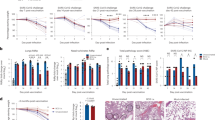
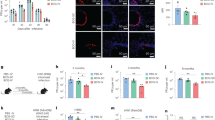
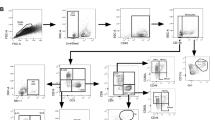
Abstract
The route of vaccine administration is associated with various immune outcomes, and the relationship between the route of administration and broad protection against heterologous pathogens remains unclear. Here, we found that subcutaneous vaccination with Bacillus Calmette-Guérin (BCG) promotes respiratory influenza clearance and T-cell responses. Group 1 innate lymphoid cells (ILC1s) express MHCII molecules and engage in antigen processing and presentation after BCG vaccination. During influenza virus infection, ILC1s in the lungs of BCG-vaccinated mice can present influenza virus antigens and prime Th1 cells. After subcutaneous vaccination with BCG, MHCII+ ILC1s migrate from the skin to the lungs and play an antigen-presenting role in influenza infection. Both the BCG and the BCG component lipomannan can induce MHCII expression and skin-to-lung migration of ILC1s via TLR2 signaling. Our study revealed an important regulatory mechanism by which subcutaneous vaccination with BCG promotes respiratory antiviral immune responses via the skin‒lung axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data, including raw sequencing data, have been deposited at the National Genomics Data Center under accession number: CRA022270 (https://ngdc.cncb.ac.cn/).
References
Lange C, Aaby P, Behr MA, Donald PR, Kaufmann S, Netea MG, et al. 100 years of Mycobacterium bovis bacille Calmette-Guerin. Lancet Infect Dis. 2022;22:e2–e12.
Prentice S, Nassanga B, Webb EL, Akello F, Kiwudhu F, Akurut H, et al. BCG-induced nonspecific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomized controlled trial. Lancet Infect Dis. 2021;21:993–1003.
Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther. 2013;35:109–14.
Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324:1980–91.
O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335–7.
Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol. 2021;22:2–6.
Ziogas A, Netea MG. Trained immunity-related vaccines: innate immune memory and heterologous protection against infections. Trends Mol Med. 2022;28:497–512.
Ziogas A, Bruno M, van der Meel R, Mulder WJM, Netea MG. Trained immunity: target for prophylaxis and therapy. Cell Host Microbe. 2023;31:1776–91.
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109:17537–42.
Boraschi D, Tagliabue A. Harnessing the power of inflammation in immunoprevention and immunotherapy. The Innovation Life. 2023;1:100025.
Arts R, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–71.
Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172:176–90.e119.
Shao F, Yu D, Xia PY, Wang S. Dynamic regulation of innate lymphoid cells in the mucosal immune system. Cellular & Molecular Immunology. 2021;18:1387–94.
Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348:1031–5.
Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature. 2022;610:744–51.
Grigg JB, Shanmugavadivu A, Regen T, Parkhurst CN, Ahmed A, Joseph AM, et al. Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature. 2021;600:707–12.
Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–95.
Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, et al. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171:795–808.e712.
Nabekura T, Riggan L, Hildreth AD, O’Sullivan TE, Shibuya A. Type 1 innate lymphoid cells protect mice from acute liver injury via interferon-gamma secretion for upregulating Bcl-xL expression in hepatocytes. Immunity. 2020;52:96–108.e109.
Kang A, Ye G, Singh R, Afkhami S, Bavananthasivam J, Luo X, et al. Subcutaneous BCG vaccination protects against streptococcal pneumonia by regulating innate immune responses in the lung. EMBO Mol Med. 2023;15:e17084.
Zhang, BZ, Shuai H, Gong HR, Hu JC, Yan B, Yuen TT, et al. Bacillus Calmette-Guerin-induced trained immunity protects against SARS-CoV-2 challenge in K18-hACE2 mice. JCI Insight. 2022;7:e157393.
Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102.
Larson EC, Ellis-Connell AL, Rodgers MA, Gubernat AK, Gleim JL, Moriarty RV, et al. Intravenous Bacille Calmette-Guerin vaccination protects simian immunodeficiency virus-infected macaques from tuberculosis. Nat Microbiol. 2023;8:2080–92.
Lobo N, Brooks NA, Zlotta AR, Cirillo JD, Boorjian S, Black PC, et al. 100 years of Bacillus Calmette-Guerin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18:611–22.
Gao X, Liu C, Wang S. Mucosal immune responses in the lung during respiratory infection: the organization and regulation of iBALT structure. hLife. 2023;1:71–82.
Ma T, Chen H, Liao YP, Li J, Wang X, Li L, et al. Pulmonary human immune responses in a humanized immune mouse model during influenza virus infection. The Innovation Life. 2023;1:315–23.
Zhao M, Shao F, Liu Z, Ma J, Yu D, Zhang H, et al. Spatially resolved transcriptomics reveals distinct pulmonary immune responses during influenza virus and SARS-CoV-2 infections. Sci Bull. 2023;68:3137–41.
Zhang X, Gao X, Liu Z, Shao F, Yu D, Zhao M, et al. Microbiota regulates the TET1-mediated DNA hydroxymethylation program in innate lymphoid cell differentiation. Nature Communications. 2024;15:4792.
Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 2020;183:771–85.e712.
Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018;172:147–61.e112.
Chen T, Ying L, Xiong L, Wang X, Lu P, Wang Y, et al. Understanding carbapenem-resistant hypervirulent Klebsiella pneumoniae: Key virulence factors and evolutionary convergence. hLife. 2024;2:611–24.
