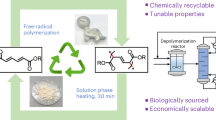
Abstract
Polymers and plastics pose environmental challenges, including marine pollution from waste and CO2 emissions from incineration. Recycling and upcycling are crucial strategies for conserving petroleum resources and reducing waste discharge. Additionally, developing sustainable polymers is essential for achieving a circular economy. Polymer degradation is a key process in both recycling and sustainable polymer development. This review examines the degradation of condensation polymers, such as polyesters and polycarbonates, when organic catalysts are used to enhance transesterification. Organic bases exhibit high catalytic efficiency in polymer degradation, whereas others facilitate the controlled polymerization of substituted cyclic esters and carbonates. Notably, 1,5,7-triazabicyclo[4.4.0]dec-7-ene has exceptional efficiency in degrading various condensation polymers, including aliphatic polycarbonates and liquid-crystalline wholly aromatic polyesters, via a dual hydrogen-bonding activation mechanism. The functionalization of aliphatic polycarbonates via side-chain modifications is a promising approach for producing functionalized degradable polymers, supported by efficient monomer synthesis and established ring-opening polymerization (ROP) techniques using organic catalysts. Precise polymer synthesis enhances mechanical and thermal properties by incorporating rigid moieties while enabling degradation control. These advancements contribute to the development of sustainable materials within a future circular economy.
Similar content being viewed by others
Introduction
The reliance on petroleum feedstock and post-use incineration contributes to greenhouse gas emissions and environmental pollution globally (Fig. 1), resulting in environmental concerns that necessitate a shift in plastic production and waste management. In a future circular economy of plastics, recycling and upcycling will be critical for reducing plastic waste and preserving petroleum-derived materials that are already in circulation. A scenario where fossil feedstocks become entirely unavailable is also a possibility. Thermal recycling should be avoided, as it does not effectively recycle plastics as a resource and leads to CO2 emissions. The European Union has mandated that plastics used in automobiles contain at least 25% recycled material [1], a trend likely to extend to other industries. Polyolefins, including polyethylene, polypropylene, polystyrene, and poly(vinyl chloride), account for more than half of the global plastic produced [2]. Although less abundant, poly(ethylene terephthalate) (PET) has been extensively studied for recycling, with material recycling serving as the dominant approach for bottle-to-bottle PET reuse owing to its cost advantages [2, 3]. However, repeated recycling degrades polymer properties, limiting plastic circularity. Maintaining polymer performance and molecular weight remains a significant challenge in material recycling. Chemical recycling has gained increasing attention for its ability to recover high-purity plastic precursors, such as monomers. Condensation polymers, including polyesters and polycarbonates (Fig. 2), are more amenable to controlled degradation into repolymerizable monomers than are polyolefins, as ester bond cleavage is energetically more favorable than is C–C bond cleavage. However, reducing costs is essential for making chemical recycling commercially viable. Catalytic degradation enables efficient chemical recycling and upcycling, potentially offering economic advantages. Consequently, numerous transesterification catalysts have been investigated for PET and other condensation polymers, such as bisphenol-A polycarbonate (BPA-PC) [4,5,6]. Organic catalysts may offer the benefit of a metal-free process for polymer degradation as a more environmentally friendly option. Since the first report by Nederberg in 2001 [7], organocatalysis in polymer chemistry (Fig. 3) has focused primarily on the ring-opening polymerization (ROP) of cyclic esters and carbonates [8, 9]. Advances in transesterification organocatalysts have led to the discovery of highly efficient catalysts with high turnover frequencies, enabling the ROP of lactide within seconds [10, 11]. This progress has encouraged researchers to explore transesterification organocatalysts for the degradation of aromatic polyesters and polycarbonates [12].
