Abstract
Petroleum-based plastics are lightweight and durable and exhibit excellent formability. However, the increase in global plastics production, coupled with the economic development of emerging countries, and the resulting marine pollution caused by plastic waste have become serious problems in recent years. Polysaccharides, such as starch and cellulose, are the most abundant biopolymers in nature and are particularly promising plastic alternatives owing to their renewability, sustainability, and biodegradability. However, owing to their lack of water resistance and adequate mechanical properties, large-scale application of polysaccharide films in single-use plastics is limited because water resistance is preferred in many daily scenarios. Further research is required to optimize bioplastics to make them economically and practically feasible. In this report, we focus on stimuli-responsive materials that form or dissociate cross-linked structures in response to slight changes in external stimuli or the environment. We developed starch-based films with different disintegration/dissolution rates in freshwater and seawater as environmentally friendly materials. Modified starch was mixed with oxidized cellulose or a water-soluble polymer to prepare a transparent, homogeneous film. After the introduction of hydrogen bonds, the starch complex film was stable in freshwater; however, in seawater, the hydrogen bond crosslinks dissociated, causing the film to dissolve rapidly. This technology balances degradability in marine environments with water resistance in everyday environments, providing an alternative means of reducing marine plastic pollution, and it is expected to be applied in a variety of industrial sectors.
Similar content being viewed by others
Introduction
Petroleum-based single-use plastics are highly prone to entering the ocean. According to recent studies, global plastic production exceeds 300 million tons annually [1], with single-use plastics, including packaging, agricultural films, and disposable consumer goods, accounting for more than half of this total [2]. These plastics are major contributors to marine litter, comprising 60–95% of such waste [3, 4]. Furthermore, the recent pandemic has driven an increased reliance on single-use plastics, exacerbating challenges in managing plastic waste [5]. To address this, there is an urgent need to replace petroleum-based plastics with alternative materials, such as marine biodegradable plastics that have a lower environmental impact even if they reach the ocean. Materials capable of biodegrading in natural environments, such as soil, before entering marine ecosystems are also essential for global environmental conservation. In response to the environmental challenges posed by discarded single-use plastics, a variety of biodegradable polymers have been developed. Aliphatic polyesters, such as polylactic acid and polycaprolactone, are particularly promising as eco-friendly substitutes for traditional synthetic polymers in single-use applications. These materials offer similar performance characteristics, such as water resistance, while demonstrating significantly improved biodegradability. However, optimal biodegradation is generally achieved only under controlled conditions, such as composting environments [6].
In seawater, many commonly used biodegradable polymers degrade at much slower rates [7, 8] due to factors such as reduced microbial diversity, lower concentrations of specific microbes, and lower temperatures compared to other environments [9]. Plastics that enter marine environments interact with ocean wildlife through entanglement, ingestion, and physical contact (e.g., collisions, obstruction, and abrasion) [10]. For these interactions to occur, plastic debris must retain a certain shape and mechanical strength. Because biodegradable polymers take considerable time to fully degrade in marine environments, they can maintain their form and strength for extended periods, potentially posing risks to marine life similar to those of conventional plastics. Consequently, these slow-degrading polyesters may impact marine ecosystems at both macroplastic and microplastic levels, mirroring the effects of traditional plastics [11,12,13,14,15,16,17]. While current biodegradable polyesters represent significant progress in addressing plastic waste, their limited effectiveness in marine ecosystems highlights the need for further improvement. Developing new biodegradable polymers that degrade more rapidly in seawater is essential to effectively combat the problem of marine plastic pollution.
Starch and cellulose are considered promising alternatives to petrochemical polymers due to their abundance, biodegradability, and renewability, making them strong candidates for marine-degradable plastics [18]. However, their application in packaging is limited by poor mechanical strength and low water durability. To address this limitation, ongoing research has focused on cost-effective methods to enhance the water resistance of starch-based packaging films. Starch consists of two types of polymers: amylose and amylopectin. Amylose, which accounts for approximately 15–30% of starch, is primarily a linear polymer of α-1,4-linked glucans with minimal branching at α-1,6 positions. In contrast, amylopectin, comprising about 70–85% of starch, contains linear chains of glucose units linked by α-1,4 glycosidic bonds and is highly branched at α-1,6 positions, with branch points occurring approximately every 10 nm along the molecular axis [19, 20]. The polymodal distribution of α-glucan chains and the clustering of branch points in amylopectin facilitate the formation of double helical structures [21]. Native starch granules exhibit a semicrystalline structure composed of lightly branched amylose and highly branched amylopectin. These granules consist of an amorphous core (primarily amylose) surrounded by alternating semicrystalline and amorphous growth rings (comprising both amylose and amylopectin) [22]. When starch is heated in water, the granules swell, amylose leaches out, and the granules partially disintegrate in a process called gelatinization. Disruption of the semicrystalline structure requires significant energy, and gelatinization typically occurs at elevated temperatures (above 80 °C). This process begins in the amorphous regions, where the loosely bound amylose chains leach out, leading to a loss of birefringence and crystalline order. Upon cooling and storage, the disaggregated amylose and amylopectin chains reorganize into a more ordered structure through a process known as retrogradation. Retrogradation is an unavoidable phenomenon in native starch: linear amylose chains reassociate during the initial cooling phase, while amylopectin undergo slow crystallization over time during storage [23].
