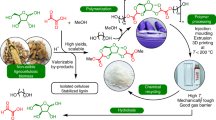
Abstract
Biomass plastics with biodegradability and suitable mechanical performance are needed to replace persistent synthetic plastics derived from fossil fuels. Lignocellulosic agricultural wastes, such as sugarcane bagasse, are promising renewable resources that offer better thermal processability when their abundant hydroxy (OH) groups are substituted with acyl groups, particularly those with longer chain lengths. However, excessive chemical modification can impair the inherent biodegradability of lignocellulose and weaken the resulting plastics. In this study, the acyl group was optimized to a decanoyl (De, C=10) group, which was the most effective in lowering the melt flow temperature of the fully substituted bagasse monoester to improve thermal moldability. The bagasse decanoate (BagDe) series were synthesized using different amounts of vinyl decanoate (VDe) ranging from 3 to 0.4 molar equivalents to the total OH content of bagasse, and their thermal/mechanical properties and degradability in soil were examined. BagDe synthesized with more than 0.6 equivalents of VDe could be hot-press molded, while the increased residual OH content improved the water uptake, degradation rate, and tensile strength. These findings indicate the potential applications of lignocellulose-based biodegradable plastics, such as agricultural mulch films.
Similar content being viewed by others
Introduction
Plastics are one of the most widely used materials and have significantly improved quality of life and revolutionized agriculture and other industries since the 1940s. Mulch films have been used in agricultural fields to improve crop yields and traits [1]. Traditional petroleum-based mulches (e.g., those made from low-density polyethylene) are predominantly used because of their low cost, ease of processing, and good mechanical performance [2]. However, they are not biodegradable in soil and present several economic and environmental drawbacks, particularly their removal and final disposal after the crop cycle [3]. Hence, biodegradable mulch has received considerable attention for sustainable agricultural development.
Biodegradable mulch films can be classified into two categories depending on the source of the raw materials: plant-based and animal-based. Plant-based sources mainly include starch, seaweed, and lignocellulose, whereas animal-based sources include gelatin from animal tissues [4]. Among these natural sources, lignocellulose is the most abundant biomass on Earth and does not compete with food production; it is available from trees, grass, crop residues, and agro-industrial waste. Substantial amounts of agricultural wastes, such as sugarcane bagasse, are generated annually but are usually burned or dumped, which has negative impacts on the environment, economy, and society. Lignocellulosic wastes are composed of cellulose, hemicellulose, and lignin, which endows them with significant potential for conversion into high-value-added products such as biomass-based thermoplastics.
The thermoplasticization of lignocellulose has been studied since the 1970s. This can be achieved via the chemical modification of hydroxy (OH) groups with new functional groups, such as acyl groups [5, 6]. In this process, efficient homogeneous reactions can be ensured via the use of ionic liquids with a powerful lignocellulose dissolution ability. Our previous study demonstrated the facile production of lignocellulose-based plastics from sugarcane bagasse via homogeneous transesterification using vinyl esters in a mixed solvent system of 1-ethyl-3-methylimidazolium acetate (EmimOAc) and dimethyl sulfoxide (DMSO) [7]. In this synthetic method, vinyl esters are used as stable and easy-to-handle acyl reagents instead of classical reagents (e.g., acid chlorides or acid anhydrides), which have higher reactivity but are corrosive and easily hydrolyzed. Furthermore, the degree of bagasse acylation can be easily controlled by the loading of vinyl esters, and fully substituted bagasse mixed esters exhibit excellent thermoplasticity without any external plasticizers [8, 9]. These lignocellulose-based plastics have also been produced from other sources, such as spruce sawdust and sisal fibers, which exhibit good thermal/mechanical properties depending on their substitutes [10,11,12]. However, in contrast to cellulose-based plastics [13], few studies have reported on the degradability of lignocellulose-based plastics in soil, and the correlation between their degradability and chemical characteristics, such as the type and degree of substitution (DS), remains unclear.