Alternative approaches to addressing current plastic-related challenges include the adoption of biobased and biodegradable polymers. Biobased materials are expected to reduce greenhouse gas emissions by eliminating the reliance on fossil resources. Compared with petroleum-based polymers, natural polymers such as cellulose, silk, and polysaccharides are both biobased and biodegradable, resulting in a lower environmental impact. However, challenges related to molecular weight control, processing, and limited modification options hinder their broader application. In contrast, synthetic polymers derived from biobased monomers have advanced significantly over recent decades [13, 14]. Improvements in biofuel production and C1 chemistry have enabled the biobased synthesis of olefin monomers and acrylic acid [15]. While biobased polyolefins and polyacrylates contribute to CO2 reduction, their waste management remains a concern, as they are nondegradable and persist in the environment. Synthetic (bio)degradable polymers offer greater potential owing to their reduced ecological impact and broader scope for modification and functionalization. Aliphatic polyesters and polycarbonates fall into this category, with their in vivo and enzymatic degradation widely studied, as exemplified by polylactides (PLAs) [16] and poly(trimethylene carbonate) (PTMC) [17, 18]. Furthermore, derivatives with diverse side chain functionalities have been developed as degradable functional polymers [17,18,19]. Organocatalysis has also contributed to this progress, enabling the selective and controlled polymerization of cyclic carbonates and esters with a carbonyl-containing substituent. Although most aliphatic polycarbonates (APCs) produced by the above polymerization are designed for biomedical applications, their underlying platforms could be adapted for environmentally friendly materials. However, the environmental impact of the degradation of synthetic (bio)degradable polymers remains insufficiently explored, and some are derived from petroleum-based chemicals. Biobased nondegradable polymers and nonbiobased degradable polymers could be viable alternatives if they can be efficiently recycled. Synthetic biobased, degradable polymers are particularly favorable as substitutes for conventional petroleum-based nondegradable polymers, provided that they offer comparable performance and economic feasibility. Currently, only a few such polymers meet these criteria and have been commercialized, highlighting the need for further development.
Organocatalysis for the degradation of polyesters and polycarbonates
Organocatalysis has emerged as an innovative approach in organic chemistry, as recognized by the 2021 Nobel Prize in Chemistry. Shortly after the pioneering works of List and MacMillan [20, 21], the first example of the organocatalyzed ROP of lactide using N,N-dimethyl-4-aminopyridine (DMAP) (Fig. 3) was reported [6]. The metal-free production of polymeric materials has been desired for semiconducting materials and biomaterial applications [22]. Hedrick and Waymouth explored a range of organocatalysts for transesterification-based ROP, including N-heterocyclic carbenes (NHCs), thioureas, and superbases [8, 9]. Organic bases play crucial roles in these catalytic systems. In contrast, Satoh and Bourissou independently developed acidic organocatalysts for ROP [23, 24], and then Hedrick and Sardon reported binary acid‒base complexes (Fig. 3) [25, 26]. ROP catalysts can be categorized into potent catalysts and controlling catalysts. Potent catalysts enable the rapid polymerization of lactide within seconds, while controlling catalysts moderate the reaction, producing polymers with narrow dispersity in a controlled manner at room temperature [8, 9]. The high turnover frequency of potent catalysts suggests their potential application in the degradation of polyesters and polycarbonates. The first example of the organocatalyzed degradation of PET was reported in 2010 using an NHC [11]. The following year, a guanidine superbase, 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), was demonstrated to be an effective catalyst for the glycolysis of PET, which was conducted for 8 min (10 mol%) and 3.5 h (1 mol%) at 190 °C, to provide bis(hydroxyethyl)terephthalate (BHET) (Fig. 4A, B) [27]. TBD is commercially available and bench-stable, whereas NHC requires in situ formation under an inert atmosphere. Computational and spectroscopic studies have elucidated the catalytic mechanisms, revealing that TBD promotes the activation of ester carbonyl and hydroxyl groups through dual hydrogen bonding—the Lewis acidic N–H group activates the carbonyl group, whereas the Lewis basic imine activates the hydroxyl group of an alcohol (Fig. 4A) [27]. The catalytic activity of organocatalysts in ROP generally correlates with their basicity and acidity (pKa values). Further studies revealed that ethylene glycol (EG), which acts as a nucleophile, serves as a hydrogen bond activator for the ester carbonyl group of PET in bidentate form [27, 28]. Notably, under glycolysis conditions, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (Fig. 3) facilitates PET degradation more efficiently than TBD does, completing it in 2 h with 1 mol% DBU at 190 °C [29]. This enhanced activity is attributed to the push‒pull theory, where the dual activation of TBD is less effective in the presence of EG, as the Lewis acidic N–H group mitigates the activation of O–H [27]. TBD, however, exhibits greater catalytic activity for PET degradation when other diols and monoalcohols are used as nucleophiles, highlighting the specificity of the interaction between EG and the catalyst [29].