Furthermore, starch is inherently hydrophilic and lacks the water resistance exhibited by biodegradable polyesters. However, the water resistance of starch-based materials can be improved through cross-linking. In previous studies, we developed starch/PVA complex films with enhanced water resistant by incorporating intermolecular crosslinks, effectively controlling the dissolution behavior in water [24]. The chemical composition of seawater differs significantly from that of freshwater (e.g., rain or tap water), which commonly contacts plastics during use. Seawater has high ionic strength and a slightly basic pH [25], both of which act as chemical stimuli capable of cleaving specific types of chemical bonds. Therefore, films with varying dissolution and disintegration behaviors in seawater and freshwater can be created by introducing chemical crosslinks with different susceptibility to these environments. In freshwater, the cross-links help retain hydrophilicity while maintaining film stability. In seawater, the cross-links can break in response to the salt environment, causing the material to return to its original hydrophilic state. Following rapid disintegration and dissolution, the separated polymer chains can further biodegrade. This biodegradation process therefore does not strongly depend on the likelihood of encountering marine organisms. By introducing microbe-independent, responsive disintegration and dissolution properties, the loss of shape and mechanical strength in seawater can be decoupled from the biodegradation process. This approach helps shorten the timeframe during which discarded materials pose an ecological risk in the marine environment.
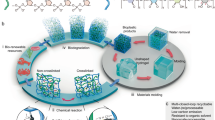
As described in this review, we aimed to develop a novel biodegradable plastic that decomposes in seawater through salt-induced changes in solubility (salt responsiveness). By focusing on starch and cellulose, the two major biopolymers, we sought to improve the physical properties of starch derivatives—such as water resistance and mechanical strength, which are traditionally weak—by combining them with cellulose nanofibers (CNFs) or water-soluble polymers. Simultaneously, marine biodegradability was introduced by developing a material design that induces the collapse and subsequent biodegradation of the composite sheet when saltwater penetrates the composite interface. Because both starch and cellulose are abundant, inexpensive, and highly biodegradable biopolymers, the developed starch composite sheet is expected to have numerous industrial applications, including in food packaging. Specifically, by adding CNFs to inexpensive starch derivatives to enhance water resistance and mechanical strength, we aimed to develop materials with practical physical properties. By controlling the chemical structure to improve water resistance, managing the interactions between starch and CNFs to enhance strength, and adjusting the composite ratio for salt responsiveness, we have developed new marine biodegradable materials (Fig. 1).
Cellulose/starch polyion complex films and their marine degradability
In our research, we focused on the differences between seawater and freshwater to design responsive crosslinks with seawater as the trigger. The key distinctions between seawater and freshwater are their dissolved salts and impurities. Seawater contains approximately 3.5% dissolved salts, primarily sodium chloride (NaCl), and is rich in various minerals, including magnesium, calcium, and potassium [26]. In contrast, freshwater has very low salinity (NaCl concentration usually less than 0.05%) and fewer minerals. Additionally, seawater typically has a slightly alkaline pH, ranging from approximately 7.5 to 8.4, due to the presence of dissolved salts and carbonates, while freshwater generally has a neutral pH, which is maintained to prevent pipe corrosion and ensure safety for daily use. Therefore, ion- and pH-responsive crosslinking is ideal for developing plastics that respond to marine conditions. Polyion complexes (PICs) are formed through the interaction of oppositely charged polyelectrolytes [27, 28]. Various factors, such as polyelectrolyte concentration, molecular weight, mixing ratio, counterions, and pH, can regulate the size, flocculation, and deformation of PICs [29]. The use of biopolymers—such as the anionic polymers alginate, hyaluronic acid, pectin, and carrageenan and the cationic polymers chitosan and gelatin—has been extensively studied in the preparation of PIC-based hydrogels and films [30,31,32]. PIC-based hydrogels have been applied in diverse fields, including in situ gelation, tissue engineering, and drug delivery [33,34,35]. In this study, we developed a CNF-reinforced starch PIC film that demonstrates good durability in freshwater and rapid degradation in marine environments [36].
