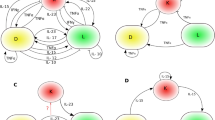
Abstract
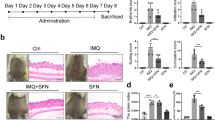
Overactivation of inflammatory signaling in keratinocytes is critical for psoriatic skin inflammation, but its regulatory mechanisms remain incompletely understood. Here, we demonstrate that the cytokine CSBF inhibits both individual and synergistic proinflammatory signaling induced by IL-17A and TNF-α (IL-17A/TNF-α) in keratinocytes, playing a protective role in psoriatic inflammation. The expression of CSBF was increased in the skin lesions and serum of psoriatic patients, and IL-17A/TNF-α enhanced its production. Csbf deletion exacerbated IMQ-induced psoriasis-like skin inflammation and led to hyperactivation of IL-17A/TNF-α signaling in keratinocytes. The CSBF protein significantly ameliorated psoriatic manifestations and suppressed IL-17A/TNF-α signaling through the receptor SUSD2. Mechanistically, CSBF-SUSD2 competed with TRAF6 and TNFR1 for interaction with ACT1, inhibiting the IL-17A/TNF-α signaling pathway. Overall, the anti-inflammatory cytokine CSBF has the potential to be a therapeutic option for psoriasis by targeting keratinocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw scRNA-seq data reported in this paper have been deposited in the Genome Sequence Archive (GSA) at the National Genomics Data Center, China National Center for Bioinformation, Beijing Institute of Genomics, Chinese Academy of Sciences, and are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human under the accession number HRA006747. The raw MS data have been deposited in OMIX (https://ngdc.cncb.ac.cn/omix: accession no. OMIX005869) [58]. Published datasets reanalyzed in this study are available under accession codes GSE150672 [29] and GSE193350 [30]. This paper does not report the original code. All other data associated with this study are presented in the main text or the supplementary materials.
References
Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. Jama. 2020;323:1945–60.
Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397:1301–15.
Ni X, Xu Y, Wang W, Kong B, Ouyang J, Chen J, et al. IL-17D-induced inhibition of DDX5 expression in keratinocytes amplifies IL-36R-mediated skin inflammation. Nat Immunol. 2022;23:1577–87.
Kurgyis Z, Vornholz L, Pechloff K, Kemény LV, Wartewig T, Muschaweckh A, et al. Keratinocyte-intrinsic BCL10/MALT1 activity initiates and amplifies psoriasiform skin inflammation. Sci Immunol. 2021;6:eabi4425.
Gupta RK, Gracias DT, Figueroa DS, Miki H, Miller J, Fung K, et al. TWEAK functions with TNF and IL-17 on keratinocytes and is a potential target for psoriasis therapy. Sci Immunol. 2021;6:eabi8823.
Guo J, Zhang H, Lin W, Lu L, Su J, Chen X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct Target Ther. 2023;8:437.
Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397:754–66.
Davidson L, van den Reek J, Bruno M, van Hunsel F, Herings R, Matzaraki V, et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg Health Eur. 2022;13:100266.
Lou F, Sun Y, Xu Z, Niu L, Wang Z, Deng S, et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity. 2020;53:204–16.e10.
Bharadwaj R, Lusi CF, Mashayekh S, Nagar A, Subbarao M, Kane GI, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998–1012.e8.
Xu M, Lu H, Lee YH, Wu Y, Liu K, Shi Y, et al. An interleukin-25-mediated autoregulatory circuit in keratinocytes plays a pivotal role in psoriatic skin inflammation. Immunity. 2018;48:787–98.e4.
Wang M, Zhang S, Zheng G, Huang J, Songyang Z, Zhao X, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66–79.e5.
Ma F, Plazyo O, Billi AC, Tsoi LC, Xing X, Wasikowski R, et al. Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat Commun. 2023;14:3455.
Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–69.