The TBD-catalyzed degradation of PET has been extended to its upcycling [30], utilizing various amines as nucleophiles to generate terephthalamides, which serve as valuable building blocks (Fig. 4B) [31]. Amines exhibit greater nucleophilicity than alcohols do, enabling noncatalytic aminolysis of PET [32]. For example, 60% of PET is degraded by benzylamine in 2 h at 150 °C without a catalyst. TBD significantly enhances the reaction efficiency by reducing the reaction time (~1 h for 99% degradation at 150 °C with 5 mol% TBD) and lowering the required temperature (120 °C) [31]. When diamines are used, small amounts of oligomers often form due to equilibrium constraints, but the presence of TBD helps minimize the oligomer content in the final product [27, 31]. The basicity of aryl amines, such as aniline, is comparable to or even lower than that of alcohols. Additionally, their high melting and boiling points increase the melting points of the terephthalamides derived from them, making solvent-free PET degradation more challenging. TBD effectively facilitates the aminolysis of PET with aryl amines, enabling the synthesis of high-melting-point aromatic terephthalamides [31].
Another advantage of the dual hydrogen bond activation of TBD was identified in benzazole formation via the intramolecular cyclization of ortho-substituted phenyl terephthalamides, which are generated through the aminolysis of PET with ortho-substituted anilines (Fig. 4B), conducted at 190 °C [33]. TBD further activates these intermediates by dual hydrogen bonding, interacting with both the amide carbonyl group and ortho substituents, such as OH and NH2 groups. Benzazole rings typically form under acidic conditions [34]. However, the TBD-catalyzed reaction mechanism follows a nucleophilic attack pathway, which has a high energy barrier, suggesting its exceptional catalytic activity [33]. This study reported the formation of nonsubstituted bis(benzimidazolyl)benzene and bis(benzoxazolyl)benzene (Fig. 4B), both of which may serve as precursors and intermediates for organic emitters and pharmaceutical compounds. Additionally, functionalized 1,2-diaminobenzenes and 2-aminophenols could be used to generate substituted derivatives with tailored properties.
The upcycled terephthalamides formed via the TBD-catalyzed aminolysis of PET possess bisfunctional structures, including diamines, diols, dienes, and diynes, making them suitable as monomers for condensation reactions and reactive building blocks [31]. One notable application of these upcycled terephthalamides is their use in supramolecular cationic nanoassemblies (Fig. 4B), which increases their antimicrobial activity [35, 36]. Terephthalamides can be converted into rigid hydrogen-bonding units by incorporating aromatic bisurea groups. This modification directs the anisotropic self-assembly of cationic block copolymers and small molecules into nanocylinders and nanofibers in aqueous environments. Moreover, cationic nanoassemblies, characterized by their high aspect ratio, demonstrate shape-dependent antimicrobial effects, highlighting their potential in biomedical and antimicrobial applications. This result motivated us to work on anisotropic polymer assemblies formed by block copolymers of poly(ethylene glycol) and PLA with similar rigid hydrogen-bonding units, which demonstrated a shape-dependent effect on cell growth [37].
The chemical recycling of PET has evolved into a maturing research field, with efforts focused on developing effective and robust catalyst systems, such as a thermally stable salt catalyst comprising TBD and methanesulfonic acid [38]. Organocatalysts facilitate transesterification, expanding their applicability to a broader range of carbonyl-containing condensation polymers, including polycarbonates and polyurethanes [39, 40]. BPA-PC is a widely used aromatic polycarbonate and a common engineering plastic found in applications ranging from windshields in transportation to housing materials for oxygenators. Compared with commodity plastics, engineering plastics possess greater physical and thermal stability, making them more challenging to recycle and reprocess. However, achieving a zero-waste future necessitates the recycling of these poorly degradable polymers, even through chemical methods. Sardon et al. explored the organocatalytic chemical recycling and upcycling of polyurethanes and BPA-PC [41, 42]. Their work revealed a unique alcoholysis mechanism for BPA-PC using diols with varying methylene spacers (Fig. 4C) [42, 43]. When diols with two to four methylene units act as nucleophiles, cyclic carbonates are preferably formed. In contrast, longer diols favor the formation of linear carbonate diols. The resulting six- and seven-membered cyclic carbonates undergo ROP, whereas linear carbonate diols undergo transesterification and polycondensation, producing APCs [42, 44]. The upcycled APCs demonstrate promising efficiency as polymer electrolytes for lithium-ion secondary batteries, highlighting their potential for sustainable material development.