The starch-based PIC film was prepared by complexing TCNF with CS, followed by solvent casting at 45 °C, as shown in Fig. 2a. TCNF, an anionic CNF with a carboxylate content of 1.3 mmol/g, and CS, a cationic starch fiber, were individually transparent in suspension. Upon complexation, the TCNF/CS mixture became opaque due to the ionic interactions between the oppositely charged cellulose and starch fibers. Variations in the ionic components of cellulose and starch influenced the opacity and homogeneity of the TCNF/CS mixtures. Modification of the carboxylate groups significantly affects the fibrillation of CNFs [37, 38], which can impact the mechanical and optical properties of the film. Therefore, adjusting the degree of substitution (DS) of the cationic groups on CS is crucial for optimizing film production. As the DS of the cationic groups increased, the opacity of the TCNF/CS mixture suspension decreased due to the distribution of polyion complexes. With a lower DS of the cationic groups, TCNF and CS formed weaker complexes. Additionally, the repulsion between the carboxylate groups on TCNF prevented the formation of large aggregates, leading to a more uniform distribution of polyion complexes [39, 40]. In contrast, a higher DS of the cationic groups resulted in stronger ionic interactions, causing the formation of larger complexes and secondary aggregation, which produced a more heterogeneous distribution. The CS suspension with a DS of 0.033 produced the most uniform TCNF/CS mixture, which was then used for further measurements. A starch-based film without ionic interactions was prepared by complexing TCNF with a noncationic starch (NCS, hydroxypropyl starch) for comparison with PIC TCNF/CS.
a Illustrative representation of the preparation method of the TCNF/CS film. b Seawater swollen films, c swelling profile, and d wet tensile modulus of TCNF/NCS and TCNF/CS films. Disintegration is indicated by the yellow arrow. Reproduced from [36] with permission from Wiley
TCNF, TCNF/NCS, and TCNF/CS were immersed in artificial seawater, and their swelling behavior and wet tensile strength were compared. Figure 2b, c shows the swollen films and their swelling profiles after 28 days of immersion. TCNF and TCNF/NCS exhibited water uptake ratios (WUR) of approximately 250% and 550%, respectively. Neither film displayed polymer leaching or disintegration after 28 days of immersion (Fig. 2b). Interestingly, TCNF/CS presented a WUR of approximately 507% after 14 days, which decreased to approximately 395% after 28 days. This reduction indicates polymer chain disintegration, as confirmed by optical images of the swollen TCNF/CS film. The wet tensile moduli of the swollen films are presented in Fig. 2d. The tensile modulus of TCNF decreased from 35 MPa on Day 1 to 25 MPa on Day 28. Similarly, the tensile modulus of TCNF/NCS decreased from 480 to 350 kPa. Notably, the tensile modulus of TCNF/CS decreased from 636 to 30 kPa. These results demonstrate that TCNF/CS almost completely lost its strength and stability, whereas TCNF and TCNF/NCS experienced only slight reductions in wet strength. The neat TCNF film contains anionic CNFs stabilized by counterions in seawater. This ionic cross-linking provides stability, reduces carboxylate repulsion, and inhibits water penetration into the network, explaining why TCNF exhibited the least amount of swelling and the highest wet tensile modulus. In TCNF/NCS, anionic CNFs are combined with nonionic starch. The blending of NCS with TCNF results in the formation of a partially crosslinked network through hemiacetal bonding between TCNF (aldehyde moieties: 25 µmol/g) and NCS. This bonding, combined with the counterion crosslinking of cellulose fibers, stabilized and strengthened the network even after 28 days of immersion in artificial seawater.
However, TCNF/NCS exhibited more swelling and a lower wet strength than neat TCNF due to water absorption by the starch component. As the starch absorbs water, the hemiacetal and ionic crosslinked networks swell, leading to increased water uptake. On the other hand, TCNF/CS contains anionic CNFs and cationic starch, which form a PIC network. PICs are weak polyelectrolytes that are unstable in high-ionic-strength solutions [41, 42]. As a result, this network loses strength and stability, leading to disintegration in the presence of counterions. Over time, polymer leaching occurs from the TCNF/CS network, causing swelling and a significant decrease in the wet tensile modulus. These results indicate that the freshwater durability and marine disintegration of TCNF/CS can be improved by modifying the degree of substitution (DS) of the cationic groups on the starch and optimizing the starch-to-TCNF ratio.