Here, we investigated the degradability of lignocellulose-based thermoplastics in soil and their correlation with the chain length and DS of their fatty acyl groups, where the DS corresponds to the conversion rate (%) of OH groups in lignocellulose derivatives. The shorter chain length and lower DS of the acyl groups in lignocellulose esters would lead to better degradability in soil, similar to the behavior of conventional cellulose esters [14,15,16]. To verify this hypothesis, bagasse monoesters with different acyl chain lengths and DSs were synthesized via homogeneous transesterification in EmimOAc/DMSO (Scheme 1), where the monoester means that a single type of ester group is substituted with bagasse-OH groups, and their hot-pressed films were subjected to soil burial tests for up to 3 months. The degradability of each sample in the soil was evaluated in terms of weight loss, and the associated changes in the mechanical properties were also monitored. These attempts aimed to assess the feasibility of using lignocellulose-based plastics as agricultural mulch films, which require multifunctional properties such as ease of melt-molding, appropriate mechanical properties during use, and degradability in soil after use.
Materials and methods
Materials
Sugarcane bagasse was supplied by Okinawa Powder Foods Co., Ltd (Okinawa, Japan). It was milled using a grinder mill (Y-308B, Osaka Chemical Co., Ltd, Osaka, Japan) and sifted to sort particles smaller than 250 μm. The obtained bagasse powder was vacuum dried at 70 °C for 24 h to reach a constant weight. It contains 50.6% cellulose, 20.2% hemicellulose, and 21.4% lignin. The total OH content ([OH]Bagasse) was estimated as 13.4 mmol g−1 based on the hypothesis that the hemicellulose was composed entirely of xylan and that the OH content of lignin was 4.5 mmol g−1 [17].
EmimOAc (≥95%; initial water content, 0.05 wt%) was obtained from Nippon Nyukazai Co., Ltd (Tokyo, Japan) and used without further purification. DMSO (anhydrous, ≥99.9%) was purchased from Sigma‒Aldrich Co., LLC (St. Louis, MO, USA). Vinyl hexanoate (VHe, >99.0%), vinyl n-octanoate (VOc, >99.0%), vinyl decanoate (VDe, >99.0%), and vinyl laurate (VLa, >99.0%) were purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). All other chemicals were commercially available and used as received unless stated otherwise. The bacteria and soil mixture used for burial tests were provided by IRIS OHYAMA Inc. (Sendai, Japan), and those mixtures contain microorganisms often used for agricultural soil reformers in Japan. The bacteria included a mixture of several microorganisms: yeast, Bacillus spp., Pseudomonas spp., filamentous fungi, and lactic acid bacteria, which express de-esterase and cellulase for the degradation of lignocellulose ester.
Synthesis of bagasse monoesters
Dried bagasse powder (6.0 g, 6 wt%/EmimOAc) in EmimOAc (100 g) was vacuum-dried at 80 °C for 24 h. DMSO (150 mL) was added to the mixture under an Ar atmosphere, and the bagasse was dissolved at 110 °C for 16 h under stirring. Vinyl esters (VEs; [VE]/[OH]Bagasse = 0.4, 0.6, 0.8, 1, and 3; mol/mol) were then added to the homogeneous solution and reacted at 80 °C for 1 h under stirring. The resulting viscous mixture was poured into distilled water (6 L) at room temperature (RT, 25 °C) to precipitate the product while an oil layer formed on the water surface. The oil layer, consisting of excess VE, was carefully removed from the solution. The precipitate obtained was vacuum-filtered and repeatedly washed with distilled water. Finally, the product was vacuum-dried at 70 °C for 24 h to yield bagasse monoester, abbreviated as BagR (R corresponds to the acyl group).
Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectra of the samples were recorded on a Thermo Fisher Scientific Nicolet iS10 spectrophotometer (Thermo Fisher Scientific, Inc., Tokyo, Japan) equipped with an attenuated total reflection (ATR) unit. The measured wavenumbers ranged from 400 to 4000 cm–1, and 64 scans were accumulated per spectrum. A resolution of 4 cm–1 was maintained throughout the measurements, with a signal-to-noise ratio (S/N) of 35,000:1.
Thermal analyses
Thermogravimetric analysis (TGA) was performed using DTG-60AH/FC-60A/TA-60 (Shimadzu Co., Kyoto, Japan) at a temperature range of 100–500 °C, employing a heating rate of 10 °C min–1 under a N2 flow rate of 50 mL min–1. For these measurements, 10 mg of each sample was dried at 120 °C for 2 h. The thermal decomposition temperature (Td-5%) was determined as the temperature at which significant (≥5%) weight loss was measured.
A capillary rheometer (melt-flow tester, CFT-500EX; Shimadzu Corp., Kyoto, Japan) was used to determine the temperature at which the samples began to melt-flow (Tflow). All the samples were vacuum-dried at 70 °C for 24 h, and 1 g of the dried sample was then loaded into a barrel and roughly pressed with a piston (diameter: 10 mm). After preheating at 50 °C for 300 s, a constant pressure of 4.9 MPa was applied to the piston at a heating rate of 3 °C min–1. Tflow is the temperature at which the sample was extruded from the die (diameter: 1.0 mm, length: 10 mm) attached to the barrel, and the temperature at which the piston moved downward by 5 mm owing to the melt flow was defined as Toffset.
Hot-press molding of films
The bagasse monoester was vacuum dried at 70 °C for 24 h to remove moisture before use. After preheating the kneader at a predetermined temperature of Tflow + 10 °C, each sample was compounded in a twin-screw extruder (DCM Xplore MC5, Xplore Instruments BV, Sittard, The Netherlands) and kneaded at 60 rpm for 5 min. The kneaded sample was extruded in a rod-like shape (diameter: 2–3 mm). The first eluted sample was discarded to prevent contamination by the residues from the previous kneading. The latter samples were collected and cut into pellets ~ 5 mm in size.
The prepared pellets were vacuum-dried at 70 °C for 24 h and molded into films through hot pressing. The dried pellets (2 g) were placed on the aluminum plate of a hot-press machine (IMC-180, Imoto Co., Ltd, Kyoto, Japan). After the sample was sufficiently melted at a predetermined temperature of Tflow + 10 °C within 5 min, it was pressed under a constant pressure of 40 kN for 3 min. In accordance with JIS K 7139, the resulting film was cut into dumbbell-shaped specimens using a punching machine (IMC-1948, Imoto Co., Ltd, Kyoto, Japan).
Tensile testing in dry and wet states
The mechanical properties of dry bagasse monoesters as dumbbell-shaped specimens were investigated through tensile testing using a universal testing machine (AG-5kNX, Shimadzu Corp., Kyoto, Japan). The test was conducted at a crosshead speed of 5 mm min–1 with an intergrip distance of 58 mm. The test was repeated five times, and the tensile properties were evaluated as the average of at least three tests.
The dumbbell-shaped specimens of bagasse monoester were soaked in 100 mL of distilled water at RT for 3 days. After wiping the water from the sample surface, the dimensions of the wet specimens were measured using a micrometer (MDC-25MX, Mitutoyo Co., Ltd, Kanagawa, Japan) and then subjected to tensile testing to investigate their mechanical properties in the wet state.
Degradation test in soil
The beds used for the soil burial tests consisted of three layers. A mixture of perlite (300 g) and water (600 mL) was used for the upper and lower layers. For the middle layer, a mixture of perlite (500 g), soil (1000 g), and water (500 mL) was prepared, and the microbial mixture was added and mixed thoroughly. The middle layer was gently layered onto the lower layer, and the specimens were buried. Finally, the upper layer was overlaid and allowed to stand at RT for up to 3 months.