APCs exhibit potential biodegradability, as exemplified by PTMC, which has been utilized in sutures because of its biodegradation in vivo and in the presence of enzymes [45, 46]. However, PTMC is not readily degraded in noncatalytic aqueous environments and remains highly resistant to decomposition, even under alkaline conditions [47]. The mass of a PTMC (Mn 69 kg mol–1) disc with a diameter of 10 mm was reduced by 26% in a lipase solution for 20 weeks at 37 °C, whereas it was unchanged in alkaline solutions at pH 10 and 13 after 8 weeks at 37 °C [45]. Consequently, APC materials may require catalytic chemical recycling strategies, particularly if they are applied as sustainable green materials. To explore this possibility, we investigated the catalytic solvolysis of PTMC (Mn 45 kg mol–1) as a model reaction (Fig. 4D) [48]. Hydrolysis was initially considered owing to the environmentally benign properties of water. Several organic acids and bases were tested, but no complete PTMC hydrolysis was achieved under ambient conditions (20–25 °C). Among the catalysts screened, the phosphazene base P2-tBu exhibited the highest catalytic activity, yielding 40% conversion, followed by TBD and DBU. The hydrolysis of polycarbonates generates carbonic acid, which mitigates the effectiveness of basic catalysts. Although acidic catalysts are expected to perform well, they also have insufficient catalytic efficiency. At elevated temperatures (100 °C), basic organocatalysts significantly enhanced hydrolysis, achieving more than 90% conversion [48]. A recent report by Yu demonstrated that PTMC was successfully degraded by DPP-catalyzed hydrolysis at 160 °C [49]. Alcoholysis is a more frequently employed method for the degradation of PET and BPA-PC, particularly when basic organocatalysts are used [29, 39]. Unlike hydrolysis, alcoholysis avoids the formation of acidic degradation products. Methanol, now regarded as a green solvent owing to advancements in C1 chemistry and CO2 transformation [50], was selected to evaluate the organocatalytic methanolysis of PTMC. Because PTMC is insoluble in methanol, the reaction occurs heterogeneously. Among the tested catalysts, TBD demonstrated the highest catalytic efficiency, completing PTMC degradation within 3 h at room temperature. The reaction rate depended strongly on the catalyst concentration: with 0.01 M TBD, degradation required 36 h, whereas increasing the concentration to 0.1 M TBD reduced the reaction time to 2.5 h, maintaining the same PTMC-to-catalyst ratio (10:1). Under identical conditions, other catalysts, including P2-tBu and DBU, achieved only 20% degradation [48]. The TBD-catalyzed methanolysis of BPA-PC under similar conditions (BPA-PC: 0.5 mmol, TBD: 0.01 mmol, methanol: 1 mL) achieved 30% degradation in 12 h at room temperature [51]. The superior catalytic activity of TBD can be attributed to its dual hydrogen bonding activation (Fig. 4A), which has been demonstrated to function effectively in solvents with high dielectric constants [27]. This mechanistic advantage explains its prominent catalytic performance in PTMC methanolysis.
PTMC analogs with side chain functionalities exhibit biodegradability in the presence of enzymes [52, 53]. Recent studies have explored the enhancement of the physical properties of PTMC analogs by incorporating liquid-crystalline (LC) aromatic triad esters, which enables the formation of multiblock structures (Fig. 5A) [54]. The LC moieties self-assemble, inducing nanosegregation, which leads to enhanced elasticity and an increased modulus. However, these self-assembled nanostructures also improve the degradation resistance. The TBD-catalyzed methanolysis of the PTMC analog (APC) was complete within a few hours, whereas the multiblock form containing LC moieties degraded significantly more slowly, requiring ~200 h. This finding suggests that the degradation of the PTMC main chain is influenced by self-assembly and rigid aromatic structures, demonstrating the impact of molecular organization on polymer stability.