Starch/poly(vinyl pyrrolidone)-crosslinked blend film with seawater-specific dissolution
While starch-based blend films exhibit low water resistance, their susceptibility to water enables them to rapidly disintegrate and dissolve after being discarded and entering marine environments. However, enhancing their water resistance could diminish this benefit, creating a trade-off between fast disintegration/dissolution and adequate water resistance for daily applications. We aimed to introduce seawater-responsive, microbe-independent disintegration and dissolution properties into starch films by incorporating crosslinks that are responsive to seawater [43]. As noted in the previous section, the introduction of crosslinks can significantly impact the dissolution and disintegration behavior of starch-based materials; thus, these properties can be controlled by adjusting crosslink formation and disruption. Compared with freshwater, which is commonly encountered in daily life, seawater has higher ionic strength and a slightly basic pH [25], and these properties can act as triggers to break chemically responsive crosslinks. Furthermore, prior research has shown that strong, water-stable hydrogen bonds can form between the carboxylic acid groups of PAAc and the carbonyl groups of PVP and that these bonds can be disrupted under elevated pH conditions due to deprotonation of the –COOH groups in PAAc. In this study, a starch-based blend film, TS-g-PAAc/PVP, was developed to exhibit distinct dissolution and disintegration behaviors in freshwater and seawater by introducing these seawater-responsive hydrogen bonds. The results demonstrated seawater-responsive dissolution and disintegration behaviors, and the underlying mechanism was thoroughly investigated.
The process for preparing the hydrogen-bonded, crosslinked TS-g-PAAc/PVP blend film is illustrated in Fig. 3a. Initially, tapioca starch-g-poly(acrylic acid) (TS-g-PAAc) was synthesized through free radical grafting polymerization. A 5.5% w/v suspension of tapioca starch (TS) in water was purged with N2, gelatinized at 80 °C for 30 min, and then cooled to 55 °C. Next, ammonium persulfate (APS) was dissolved in 5 mL of water, and the acrylic acid monomer was gradually added. The reaction was carried out at 55 °C for 3 h in a sealed reaction vessel. The resulting product was precipitated in acetone, separated via centrifugation, and washed five times with acetone using the same method. The final precipitate was vacuum-dried and subsequently oven-dried. To prepare TS-g-PAAc/PVP blend films, the solution casting method was employed. For this purpose, 3% w/v DMSO solutions of TS-g-PAAc and PVP were prepared by directly dissolving each polymer in DMSO. The solutions were then mixed, cast onto a Petri dish, and dried in a well-ventilated oven. The formation of hydrogen bonds between TS-g-PAAc and PVP was confirmed by analyzing the shifts in the –C=O stretching peaks in the FTIR spectra of the polymers. After mixing and drying, the –C=O stretching peak of TS-g-PAAc shifted to 1718 cm−1, while that of PVP shifted to 1632 cm−1, aligning with previous studies [44]. These results suggest that hydrogen bonds formed between the –COOH groups of TS-g-PAAc and the –C=O groups of PVP during the drying process.
a Illustrative representation of the preparation process of TS-g-PAAc/PVP blend films. b Dissolution behavior of the TS-g-PAAc/PVP (2:1) blend film in DIW, 3.5% w/v NaCl solution, and seawater. c Seawater-specific dissolution behavior controlled by –COOH deprotonation. d TS-g-PAAc/PVP 2:1 film after 24 h of immersion in different water solutions. Salts: Solutions containing the major salt species in seawater at their typical concentrations. Acid: HCl solution with different concentrations. Base: NaOH solution with different concentrations. Buffers: pH 5 acetate buffer and pH 6, 7, and 7.6 phosphate buffer. Reproduced from [43] with permission from Elsevier
The dissolution and disintegration behaviors of TS-g-PAAc/PVP (weight ratio 2:1) blend film samples were evaluated in deionized water (DIW), a 3.5% NaCl solution, and artificial seawater (SW) (Fig. 3b). While the unmodified starch film dissolved rapidly in DIW, the TS-g-PAAc/PVP film displayed swelling without any dissolution when immersed in DIW. Since both PAAc and PVP are readily soluble in water, this resistance to dissolution indicates that the water-stable hydrogen bonds between TS-g-PAAc and PVP, combined with ladder-like structures and hydrophobic interactions [45], help prevent the film from dissolving in DIW. When immersed in SW, the film gradually eroded from the edges and eventually dissolved. This behavior is likely due to the disruption of crosslinks caused by deprotonation. As the polymer chains detached and dispersed, the film dissolved completely. However, no dissolution occurred in the 3.5% w/v NaCl solution, despite its salinity being similar to that of SW. This suggests that the higher pH of SW is the primary factor driving the dissolution of the film under marine conditions.
To investigate the dissolution mechanism of the TS-g-PAAc/PVP blend film in artificial seawater (SW), the film was immersed in various solutions, including solutions containing the major salt species of SW at their typical concentrations, as well as strong acid, strong base, and buffer solutions. Figure 3d shows images of the TS-g-PAAc/PVP 2:1 film samples after 24 h of immersion. The film samples did not dissolve in solutions of most major salt species at SW concentrations, except in the case of NaHCO₃, where dissolution was observed. This suggests that the type and valency of metal ions alone do not trigger dissolution, but NaHCO₃ has a unique effect.