After 1 and 3 months of testing, the specimens were carefully washed with distilled water and vacuum-dried for 1 day. The dried specimens were weighed to determine the weight loss percentage to examine their degradability in the soil. These specimens were further analyzed via tensile testing and ATR-mode FT-IR spectroscopy to investigate the effects of degradation on their mechanical strength and chemical structure.
Results and discussion
Effects of acyl chain length and residual OH content on the thermal moldability of bagasse monoesters
The FT-IR spectra (Fig. S1) of bagasse monoesters synthesized with different types of VEs exhibited a sharp band at 1740 cm−1, which was attributed to C=O stretching of the acyl groups. No bands corresponding to O–H stretching were observed at 3460 cm−1 [18]. These results indicate that almost all OH groups in the bagasse monoesters were substituted with acyl groups. The product masses and isolated yields are listed in Table 1. The mass yields of bagasse monoesters synthesized with excess VEs ([VE]/[OH]Bagasse = 3) increased with increasing acyl chain length. Theoretical yields were calculated when all OH groups in the bagasse were replaced by VE-derived acyl groups, assuming that the composition of the original bagasse remained constant. The isolated yields, which ranged from 83 to 93%, were calculated by dividing the product mass by the theoretical yield. These values were acceptably high, although they did not reach 100%; this may be due to undesired losses during the recovery process, such as precipitation in water and subsequent filtration, rather than synthetic issues.
For the bagasse decanoate (BagDe) series, the product mass decreased with decreasing VDe amount used for the synthesis; however, the isolated yields were as high as those of the fully substituted bagasse monoesters (Table 1). The FT-IR spectra of the BagDe series (Fig. S1) showed two sharp bands at 1740 and 2920 cm−1, which corresponded to C=O stretching and C-H stretching in the methyl and methylene groups of the De groups, respectively [18]. Furthermore, a broad O-H stretching band with higher intensity was observed with lower VDe amounts ([VDe]/[OH]Bagasse ≤ 1), suggesting that partial decanoylation occurred.
The residual OH content of the BagDe series was quantified via 31P NMR analysis after phosphytilation (Fig. S2 and Table S1), according to our previous study [8], and the DS was estimated as the degree of OH conversion from the original [OH]Bagasse. BagDe-3 exhibited a DS of 91 mol%, whereas those of BagDe-0.8 and -0.6 were 77 mol% and 60 mol%, respectively. These results indicate that BagDe-3 was almost fully acylated, whereas the latter two were partially acylated. Typically, the hemicellulose backbone in bagasse is formed by β-(1→4)-D-xylopyranosyl units with a certain number of acetyl (Ac) groups, and the Ac content was determined to be 8.7% in a previous study [19]. The Ac content of BagDe-3 was subsequently quantified via 1H NMR analysis (Figs. S3–S4), revealing that only 6.7% of the acyl groups in BagDe-3 were Ac groups. This result suggested that the hemicellulose-derived Ac groups were retained even after the acylation of bagasse.
For the bagasse monoesters listed in Table 1, Td−5% was measured through TGA, whereas Tflow and Toffset were determined using a melt-flow tester (Figs. S5–6 and Table S2). As shown in Fig. 1, the Td−5% of fully substituted bagasse monoesters decreased as the carbon number of the acyl groups increased. Furthermore, the melt-flowability improved, as indicated by the decreases in Tflow and Toffset. However, both parameters were slightly greater for BagLa-3 (C=12) than for BagDe-3 (C=10). A similar phenomenon has been reported for fully substituted cellulose esters when the carbon number of the acyl groups exceeds 10 or 12 [20]. Consequently, BagDe-3 exhibited the largest thermal processing window of 108 °C (difference between Td−5% and Toffset). The effectiveness of the De group in imparting thermal moldability is consistent with previous reports on bagasse and cellulose mixed esters, which were substituted with various long-chain acyl (C=6–18) groups combined with rich Ac (C=2) groups [8, 21].