Chen A, Luo Y, Xu J, Guan X, He H, Xuan X, et al. Latest on biomaterial-based therapies for topical treatment of psoriasis. J Mater Chem B. 2022;10:7397–417.
Pan W, Cheng Y, Zhang H, Liu B, Mo X, Li T, et al. CSBF/C10orf99, a novel potential cytokine, inhibits colon cancer cell growth through inducing G1 arrest. Sci Rep. 2014;4:6812.
Suply T, Hannedouche S, Carte N, Li J, Grosshans B, Schaefer M, et al. A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci Signal. 2017;10:eaal0180.
Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. 2013;340:1456–9.
Ocón B, Pan J, Dinh TT, Chen W, Ballet R, Bscheider M, et al. A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front Immunol. 2017;8:1111.
Song J, Zheng H, Xue J, Liu J, Sun Q, Yang W, et al. GPR15-C10ORF99 functional pairing initiates colonic Treg homing in amniotes. EMBO Rep. 2022;23:e53246.
Schramm S, Liu LJ, Saad M, Dietz L, Dedden M, Müller TM, et al. Blocking GPR15 counteracts integrin-dependent T-cell gut homing in vivo. J Crohns Colitis. 2024;18:1162–72.
Sezin T, Kempen L, Meyne LM, Mousavi S, Zillikens D, Sadik CD. GPR15 is not critically involved in the regulation of murine psoriasiform dermatitis. J Dermatol Sci. 2019;94:196–204.
Guo P, Luo Y, Mai G, Zhang M, Wang G, Zhao M, et al. Gene expression profile based classification models of psoriasis. Genomics. 2014;103:48–55.
Dainichi T, Nakano Y, Doi H, Nakamizo S, Nakajima S, Matsumoto R, et al. C10orf99/GPR15L regulates proinflammatory response of keratinocytes and barrier formation of the skin. Front Immunol. 2022;13:825032.
Li X, Fan R, Tong A, Yang M, Deng J, Zhou L, et al. In situ gel-forming AP-57 peptide delivery system for cutaneous wound healing. Int J Pharm. 2015;495:560–71.
Bassilana F, BB, Carte NMT, Detheux M, Falchetto R, Hannedouche S. Organic compounds. 2015. Google Patents.
Chen C, Wu N, Duan Q, Yang H, Wang X, Yang P, et al. C10orf99 contributes to the development of psoriasis by promoting the proliferation of keratinocytes. Sci Rep. 2018;8:8590.
Tseng PY, Hoon MA. GPR15L is an epithelial inflammation-derived pruritogen. Sci Adv. 2022;8:eabm7342.
Hughes TK, Wadsworth MH, Gierahn TM, Do T, Weiss D, Andrade PR, et al. Second-strand synthesis-based massively parallel scRNA-seq reveals cellular states and molecular features of human inflammatory skin pathologies. Immunity. 2020;53:878–94.e7.
Wang J, Li X, Zhang P, Yang T, Liu N, Qin L, et al. CHRNA5 is overexpressed in patients with psoriasis and promotes psoriasis-like inflammation in mouse models. J Investig Dermatol. 2022;142:2978–87.e6.
Russell CB, Rand H, Bigler J, Kerkof K, Timour M, Bautista E, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828–36.
McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906.
Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–75.
Jegodzinski L, Sezin T, Loser K, Mousavi S, Zillikens D, Sadik CD. The G protein-coupled receptor (GPR) 15 counteracts antibody-mediated skin inflammation. Front Immunol. 2020;11:1858.
Draberova H, Janusova S, Knizkova D, Semberova T, Pribikova M, Ujevic A, et al. Systematic analysis of the IL-17 receptor signalosome reveals a robust regulatory feedback loop. EMBO J. 2020;39:e104202.
Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, et al. Act1, an NF-kappa B-activating protein. Proc Natl Acad Sci USA. 2000;97:10489–93.