To examine the dissolution behavior of the TS-g-PAAc/PVP film, it was immersed in strong base (NaOH) and strong acid (HCl) solutions at various concentrations. No dissolution occurred until the NaOH concentration reached 10⁻³ M, where the pH was significantly higher than that of seawater (SW), suggesting that the deprotonation pH of PAAc corresponds to its pKa. In contrast, the film dissolved in commercial buffer solutions with pH values between 5 and 7.6 and an ionic strength of 0.1 mol/L, regardless of buffer type or pH. To further investigate this dissolution mechanism, additional tests were conducted in the same buffer conditions. Unlike its behavior in strongly acidic and basic solutions, the film dissolved in all the tested buffers, indicating that its dissolution is not solely pH-dependent, but is influenced by the deprotonation kinetics of the PAAc chain segments. Specifically, weak acid anions accelerate deprotonation, thereby shortening the dissolution time. In solutions containing weak acid anions (e.g., buffer solutions, artificial seawater, and NaHCO₃ solution), the concentration of weak acid anions [A⁻] is higher than that of hydroxide ions [OH⁻] in strong base solutions with a similar pH. Consequently, protons from PAAc segments transfer rapidly to [A⁻]. Rather than resulting from a shift in protonation/deprotonation equilibrium at high ionic strength, the dissolution of the TS-g-PAAc/PVP film is more likely driven by accelerated deprotonation kinetics induced by weak acid anions, which significantly increase the deprotonation rate and promote film dissolution.
Additionally, the response to varying NaHCO₃ concentrations was assessed by immersing the film in solutions with NaHCO₃ concentrations 0.01, 0.1, 1, and 10 times the Na₂CO₃ concentration in SW. Within 24 h, the films in the 0.01× and 0.1× NaHCO₃ solutions did not dissolve, while those in the higher-concentration solutions did. These findings suggest that a threshold concentration of NaHCO₃ is necessary to induce film dissolution. The strong, water-stable hydrogen bonds between PVP and the grafted PAAc chains of TS-g-PAAc prevented the film from dissolving in deionized water (DIW). However, in artificial seawater, the carboxyl groups of TS-g-PAAc rapidly deprotonated, breaking the hydrogen bond network and enabling the dissolution of TS-g-PAAc. This rapid deprotonation was driven by interactions with bicarbonate groups in the artificial seawater. This method demonstrates a potential approach to mitigate oceanic plastic pollution and highlights the suitability of such materials for single-use applications in packaging, healthcare, and agriculture.
Dual-crosslinked starch/carboxymethyl cellulose blend film with ion-responsive dissolution properties
This study explored a means of balancing the trade-off between rapid disintegration/dissolution in seawater and water resistance during daily use by introducing seawater-responsive hydrogen bond crosslinks. To expand the application of this concept, an alternative crosslinking mechanism was developed to complement seawater-responsive hydrogen bond crosslinking. PICs are known to have stability influenced by ionic strength due to the dissociation of these bonds [46]. Given the significantly higher ionic strength of seawater than those of freshwater and most daily-use solutions, a starch-based PIC film could exhibit water resistance during regular use and rapid disintegration upon entering the sea. In this study, a dual-crosslinked dialdehyde starch (DAS)/carboxymethyl cellulose (CMC) blend film was prepared using a modified solution-casting method. This film incorporated both ionic and acetal crosslinks and exhibited seawater-responsive dissolution and disintegration behavior, which was further investigated.
The preparation process for the DAS/CMC/GT/HCl blend films is shown in Fig. 4a. DAS was synthesized via periodate oxidation. A 4% tapioca starch (TS) water suspension was heated to 90 °C for approximately 30 min to gelatinize the TS and then cooled to room temperature (RT). Afterward, 0.05 g/mL NaIO₄ was added to the cooled starch paste, which was allowed to react for 1 h at RT in the dark with stirring. The resulting DAS paste was dialyzed against deionized water (DIW) for three days using a dialysis tube (MWCO 10 kDa) and subsequently subjected to two rounds of high-pressure homogenization at 200 MPa. Next, the dual-crosslinked DAS/CMC/GT/HCl film was prepared. A 3% CMC solution was mixed with the homogenized DAS suspension, stirred, and heated at 45 °C for 30 min before being cooled to RT and further chilled in an ice-water bath. The pH of the solution was adjusted with 1 M HCl, and Girard’s reagent T (GT) was added to introduce quaternary ammonium groups into DAS by forming imine bonds, converting DAS into a cationic polymer [24]. Fifteen milliliters of the mixture was cast into a fluoropolymer Petri dish and dried overnight at 45 °C. The resulting film was washed with DIW and glycerol solution and dried again overnight at 45 °C. The reaction between DAS and GT was confirmed by comparing the FTIR spectra of the DAS/CMC and DAS/CMC/GT/HCl films. Peaks corresponding to the GT structure—923 cm⁻¹ (N=N) and 1691 cm⁻¹ (C=O)—were observed in the DAS/CMC/GT/HCl spectrum [47], indicating that GT reacted with DAS during the drying process.