Degradation of aromatic ester-containing polymers. A Aromatic ester (ArE)-APC multiblock polymers (MBPs). Adapted with permission from ref. [54]. Copyright 2022 American Chemical Society. B TBD-mediated methanolysis of Vectra®, a wholly aromatic polyester. Adapted with permission from ref. [55]. Copyright 2024 American Chemical Society
The aforementioned study also revealed that the rigid aromatic triad ester underwent degradation under the tested conditions, an unexpected result that expanded the scope of degradation to wholly aromatic polyesters, including LC polyesters. These materials, classified as super engineering plastics, are known for their exceptional thermal and mechanical properties, making their recycling and degradation significantly more challenging than those of PET and BPA-PC because of their superior physical stability. However, achieving effective recycling and degradation of these high-performance plastics, which are widely used in electronic devices, aerospace materials, and medical devices, is essential for advancing a circular plastic economy. To address this challenge, the organocatalytic degradation of LC polyesters was examined via Vectra®, a poly(4-hydroxybenzoate-co-9-hydroxynaphthoate) copolymer [55]. TBD demonstrated superior catalytic activity compared with all the other tested organic acids and bases, efficiently promoting the methanolysis of Vectra® at 73 °C. Unlike PET degradation, which proceeds with catalytic amounts of TBD, the complete degradation of Vectra® requires an equimolar ratio of TBD to ester bonds. Additionally, a relatively long reaction time (~100 h) is necessary to achieve full degradation (Fig. 5B). These specific results are explained by degradation products containing acidic phenol moieties mitigating TBD and the self-assembled structure of the LC polymer, which compromises the access of methanol molecules to ester bonds. Nonetheless, compared with other catalytic systems, TBD is highly efficient at degrading super engineering plastics, which typically require excess catalyst loading and severe heating above 100 °C [56, 57].
The efficient separation and recovery of degradation products are important for industrial feasibility. A 2011 report demonstrated that the TBD-catalyzed glycolysis of PET results in high-purity BHET from the reaction mixture upon cooling [27]. TBD dissolves in EG, and thus, a crystallized form of BHET is recovered in a straightforward manner by simple filtration. The TBD-containing EG can be reused for the glycolysis of PET several times, although the catalytic activity is reduced [27]. Alternatively, TBD is removed by ion-exchange resins to clean EG. Terephthalamides produced by the aminolysis of PET (Fig. 4B) are also readily separated from the reaction mixture [31]. They often solidify after the reaction and do not dissolve in common solvents, including water. Thus, solvents are used to remove the unreacted amines and TBD to recover the product, terephthalamides, with trituration. Methanolysates of an LC polyester (Fig. 5B) can also be cleaned by ion-exchange resins [55]. No side products are formed during methanolysis. Thus, high-purity monomers are obtained after the evaporation of methanol as the reaction medium. Alkaline reagents such as NaOH are economically feasible for degrading polyesters and polycarbonates [32, 51]. However, the approach used to recover the degradation products may not be as straightforward as organocatalytic approaches are [58, 59]. This is another advantage of using organocatalysts in chemical recycling and upcycling.
Aliphatic polycarbonates and polyesters with side-chain-promoted degradability
Aliphatic polyesters and polycarbonates with side-chain functionalities have been extensively developed for functional degradable biomaterial applications [60, 61]. Functionalized APCs with diverse side groups derived from 2,2-bis(hydroxymethyl)propionic acid (bisMPA) have attracted significant attention owing to their well-established synthesis and controlled polymerization of cyclic carbonates with a substituent linked via an ester bond [18, 61]. The use of organocatalysts has facilitated their controlled polymerization under mild conditions, effectively minimizing side reactions [8, 9, 62]. Since the pendant ester bond is potentially active for transesterification, inappropriate polymerization conditions and catalysts induce chain transfer, resulting in cross-linking and a branched structure [63]. Thus, the APCs and polyesters with carbonyl-containing side groups presented hereinafter could not have been developed without organocatalysts. Efficient monomer synthesis is crucial for ensuring economic feasibility in polymer production. Conventional methods, which involve protection and deprotection steps, require four to five synthetic steps to obtain the target functionalized monomers, MPA-CCs [64]. A breakthrough in cyclic carbonate synthesis was achieved with bis(pentafluorophenyl)carbonate (PFC), which enabled the two-step synthesis of MPA-CCs directly from bisMPA (Fig. 6A) [65]. However, PFC-based synthesis has limitations, including high cost and the formation of the toxic byproduct pentafluorophenol, necessitating alternative approaches. A notable advancement in monomer synthesis involved the development of the ammonium carboxylate form of bisMPA-based cyclic carbonate (MPA-CCTEA). This approach enables one-step synthesis of cyclic carbonate monomers from bisMPA while also serving as a universal precursor for MPA-CCs via SN2-type esterification with alkyl bromides [66]. The availability of multiple synthetic pathways for cyclic carbonate monomers expands access to a wide range of side-chain functionalities in APCs. Consequently, bisMPA-based APCs (MPA-PCs) have been widely developed and utilized as functionalized degradable biomaterials.