May MJ. IL-17R signaling: new players get in on the Act1. Nat Immunol. 2011;12:813–5.
Luo Q, Liu Y, Shi K, Shen X, Yang Y, Liang X, et al. An autonomous activation of interleukin-17 receptor signaling sustains inflammation and promotes disease progression. Immunity. 2023;56:2006–2020.e6.
Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–56.
Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–40.
Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, et al. Act1, a negative regulator in CD40- and BAFF-mediated B-cell survival. Immunity. 2004;21:575–87.
Qian Y, Zhao Z, Jiang Z, Li X. Role of NF kappa B activator Act1 in CD40-mediated signaling in epithelial cells. Proc Natl Acad Sci USA. 2002;99:9386–91.
Giltiay NV, Lu Y, Allman D, Jørgensen TN, Li X. The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B-cell selection during transitional B-cell maturation. J Immunol. 2010;185:99–109.
Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2:ra63.
Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T-cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–58.
Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–91.
Brockmann L, Tran A, Huang Y, Edwards M, Ronda C, Wang HH, et al. Intestinal microbiota-specific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. 2023;56:2719–35.e7.
Zhao B, Gong W, Ma A, Chen J, Velegraki M, Dong H, et al. SUSD2 suppresses CD8(+) T-cell antitumor immunity by targeting IL-2 receptor signaling. Nat Immunol. 2022;23:1588–99.
Zhang W, Pajulas A, Niese M, Zhou H, Zhao J, Akhtar N, et al. Diminished γδ T cells during murine allergic skin inflammation is mediated by IL-4 signaling in keratinocytes. J Immunol. 2024;213:125–34.
Wang J, Pajulas A, Fu Y, Adom D, Zhang W, Nelson AS, et al. γδ T-Cell‒mediated wound healing is diminished by allergic skin inflammation. J Invest Dermatol. 2022;142:2805–16.e4.
Augustin I, Gross J, Baumann D, Korn C, Kerr G, Grigoryan T, et al. Loss of epidermal Evi/Wls results in a phenotype resembling psoriasiform dermatitis. J Exp Med. 2013;210:1761–77.
Herjan T, Hong L, Bubenik J, Bulek K, Qian W, Liu C, et al. IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nat Immunol. 2018;19:354–65.
Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–7.
Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5.
Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–25.
Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276:36530–4.
Kanamori M, Kai C, Hayashizaki Y, Suzuki H. NF-kappaB activator Act1 associates with IL-1/Toll pathway adaptor molecule TRAF6. FEBS Lett. 2002;532:241–6.
Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genom Proteom Bioinform. 2021;19:578–83.
Acknowledgements
We thank Prof. Dalong Ma (Peking University) for providing advice. We thank Prof. Peter Draber (Charles University) for sharing the 6 × His-2 × Strep-Flag-IL17A plasmid. This work was supported by grants from the Beijing Municipal Natural Science Foundation (No. 7232095) and the National Natural Science Foundation of China (Nos. 82171750, 82150104, 82203938, 82030095, and 82071850).
Author information
Authors and Affiliations
Contributions
WH, PW, RL, XL, KZ, and TL designed the study; XL, KZ, XY, and YC carried out the experiments and performed the data analysis; SH, YH, HD, TL, WD, XM, and JZ helped with the experiments and manuscript writing; PW and YH helped with the bioinformatics analysis; RL and KZ provided samples of patients; XL wrote the manuscript; WH, PW, RL, KZ and SH revised the manuscript; and WH, PW and RL supervised the study. All the authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhang, K., Yang, X. et al. The cytokine CSBF inhibits the IL-17A and TNF-α inflammatory pathways via SUSD2-ACT1 in keratinocytes and alleviates IMQ-induced psoriasis. Cell Mol Immunol 22, 1109–1122 (2025). https://doi.org/10.1038/s41423-025-01325-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41423-025-01325-3