a Illustration of the preparation process of the DAS/CMC/GT/HCl blend film. b Swelling curves of DAS/CMC/GT/HCl films in DIW, 3.5% NaCl, and SW. Reproduced from [49] with permission from Elsevier
The ionic swelling-responsive behavior of the DAS/CMC/GT/HCl film was evaluated by observing its swelling and dissolution in freshwater and two seawater models: a 3.5% NaCl solution and artificial seawater (SW) (Fig. 4b). While the pure starch film dissolved quickly in DIW, the DAS/CMC/GT/HCl film achieved swelling equilibrium without dissolving. However, in 3.5% NaCl solution and SW, the film exhibited rapid swelling and disintegration. This behavior is attributed to the cleavage of ionic bonds triggered by ion exchange, leaving only acetal crosslinks. These crosslinks alone were insufficient to resist swelling, causing the film to swell and disintegrate rapidly.
DAS/CMC blend films without crosslinks or with only one type of crosslink were also prepared to evaluate the contribution of each crosslink type to the ion-responsive swelling and dissolution processes. The swelling curves of the four film types are presented in Fig. 5. The DAS/CMC film, which lacked crosslinks, exhibited the typical swelling behavior of superabsorbent polymers. It continued to swell in both NaCl solution and DIW, although swelling was less pronounced in the NaCl solution due to the reduced osmotic pressure difference and the counterion shielding effect [48]. The DAS/CMC/HCl film, containing only acetal bonds, exhibited a molecular structure similar to that of the DAS/CMC film. Despite the presence of acetal crosslinks, this film did not reach swelling equilibrium in DIW, indicating that acetal bonds alone do not provide sufficient binding strength to counteract the osmotic expansion forces.
Swelling curves of a DAS/CMC0.5, b DAS/CMC0.5/HCl, c DAS/CMC0.5/GT, and d DAS/CMC0.5/GT/HCl films in DIW and 3.5% NaCl solution. Reproduced from [49] with permission from Elsevier
In contrast, films containing ionic bonds, such as DAS/CMC/GT and DAS/CMC/GT/HCl, reached swelling equilibrium in DIW but swelled continuously and disintegrated in the NaCl solution. This behavior highlights the importance of ionic bonds in ensuring water resistance in low-ionic-strength environments. Ionic bonds add an additional binding force to the network, countering expansion forces and shielding carboxylate groups, which further reduces swelling. Moreover, films containing both ionic and acetal bonds swelled significantly less in both DIW and NaCl solution compared to their counterparts without acetal bonds. This demonstrates that in addition to ionic bonds, acetal bonds provide supplementary binding forces across different solutions. While the ionic-responsive dissolution and disintegration properties of the films are attributed primarily to ionic crosslinks, both ionic and acetal crosslinks contribute to water resistance in low-ionic-strength environments such as DIW. These findings are expected to aid in the development of starch-based single-use materials with reduced risks to marine ecosystems.
Summary and perspective
In this review, we explored strategies to control the dissolution and disintegration behavior of starch-based complex films by forming polyion complexes and introducing various types of intermolecular crosslinks, including covalent, noncovalent, and environlentally responsive types. The results demonstrated that the dissolution/disintegration behavior can be effectively regulated through the formation and selective disruption of these crosslinks. Furthermore, controlling the dissolution/disintegration behavior in different aqueous environments—specifically seawater and freshwater—can be achieved by selecting crosslink types that respond to the chemical characteristics of seawater and by carefully choosing suitable modification methods. While the direct blending of polycations and polyanions to form ionic crosslinks can result in aggregation that hinders film formation, this issue was resolved using a modified solution-casting process. Although ionic crosslinks primarily provide ionic-responsive dissolution/disintegration properties, both ionic and other types of crosslinks contribute to maintaining water resistance in low-ionic-strength solutions such as DIW. These findings suggest that the tailored control of dissolution and disintegration in both freshwater and seawater environments offers a promising strategy to balancing ecological safety with practical utility in daily life.
This research is currently being extended to further enhance water resistance and mechanical strength under freshwater conditions. Polysaccharide-based composite materials combined with hydrophilic polymers are being developed. By introducing reversible hemiacetal/acetal bonds between polymer chains, chemical crosslinks are formed, and strong physical multipoint interactions are simultaneously created. Under mild conditions—such as exposure to salt—these reversible chemical bonds and physical interactions can be dissociated, enabling easy disassembly of the material and resource recycling through recombination. The crosslinking structure is being fine-tuned to improve water resistance, strengthen multipoint interactions, and optimize the composite formulation for salt responsiveness. Additionally, based on an original concept proposed by the primary investigator, environmental responsiveness will be integrated into the polysaccharide-based composite materials to demonstrate the functionality of the switching function. These advancements are expected to contribute to the development of starch-based single-use materials that present lower risks to marine ecosystems.