Thermal processing window (ΔTd−5%−Toffset or Tflow) of bagasse monoesters: ×, Td−5%; ∘, Toffset; \(\bullet\), Tflow. The effects of the carbon numbers of acyl groups in fully substituted bagasse monoesters (left) and the VDe amount used for the synthesis of the BagDe series (right) on the thermal properties
In the BagDe series (Fig. 1), Td−5% increased, whereas Tflow and Toffset decreased proportionally with increasing VDe amounts used for the synthesis. BagDe-0.4, which was synthesized with the lowest amount of VDe, exhibited the highest Toffset, resulting in the narrowest thermal processing window and the greatest difficulty in hot-press molding. In contrast, other BagDe series were suitable for kneading and subsequent hot-press molding into dark-brown and translucent films at Tflow + 10 °C, indicating a favorable effect of decanoylation on thermal moldability.
Wettability and wet-to-dry strength ratio of bagasse monoesters
Dumbbell-shaped hot-pressed bagasse monoester films in the dry state were subjected to tensile testing (Fig. S7). The average tensile strength, elongation at break, and Young’s modulus are shown in Fig. 2. For the fully substituted bagasse monoesters, as the carbon number of the acyl groups increased from 6 to 10, both the tensile strength and modulus decreased, whereas the elongation at break increased. This result could be attributed to the decreased glass transition temperature as the length of the flexible n-alkyl side chains of the acyl groups increased [21]. However, further increasing the carbon number to 12 yielded the opposite result (Fig. 2). The increase in tensile strength and decrease in elongation at break may be attributed to the enhanced interaction between the La groups, similar to the suppressed melt flowability (Fig. 1). A comparison of the BagDe series indicated that a higher residual OH content resulted in an increase in the tensile strength and modulus and a decrease in the elongation at break. This result indicates that the enhanced hydrogen bonding due to the increased OH content may have improved the stiffness of BagDe in the dry state.
The wet-to-dry strength ratio of plastic materials is crucial for outdoor applications, such as their use as agricultural mulch films [22]. The dumbbell-shaped hot-pressed bagasse monoester films were then soaked in water for 3 days, and the wet specimens were subjected to tensile testing (Fig. 3). The water uptake of the fully substituted bagasse monoesters ranged from 2–5 wt% (Fig. S8), and their tensile strengths and moduli in the wet state were lower than those in the dry state (Figs. 2 and S7). In contrast, the elongation at break increased. However, the resulting wet-to-dry strength ratio ranged from 0.76 to 0.88 (Table 2). These values suggest an acceptably high water resistance in terms of strength retention, possibly owing to the hydrophobic fatty acyl groups [23].
Stress-strain curves of bagasse monoesters fully substituted with acyl groups of different chain lengths (left) and partially substituted with the De group (right), measured via tensile testing for dumbbell-shaped specimens of hot-pressed films in dry and wet states (solid and dotted lines, respectively)
The higher content of hydrophilic OH groups in the BagDe series resulted in a higher water uptake of up to 8 wt% (Fig. S8) and a decrease in the values of all the tensile properties in the wet state (Fig. 3). Specifically, BagDe-0.6 presented the lowest wet-to-dry strength ratio of 0.50 (Table 2); this indicates that its water resistance is relatively low, possibly due to the suppression effect of water screening by the De groups [24]. However, it exhibited a higher tensile strength in the dry state than the other BagDe series did (Figs. 2 and S7), possibly owing to intermolecular hydrogen bonding. Interestingly, with increasing residual OH content, the water contact angle of the film surface increased (Fig. S8). The BagDe-0.6 film presented the highest value of 110° among all the bagasse monoesters, suggesting its relatively high hydrophobicity. The mechanism for this phenomenon is still under investigation; however, this result implies that the BagDe-0.6 film surface can repel raindrops and thereby suppress the moisture-induced degradation of its mechanical performance during outdoor use.