Ether-functionalized hydrolyzable polymers with hydration-driven degradability. A Efficient synthetic pathway for MPA-CC and its applications in ether-functionalized biocompatible APCs. B Biocompatible and biodegradable MPA-PC (ME). Adapted with permission from ref. [52]. Copyright 2017 American Chemical Society. C Ether-functionalized APC (PMDO) and polydioxanone (PMAcDX) derived from glycerol. D Schematic representation of hydrolysis promoted by the hydration of ether side groups at the interface
The biological functions of MPA-PCs have been extensively evaluated; however, the impact of side chain modifications on their degradability remains largely unexplored. Recent studies have highlighted the importance of hydration at the biointerface in the design of biomaterials with enhanced blood compatibility [67, 68]. Inspired by poly(2-methoxyethyl acrylate), a clinically applied blood-compatible polymer [69], several ether-functionalized MPA-PCs were synthesized (Fig. 6A) [52, 66]. Compared with PTMC, these materials exhibit reduced platelet adhesion, a property attributed to surface hydration, as supported by water contact angle measurements and the thermal properties of hydrated polymers. Additionally, ether-functionalized MPA-PC (ME) demonstrated lower enzymatic susceptibility to lipase-mediated degradation than PTMC did because of its hydrated surface, which hindered enzyme recognition (Fig. 6B) [52]. This result inspired the possible control of the hydrolysis of APCs by side-chain hydration. Other ether-functionalized polymers, including polycarbonate (PMDO) and polyester (PMAcDX) derived from glycerol, a renewable biomass resource, were also originally designed to exhibit high biocompatibility, facilitated by side-chain hydration (Fig. 6C). Additionally, they showed high susceptibility to hydrolysis [70, 71]. Notably, all these polymers remain non-water soluble. Therefore, these results suggest that hydration enhanced by the ether side groups at the polymer interface sufficiently promotes the hydrolysis of aliphatic polymers (Fig. 6D).
The potential for controlling aliphatic condensation polymer degradation through side-chain engineering was subsequently investigated. BisMPA-based APCs featuring different ether side chains were developed through postfunctionalization of a common APC precursor with a consistent degree of polymerization (DP) (Fig. 7) [72]. The side-chain exchange reaction proceeded quantitatively via amidation of an activated ester, specifically pentafluorophenyl ester, although the reaction rate was influenced by amine bulkiness and nucleophilicity. The hydrophilicity of the side chains was directly correlated with the hydration properties and wettability of MPA-PCs, which in turn affected their hydrolytic susceptibility. Notably, MPA-PCs with highly hydrated surfaces degraded within 1 month under neutral conditions (pH 7.4 in phosphate saline buffer, 37 °C; inset table in Fig. 7). Additionally, the amide linker in the MPA-PC side chains possibly functioned as a catalyst, promoting hydrolytic degradation of the aliphatic carbonate backbone [73]. Under alkaline conditions (0.1 M NaOD, 37 °C; inset table in Fig. 7), MPA-PCs containing amide linkers underwent complete degradation, regardless of their side group structure, whereas MPA-PCs with ester linkers (ME, Fig. 6B) and PMDO (Fig. 6C) degraded with an efficiency of less than 30%, and PTMC remained largely intact. While amide groups increase hydration, the significantly greater hydrolyzability of MEA than that of ME can be attributed to the catalytic contribution of the amide linker (Fig. 7).
Poly(ε-caprolactone) (PCL) is recognized as a marine-degradable polymer [74, 75], and its functionalized derivatives hold promise for sustainable and biomedical applications [76]. Ether-functionalized PCL analogs are also expected to show biocompatibility and hydrolyzability driven by side chain hydration. Thus, an ε-caprolactone (CL) analog with an ether pendant group (CL1) was synthesized, and its ROP was investigated (Fig. 8) [77]. The ring-opening of CL1 proceeded efficiently in the presence of multiple catalysts, including organic bases, acids, and organometallic compounds. However, the resulting products did not consist predominantly of polymeric compounds with high DPs. Detailed experimental and computational analyses revealed that the reaction yielded five-membered lactones (GBL(Ax) and GBL1) via internal transesterification of the ring-opened species (CL1-O). This occurs when the terminal OH group preferentially attacks the side ester bond located four bonds away from the OH group over the lactone ester group of another monomer (Fig. 8A). The formation of five-membered lactones is thermodynamically driven, as they exhibit high stability and are generally nonpolymerizable, except in rare cases [78]. Comparative studies have shown that basic and organometallic catalysts slightly enhance intramolecular cyclization relative to acidic catalysts, suggesting that terminal OH group activation plays a more critical role than carbonyl activation does (Fig. 8B–D). A similar preference was observed in the ring-opening reaction of an amide-substituted CL analog of CL1 [77], deviating from Lang’s findings [76], where bulky substituents inhibited intramolecular cyclization, forming PCL derivatives. This is exceptional for ester formation from amide compounds, which is thermodynamically unfavorable. Finally, copolymerization with CL provided linear polymers with Mn values of 4–6 kg mol–1 [77]. These results demonstrate that specific side chain configurations thermodynamically favor depolymerization by backbiting reactions. In contrast, these substituted CLs may expand to a degradation system promoted by side chains, placing less hindered pendant groups with ester and amide linkers at the γ-position.