References
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3:e1700782.
Hopewell J, Dvorak R, Kosior E. Plastics recycling: challenges and opportunities. Philos Trans R Soc B Biol Sci. 2009;364:2115.
Xanthos D, Walker TR. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar Pollut Bull. 2017;118:17.
Schnurr REJ, Alboiu V, Chaudhary M, Corbett RA, Quanz ME, Sankar K, et al. Reducing marine pollution from single-use plastics (SUPs): a review. Mar Pollut Bull. 2018;137:157.
Vanapalli KR, Sharma HB, Ranjan VP, Samal B, Bhattacharya J, Dubey BK, et al. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci Total Environ. 2021;750:141514.
Emadian SM, Onay TT, Demirel B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017;59:526.
Bagheri AR, Laforsch C, Greiner A, Agarwal S. Fate of so-called biodegradable polymers in seawater and freshwater. Glob Chall. 2017;1:1700048.
Dilkes-Hoffman LS, Lant PA, Laycock B, Pratt S. The rate of biodegradation of PHA bioplastics in the marine environment: a meta-study. Mar Pollut Bull. 2019;142:15.
Wang G-X, Huang D, Ji J-H, Völker C, Wurm FR. Seawater-degradable polymers—fighting the marine plastic pollution. Adv Sci. 2021;8:2001121.
Law KL. Plastics in the marine environment. Annu Rev Mar Sci. 2017;9:205.
Balestri E, Menicagli V, Vallerini F, Lardicci C. Biodegradable plastic bags on the seafloor: a future threat for seagrass meadows?. Sci Total Environ. 2017;605–606:755.
Anderson G, Shenkar N. Potential effects of biodegradable single-use items in the sea: polylactic acid (PLA) and solitary ascidians. Environ Pollut. 2021;268:115364.
Green DS, Boots B, Sigwart J, Jiang S, Rocha C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer and nutrient cycling. Environ Pollut. 2016;208:426.
Jiang M, Hu L, Lu A, Liang G, Lin Z, Zhang T, et al. Strong sorption of two fungicides onto biodegradable microplastics with emphasis on the negligible role of environmental factors. Environ Pollut. 2020;267:115496.
Weinstein JE, Dekle JL, Leads RR, Hunter RA. Degradation of bio-based and biodegradable plastics in a salt marsh habitat: another potential source of microplastics in coastal waters. Mar Pollut Bull. 2020;160:111518.
Zuo L-Z, Li H-X, Lin L, Sun Y-X, Diao Z-H, Liu S, et al. Sorption and desorption of phenanthrene on biodegradable poly(butylene adipate co-terephtalate) microplastics. Chemosphere. 2019;215:25.
Wang C, Yu J, Lu Y, Hua D, Wang X, Zou X. Biodegradable microplastics (BMPs): a new cause for concern?. Environ Sci Pollut Res. 2021;28:66511.
Plackett D. Biopolymers: new materials for sustainable films and coatings. Chichester, UK: John Wiley & Sons, Ltd; 2011. pp. 1–20.
Durrani CM, Donald AM. Physical characterisation of amylopectin gels. Polym Gels Netw. 1995;3:1.
Jenkins PJ, Cameron RE, Donald AM. A universal feature in the structure of starch granules from different botanical sources. Starch. 1993;45:417.
Ellis RP, Cochrane MP, Dale MFB, Duffus CM, Lynn A, Morrison IM, et al. Starch production and industrial use. J Sci Food Agric. 1998;77:289.
Cornejo-Ramírez YI, Martínez-Cruz O, Del Toro-Sánchez CL, Wong-Corral FJ, Borboa-Flores J, Cinco-Moroyoqui FJ. The structural characteristics of starches and their functional properties. CyTA J Food. 2018;16:1003.
Wang S, Li C, Copeland L, Niu Q, Wang S. Starch retrogradation: a comprehensive review. Compr Rev Food Sci Food Saf. 2015;14:568.
Jia Y, Asoh T-A, Hsu Y-I, Uyama H. Wet strength improvement of starch-based blend films by formation of acetal/hemiacetal bonding. Polym Degrad Stab. 2020;177:109197.
Ishizu M, Miyazawa Y, Tsunoda T, Ono T. Long-term trends in pH in Japanese coastal seawater. Biogeosciences. 2019;16:4747.
Culkin F, Cox RA. Sodium, potassium, magnesium, calcium and strontium in sea water. Deep Sea Res. 1966;13:789.
Starchenko V, Müller M, Lebovka N. Sizing of PDADMAC/PSS complex aggregates by polyelectrolyte and salt concentration and PSS molecular weight. J Phys Chem B. 2012;116:14961.