Degradability of bagasse monoesters in soil and changes in tensile properties
Biodegradable plastics should have controllable biodegradation rates suitable for specific applications while maintaining sufficient mechanical properties throughout their usage [25]. Figure 4 shows the weight loss percentage of bagasse monoesters after 1 and 3 months of soil burial tests and the resulting tensile properties in the dry state (Fig. S9). All the specimens exhibited weight losses in the range of 1–10 wt%, indicating their potential degradability in soil, regardless of the n-alkyl side chain length and DS of the acyl groups. As hypothesized, the weight loss rate of the BagDe series increased with decreasing DS. BagDe-0.6 resulted in the greatest weight loss, ~10 wt%, which was correlated with the highest water uptake among all the samples (Fig. S8); this suggests that greater water absorption may provide a more accessible environment for soil microorganisms in bagasse monoesters.
In general, the extended acyl groups of polysaccharide esters tend to prevent ready access to enzymes [13], thereby decreasing the biodegradation rate [26]. In contrast, the weight loss rate of bagasse monoesters in the soil burial tests increased with increasing acyl chain length (Fig. 4). Furthermore, the tensile strengths of all the specimens increased as the weight loss percentage increased.
ATR-mode FT-IR analysis was performed on specimens of BagDe-3, -0.8, and -0.6 before and after the soil burial tests. The FT-IR spectrum of BagDe-3 after 3 months (Fig. 5) revealed a slight decrease in the intensity of the C=O and C-H stretching bands at 1740, 2850, and 2920 cm−1 owing to the De groups. In contrast, the intensity of the O-H stretching bands at 3300–3500 cm−1 increased. The same trend was observed for the other fully substituted bagasse monoesters (BagHe-3 and BagLa-3, Fig. S10). These changes in the FT-IR spectrum were more pronounced for BagDe-0.6 and BagDe-0.8 (Fig. 5), and local whitening was observed on the sample surface (Fig. S11). These results suggest that the weight loss of bagasse monoesters was primarily due to deacylation in the soil. Therefore, bagasse monoesters substituted with longer acyl groups likely led to higher weight loss percentages in the soil (Fig. 4) because of the higher molecular weight of the leaving groups produced during deacylation. Furthermore, the increased water absorbency of the specimens may promote deacylation, as it is a hydrolysis reaction; this was demonstrated by the highest weight loss of BagDe-0.6, which exhibited the highest water uptake (Fig. S8). Moreover, deacylation resulted in an increase in the number of free OH groups in bagasse monoesters, increasing intermolecular hydrogen bonding, which could be the mechanism of the improved mechanical properties after the soil burial tests (Fig. 4).
These results indicate that, in both the partially and fully substituted bagasse monoesters, the substituents first undergo hydrolysis in the soil. Through gradual deacylation, their semisynthetic chemical structures eventually become similar to those of unmodified bagasse, and their biodegradation rate is expected to increase over time. Therefore, bagasse monoesters can be considered biodegradable plastics in soil. Notably, for the initial 3 months, the partial biodegradation of the bagasse monoesters improved their mechanical properties rather than deteriorated them, which is significantly conducive for their practical application. In this study, the late stages of the soil burial tests were not monitored because of the limitations of the testing period. However, the initial degradation rate via deacylation can be regulated by adjusting the n-alkyl side chain length and the DS of the fatty acyl groups, making them suitable for various field applications with different usage durations.