Liu Q, Li W, Qian Y, Wang C, Xia P. AA467197 controls the hyperactivation of the NLRP3 inflammasome during infection. The Innovation Life. 2023;1:100012.
Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–7.
Zhao M, Shao F, Yu D, Zhang J, Liu Z, Ma J, et al. Maturation and specialization of group 2 innate lymphoid cells through the lung-gut axis. Nat Commun. 2022;13:7600.
Shand FH, Ueha S, Otsuji M, Koid SS, Shichino S, Tsukui T, et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci USA. 2014;111:7771–6.
Zhang S, Wan D, Zhu M, Wang G, Zhang X, Huang N, et al. CD11b + CD43 hi Ly6C lo splenocyte-derived macrophages exacerbate liver fibrosis via spleen-liver axis. Hepatology. 2023;77:1612–29.
Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G. The acylation state of mycobacterial lipomannans modulates innate immunity response through Toll-like receptor 2. Chem Biol. 2006;13:39–47.
Lin Y, Hu Z, Fu Y-X, Peng H. Mucosal vaccine development for respiratory viral infections. hLife. 2024;2:50–63.
Islam SA, Luster AD. T-cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–15.
Yu D, Zhang JQ, Wang S. Trained immunity in the mucosal diseases. Wires Mech Dis. 2022;14:1543.
Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–52.
de Castro MJ, Pardo-Seco J, Martinon-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis. 2015;60:1611–9.
Chen J, Gao L, Wu X, Fan Y, Liu M, Peng L, et al. BCG-induced trained immunity: history, mechanisms and potential applications. J Transl Med. 2023;21:106.
Arts R, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100.e105.
Cirovic B, de Bree L, Groh L, Blok BA, Chan J, van der Velden W, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28:322–334 e325.
Moorlag S, Folkman L, Ter Horst R, Krausgruber T, Barreca D, Schuster LC, et al. Multiomics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity. Immunity. 2024;57:171–187 e114.
Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15:615–25.
Soto JA, Gálvez N, Andrade CA, Ramírez MA, Riedel CA, Kalergis AM, et al. BCG vaccination induces cross-protective immunity against pathogenic microorganisms. Trends Immunol. 2022;43:322–35.
Kaufmann E, Khan N, Tran KA, Ulndreaj A, Pernet E, Fontes G, et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38:110502.
Faustman DL, Lee A, Hostetter ER, Aristarkhova A, Ng NC, Shpilsky GF, et al. Multiple BCG vaccinations for the prevention of COVID-19 and other infectious diseases in type 1 diabetes. Cell Rep Med. 2022;3:100728.
Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183:315–23.e319.
Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, van Crevel R, et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212:1930–8.
Rivas, MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131:e145157.
Lee A, Floyd K, Wu S, Fang Z, Tan TK, Froggatt HM, et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat Immunol. 2024;25:41–53.
Xia P, Liu J, Wang S, Ye B, Du Y, Xiong Z, et al. WASH maintains NKp46(+) ILC3 cells by promoting AHR expression. Nat Commun. 2017;8:15685.
Bai, L, Vienne M, Tang L, Kerdiles Y, Etiennot M, Escalière B, et al. Liver type 1 innate lymphoid cells develop locally via an interferon-gamma-dependent loop. Science. 2021;371:eaba4177.
Sparks IL, Kado T, Prithviraj M, Nijjer J, Yan J, Morita YS. Lipoarabinomannan mediates localized cell wall integrity during division in mycobacteria. Nature Communications. 2024;15:2191.
Graham DB, Luo C, O'Connell DJ, Lefkovith A, Brown EM, Yassour M, et al. Antigen discovery and specification of immunodominance hierarchies for MHCII-restricted epitopes. Nat Med. 2018;24:1762–72.
Wan X, Vomund AN, Peterson OJ, Chervonsky AV, Lichti CF, Unanue ER. The MHC-II peptidome of pancreatic islets identifies key features of autoimmune peptides. Nat Immunol. 2020;21:455–63.
Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–22.
Acknowledgements
We thank Prof. George F Gao (Institute of Microbiology, Chinese Academy of Sciences) for providing the influenza A/Puerto Rico/8/34 (H1N1, PR8) virus strain. We thank Prof. Cuihua Liu (Institute of Microbiology, Chinese Academy of Sciences) for providing Bacillus Calmette-Guérin (BCG). We thank Prof. Zongfang Li (Xi’an Jiaotong University, China) for providing KikGR mice. We thank Prof. Pengyan Xia and Qiannv Liu (Peking University, China) for their technical support. We thank Dr. Tong Zhao and Dr. Jingfang Liu (Institute of Microbiology, Chinese Academy of Sciences) for their technical support. We thank Prof. Changyong Tian (Technical Institute of Physics and Chemistry, Chinese Academy of Sciences) for providing the 405 nm laser. This work was supported by the National Key R&D Program of China (2022YFC2302900, 2021YFA1300202, 2023YFC2306204), the Beijing Natural Science Foundation (JQ24043), and the CAS Project for Young Scientists in Basic Research (YSBR-010). The cartoons in Figs. 1a, 3c, f, 4a, 5b, 5f and the supplementary information Fig. S7 were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
DY performed the experiments and analyzed the data; XG, FS, ZL, AL, MZ, ZT, and YG performed the experiments; SW initiated the study and organized, designed, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, D., Gao, X., Shao, F. et al. Antigen-presenting innate lymphoid cells induced by BCG vaccination promote a respiratory antiviral immune response through the skin‒lung axis. Cell Mol Immunol 22, 390–402 (2025). https://doi.org/10.1038/s41423-025-01267-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01267-w