Dautzenberg H. Polyelectrolyte complex formation in highly aggregating systems. 1. Effect of Salt: polyelectrolyte complex formation in the presence of NaCl. Macromolecules. 1997;30:7810.
Müller M. Sizing, shaping and pharmaceutical applications of polyelectrolyte complex nanoparticles. Adv Polym Sci. 2012;256:197.
Farris S, Schaich KM, Liu LS, Cooke PH, Piergiovanni L, Yam KL. Gelatin–pectin composite films from polyion-complex hydrogels. Food Hydrocoll. 2011;25:61.
Farris S, Schaich KM, Liu LS, Piergiovanni L, Yam KL. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: a review. Trends Food Sci Technol. 2009;20:316.
Asma C, Meriem E, Mahmoud B, Djaafer B. Physicochemical characterization of gelatin-CMC composite edible films from polyion-complex hydrogels. J Chil Chem Soc. 2014;59:2279.
Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromol. 2014;64:353.
Yataka Y, Suzuki A, Iijima K, Hashizume M. Enhancement of mechanical properties of polysaccharide composite films utilizing cellulose nanofibers. Polym J. 2020;52:645.
Lam J, Clark EC, Fong ELS, Lee EJ, Lu S, Tabata Y, et al. Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(L-lysine) for applications in cartilage tissue engineering. Biomaterials. 2016;83:332.
Soni R, Hsu Y-I, Asoh T-A, Uyama H. Cellulose nanofiber reinforced starch film with rapid disintegration in marine environments. J Appl Polym Sci. 2022;139:e52776.
Levanič J, Šenk VP, Nadrah P, Poljanšek I, Oven P, Haapala A. Analyzing TEMPO-oxidized cellulose fiber morphology: new insights into optimization of the oxidation process and nanocellulose dispersion quality. ACS Sustain Chem Eng. 2020;8:17752.
Hastuti N, Kanomata K, Kitaoka T. Characteristics of TEMPO-oxidized cellulose nanofibers from oil palm empty fruit bunches produced by different amounts of oxidant. IOP Conf Ser Earth Environ Sci. 2019;359:012008.
Insua I, Wilkinson A, Fernandez-Trillo F. Polyion complex (PIC) particles: preparation and biomedical applications. Eur Polym J. 2016;81:198.
Dautzenberg H. Polyelectrolyte complex formation in highly charged systems: a kinetic study. Macromolecules. 1997;30:7810.
Asoh TA, Kikuchi A. Electrophoretic adhesion of stimuli-responsive hydrogels. Chem Commun. 2010;46:7793.
Asoh TA, Kawai W, Kikuchi A. Electrophoretic adhesion of biodegradable hydrogels through the intermediary of oppositely charged polyelectrolytes. Soft Matter. 2012;8:1923.
Jia Y, Hsu Y-I, Uyama H. A starch-based, crosslinked blend film with seawater-specific dissolution characteristics. Carbohydr Polym. 2023;299:120181.
Guan Y, Yang S, Zhang Y, Xu J, Han CC, Kotov NA. Fabry−Perot fringes and their application to study the film growth, chain rearrangement, and erosion of hydrogen-bonded PVPON/PAA films. J Phys Chem B. 2006;110:13484.
Tsuchida E, Osada Y, Ohno H. Formation of interpolymer complexes. J Macromol Sci Part B Phys. 1980;17:683.
El-Ayaan U, Kenawy IM, Abu El-Reash YG. Synthesis, thermal and spectral studies of first-row transition metal complexes with Girard-T reagent-based ligand. J Mol Struct. 2007;871:14.
Sirviö J, Honka A, Liimatainen H, Niinimäki J, Hormi O. Synthesis of highly cationic water-soluble cellulose derivative and its potential as novel biopolymeric flocculation agent. Carbohydr Polym. 2011;86:266.
Zhang W, Wang P, Liu S, Chen J, Chen R, He X, et al. Factors affecting the properties of superabsorbent polymer hydrogels and methods to improve their performance: a review. J Mater Sci. 2021;56:16223.
Jia Y, Hsu Y-I, Uyama H. Dual-crosslinked starch/carboxymethyl cellulose blend film with ion-responsive dissolution properties. Polym Degrad Stab. 2023;215:110453.
Acknowledgements
The author sincerely thanks Prof. Hiroshi Uyama for his kind advice.
Funding
This work was supported by JSPS KAKENHI (Grants 22K21348 and 23K26717), a Japan Science and Technology Agency PRESTO Grant (JPMJPR23N4), and the Environment Research and Technology Development Fund of the Environmental Restoration and Conservation Agency of Japan (JPMEERF21S11900). Open Access funding provided by The University of Osaka.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsu, YI. Development of functional degradable materials by precise crosslinking design of biobased polymers. Polym J 57, 1095–1105 (2025). https://doi.org/10.1038/s41428-025-01051-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01051-7