Conclusions
This study elucidated the effects of the acyl chain length and residual OH content of bagasse monoesters on their degradability in soil and the resulting mechanical properties. Fully substituted bagasse monoesters with fatty acyl groups of C6–C12 and partially substituted ones with different DSs were synthesized using various amounts of VEs. The longer acyl groups of the fully substituted bagasse monoesters reduced the tensile strength in the dry state; however, the dry-to-wet strength ratio remained constant at 0.8, indicating that their mechanical properties are insensitive to water. In contrast, the higher OH content in the partially substituted BagDe resulted in higher dry strength, possibly due to enhanced hydrogen bonding. However, as the water uptake increased, the dry-to-wet strength ratio decreased to 0.5. In soil burial tests conducted for 1 and 3 months, the higher OH content and water absorbency of partially substituted BagDe resulted in greater weight loss than those of fully substituted bagasse monoesters. Notably, all bagasse monoesters exhibited weight loss, indicating their potential degradability in soil. Furthermore, FT-IR analysis revealed that the initial weight loss was predominantly caused by the hydrolysis of the acyl groups; therefore, the weight loss of bagasse monoesters increased with increasing length of the acyl substituents. Furthermore, the mechanical strength of all the specimens improved with increasing weight loss. This may be because gradual deacylation increased the number of free OH groups, enhancing hydrogen bonding. These results demonstrate that the wettability, mechanical strength, and degradation rate of bagasse monoesters can be controlled by adjusting the acyl chain length and DS (i.e., residual OH content), thereby ensuring their potential application as lignocellulose-based plastics with degradability in soil and sufficient durability.
References
Liu E, Zhang L, Dong W, Yan C. Biodegradable plastic mulch films in agriculture: feasibility and challenges. Environ Res Lett. 2021;16:011004.
Merino D, Athanassiou A. Biodegradable and active mulch films: hydrolyzed lemon peel waste and low methoxyl pectin blends with incorporated biochar and neem essential oil. ACS Sustain Chem Eng. 2022;10:10789–802.
Menossi M, Cisneros M, Alvarez VA, Casalongué C Current and emerging biodegradable mulch films based on polysaccharide bio-composites. A review. Agron Sustain Dev. 2021;41. https://doi.org/10.1007/s13593-021-00685-0.
Li X, Zheng G, Li Z, Fu P. Formulation, performance and environmental/agricultural benefit analysis of biomass-based biodegradable mulch films: a review. Eur Polym J. 2024;203:112663.
Li J, Baker T, Sacripante GG, Lawton DJW, Marway HS, Zhang H, et al. Solvent-free production of thermoplastic lignocellulose from wood pulp by reactive extrusion. Carbohydr Polym. 2021;270:118361.
Li X, Ye J, Hong J, Fu Y. A two-step physical method for fabrication of injection-moulded wood-based composite with NaOH/urea solution. Eur J Wood Prod. 2022;80:923–31.
Suzuki S, Takahashi K. Ionic liquids as Organocatalysts and solvents for lignocellulose reactions. Chem Rec. 2023;23:e202200264.
Suzuki S, Hamano Y, Hernandez SC, Wada N, Takahashi K. Green conversion of total lignocellulosic components of sugarcane bagasse to thermoplastics through transesterification using ionic liquid. ACS Sustain Chem Eng. 2021;9:15249–57.
Suzuki S, Hamano Y, Wada N, Takahashi K. Controlled allocation of aromatic/aliphatic substituents to polysaccharides and lignin in sugarcane bagasse via successive homogeneous transesterification using ionic liquid. ACS Omega. 2023;8:18582–90.
Feng Y, Zhang D, Liang Y, Yin X, Lei B. A facile strategy for preparing lignocellulose-based bioplastic by grafting with quaternary ammonium salts. Ind Crops Prod. 2021;174:114160.
Satria Sejati PS, Obounou Akong F, Torloting C, Fradet F, Gérardin P. Fully wood based novel translucent and thermoplastic materials by solvent-free esterification. RSC Adv. 2022;12:35206–14.
Sejati PS, Obounou Akong F, Torloting C, Fradet F, Gérardin P. Thermoplastic translucent film from wood and fatty acids by solvent free esterification: influence of fatty acid chain length. Eur Polym J. 2023;196:112276.
