levels of EGFR, they hypothesized that this region may be more susceptible to stress-induced EGFR-dependent changes. To test this, they created an adeno-associated virus-based CRISPR-Cas9 construct to knock down (KD) Egfr in amygdala astrocytes. As expected, genetically-manipulated mice displayed fear behavior when subjected to stress at an earlier time than previously reported. In addition, the authors found that Egfr KD led to the activation of astrocytic inflammatory pathways in the amygdala following stress, including induction of the NF-κB pathway, as demonstrated by increased transcriptional signals of receptor protein tyrosine phosphatases, including the upregulation of protein tyrosine phosphatase receptor type S (Ptprs). Together, these data suggest that Egfr+ astrocytes in the amygdala act as negative regulators of inflammation, helping to reduce stress-induced behavior.
To understand how these inflammation-sensing astrocytes communicate with neurons, the authors examined the transcriptional state of neurons from mice with astrocytic Egfr KD. They revealed an upregulation of SLIT and NTRK-like protein 2 (Slitrk2), a single-pass transmembrane receptor that is a binding partner of PTPRS. To confirm this astrocyte-neuron link, the authors performed an in vitro knockdown of astrocyte Ptprs and neuronal Slitrk2, discovering the downregulation of the neuronal transcription factor nuclear receptor subfamily 2 group F member 2 (NR2F2). This transcription factor is critical for stress-induced fear behavior, as the in vivo knockdown of Nr2f2 reduced fear behavior. Leveraging spatial transcriptomics, the authors found that excitatory neurons expressing Nr2f2 are located near astrocytes that express low levels of EGFR within the BLA, further implicating astrocyte-neuron communication in stress susceptibility.
Crucially, the authors show that the psychedelics psilocybin and 3,4-methylenedioxymethamphetamine (MDMA) can disrupt this neuroimmune communication. Both compounds reduced fear behavior and the recruitment of various immune cells, including monocytes, to the meninges when administered to mice after stress. When immune cells were exposed to psilocybin and an MDMA racemate in vitro, they exhibited a reduced inflammatory profile, which was reversed by treatment with a serotonin type 2 (5-HT2) receptor antagonist. These findings suggest that the observed psychedelic effects are at least partially mediated by the direct expression of 5-HT2 receptors on immune cells (Fig. 1).
This schematic illustrates research completed by Chung et al., who identify a novel interaction between proinflammatory monocytes, astrocytes, and neurons following chronic stress that is inhibited by psychedelics to limit fear behavior. Chronic stress increased meningeally-recruited proinflammatory monocytes and elevated the level of proinflammatory cytokines, including IL-1β, in circulation. The authors propose that this dysregulated inflammatory state leads to the decreased expression of EGFR and an increased expression of PTPRS in astrocytes of the amygdala. In addition, this inflammatory state leads to an increased expression of the binding partner of PTPRS, SLITRK2, and the upregulation of the transcription factor NR2F2 in excitatory neurons of the amygdala. This neuroimmune communication contributes to the development of fear behavior. The administration of the psychedelics psilocybin and MDMA after stress decreases meningeally-recruited monocytes and reduces this inflammatory pathway, leading to a reduction of fear behavior. Created in BioRender.com Alvarez, J. (2025) https://BioRender.com/s8ppe7x.
The authors found that other cell types within the meninges express 5-HT2 receptors in addition to immune cells. Coupled with the known vasoconstrictive properties of psychedelics, the authors explored additional explanations for the antidepressant effects of psychedelic compounds. Strikingly, they determined that when used in combination with a vasodilator, psychedelics do not reduce the recruitment of immune cells to the meninges after stress, highlighting the potential complexities of understanding psychedelic mechanisms influencing neuroimmune communication.
To confirm whether this pathway is conserved in humans, Chung et al. first performed single-nuclei RNA sequencing on the amygdala of individuals with MDD and healthy controls. They identified an expanded astrocyte subpopulation in MDD compared to healthy individuals that demonstrated a downregulation of EGFR signaling. The authors also identified a subpopulation of excitatory neurons that is increased in MDD individuals and regulated by transcription factor NR2F2. In vitro studies confirmed that human primary monocytes reduce their inflammatory profile when exposed to lipopolysaccharide, an inflammatory inducer, in combination with psilocybin and MDMA racemate. Together, these findings mirror observations in rodents, highlighting the translational potential of their studies.
Psychedelics are promising treatment options for those with stress-associated psychiatric disorders. Thus far, clinical9 and preclinical10,11 studies have focused on profiling the effects of psychedelics on neural circuits and behavior. Altogether, the exciting findings by Chung et al. make it imperative to go beyond conventional approaches and consider alternative mechanisms by which psychedelics produce their therapeutic effects — i.e., by modulating communication between the peripheral immune system and CNS. This research, combined with ongoing exploration of the neuroimmune axis in health and disease, will enable the development of refined therapeutic compounds to improve outcomes for individuals with these debilitating disorders.
References
GBD 2019 Mental Disorders Collaborators. Lancet Psychiatry 9, 137–150 (2022).
Poller, W. C. et al. Nature 607, 578–584 (2022).
Chan, K. L., Poller, W. C., Swirski, F. K. & Russo, S. J. Nat. Rev. Neurosci. 24, 591–604 (2023).
Cathomas, F. et al. Nature 626, 1108–1115 (2024).
Costi, S. et al. Transl. Psychiatry11, 565 (2021).
McIntyre, R. S. et al. World Psychiatry 22, 394–412 (2023).
Chung, E. N. et al. Nature 641, 1276–1286 (2025).
Wheeler, M. A. et al. Science 379, 1023–1030 (2023).
Zhang, X. et al. JAMA Netw. Open 8, e257803 (2025).
Lu, J. et al. Mol. Psychiatry 26, 6237–6252 (2021).
Shao, L. X. et al. Nature 642, 411–420 (2025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alvarez, J., Russo, S.J. Psychedelics target neuroimmune interactions to limit fear. Cell Res 35, 928–929 (2025). https://doi.org/10.1038/s41422-025-01154-z
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41422-025-01154-z