Glasser WG, McCartney BK, Samaranayake G. Cellulose derivatives with low degree of substitution. 3. The biodegradability of cellulose esters using a simple enzyme assay. Biotechnol Prog. 1994;10:214–9.
Komarek RJ, Gardner RM, Buchanan CM, Gedon S. Biodegradation of radiolabeled cellulose acetate and cellulose propionate. J Appl Polym Sci. 1993;50:1739–46.
Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC, et al. Advances in cellulose ester performance and application. Prog Polym Sci. 2001;26:1605–88.
Erdal NB, Hakkarainen M. Degradation of cellulose derivatives in laboratory, man-made, and natural environments. Biomacromolecules. 2022;23:2713–29.
Suzuki S, Ishikuro A, Hirose D, Ninomiya K, Takahashi K. Dual catalytic activity of an ionic liquid in lignin acetylation and deacetylation. Chem Lett. 2018;47:860–3.
Schwanninger M, Rodrigues JC, Pereira H, Hinterstoisser B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc. 2004;36:23–40.
Morais de Carvalho D, Martínez-Abad A, Evtuguin DV, Colodette JL, Lindström ME, Vilaplana F, et al. Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw. Carbohydr Polym. 2017;156:223–34.
Crépy L, Miri V, Joly N, Martin P, Lefebvre J-M. Effect of side chain length on structure and thermomechanical properties of fully substituted cellulose fatty esters. Carbohydr Polym. 2011;83:1812–20.
Suzuki S, Hikita H, Hernandez SC, Wada N, Takahashi K. Direct conversion of sugarcane bagasse into an injection-moldable cellulose-based thermoplastic via homogeneous esterification with mixed acyl groups. ACS Sustain Chem Eng. 2021;9:5933–41.
Tan Q, Yang L, Wei F, Chen Y, Li J. Comparative life cycle assessment of polyethylene agricultural mulching film and alternative options including different end-of-life routes. Renew Sustain Energy Rev. 2023;178:113239.
Kallakas H, Kattamanchi T, Kilumets C, Tarasova E, Krasnou I, Savest N, et al. Tensile and surface wettability properties of the solvent cast cellulose fatty acid ester films. Polym (Basel). 2023;15:2677.
Nilsson R, Özeren HD, Putra OD, Hedenqvist M, Larsson A. Experimental and simulated distribution and interaction of water in cellulose esters with alkyl chain substitutions. Carbohydr Polym. 2023;306:120616.
Serrano-Ruiz H, Martin-Closas L, Pelacho AM. Biodegradable plastic mulches: impact on the agricultural biotic environment. Sci Total Environ. 2021;750:141228.
Seok JH, Enomoto Y, Iwata T. Synthesis of paramylon ester-graft-PLA copolymers and its two-step enzymatic degradation by proteinase K and β-1,3-glucanase. Polym Degrad Stab. 2022;197:109855.
Acknowledgements
This work was supported by the Japan Science and Technology Agency (JST) COI-NEXT Program (grant number: JPMJPF2102 for KT) and the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers: 18H02253 and 22H02404 for KT, 21K05704 for NW, and 23H03828 and 24K17935 for SS).
Funding
Open Access funding provided by Kanazawa University.
Author information
Authors and Affiliations
Contributions
Shiori Suzuki: Data curation, visualization, writing—original draft preparation. Shogo Ishikura: Methodology, formal analysis, investigation, validation. Shoichi Ikebata: Investigation. Naoki Wada: Conceptualization, supervision, writing— review and editing. Kenji Takahashi: Writing—review and editing, funding acquisition, project administration. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki, S., Ishikura, S., Ikebata, S. et al. Degradation behavior in soil and mechanical properties of bagasse monoesters with different acyl chain lengths and residual hydroxy contents. Polym J 57, 761–769 (2025). https://doi.org/10.1038/s41428-025-01031-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01031-x