A Proposed mechanism for the formation of five-membered lactones GBL(Ax) and GBL1 via the ring-opening reaction of CL1. Plausible activation models of the ring-opened structure CL1-O by acidic catalysts (B), basic catalysts (C), and Sn(Oct)2 (D). Adapted with permission from ref. [77]. Copyright 2023 Royal Society of Chemistry
Degradable polymers should be designed for the efficient recovery of monomers and degradation products to contribute to the future circular economy of polymers. ArE/APC-MBP (Fig. 5A) is degraded by TBD-catalyzed methanolysis. However, the degradates contain several components because the polymer comprises multiple units with different cleavable bonds [54]. MPA-PCs with an ester linker (Fig. 6A, B) may also not be suitable for the facile recovery of the degradation products because more than two components are formed, including oligomers. In contrast, those with an amide linker (Fig. 7), which were completely degraded by alkaline hydrolysis in 1 month, provided almost a single degradation product, maintaining the amide-linked pendant group [72]. The recovery of cyclic carbonate monomers from the degradates should be possible. Thus, this platform is promising for polymer circulation, although actual trials have not been conducted. The polydioxanone derivative PMAcDX is depolymerizable via a backbiting reaction, forming the corresponding cyclic monomers, owing to the ring-chain equilibrium at a relatively low temperature range [71]. These analogs may serve as recyclable polymers. Similarly, P(CL1) may be regarded as an upcyclable polymer, provided that effective applications of GBL1 are found (Fig. 8A).
Conclusion
The recycling and upcycling of plastics, along with the development of degradable functionalized polymers, play critical roles in achieving net-zero emissions. Carbonyl-containing condensation polymers, such as polyesters and polycarbonates, are particularly suitable targets for chemical recycling and upcycling, as their degradation via transesterification is relatively facile. These processes enable the recovery of high-purity monomers and derivatives, enhancing the circularity of polymer materials. Super engineering plastics are essential in advanced technologies, including communication devices and aerospace applications; however, their recycling remains a significant challenge. The use of organic catalysts offers a promising approach to improve the efficiency of degradation processes for these high-performance materials, as well as the recovery of degradation products. Moreover, aliphatic polyesters and polycarbonates serve as ideal platforms for degradable functional polymers. Significant advancements have been made in monomer synthesis and selective and controlled polymerization via organocatalysis; however, further progress is needed to develop greener synthetic methods and increase the utilization of renewable resources as starting materials. Additionally, the degradation behavior of these polymers, including the environmental impact of degradation products, must be thoroughly investigated to ensure their long-term sustainability and circularity. The macromolecular design should also consider how efficiently the monomers and degradation products can be recovered for reuse. The observed transesterification behavior using the organic catalysts may be exploited to develop other sustainable and recyclable materials, such as vitrimers.
References
https://environment.ec.europa.eu/topics/waste-and-recycling/end-life-vehicles_en. Accessed 14 Mar 2023.
Global Plastics Outlook: economic drivers, environmental impacts and policy options. Paris: OECD Publishing; 2022. p. 31–59. https://doi.org/10.1787/de747aef-en. Accessed 14 Mar 2023.
An introduction to plastic recycling. Tokyo: Plastic Waste Management Institute (PWMI); 2022. p. 16–27. https://www.pwmi.or.jp/ei/plastic_recycling_2022.pdf Accessed 14 Mar 2023.
Stewart J, Fuchs M, Payne J, Driscoll O, Kociok-Köhn G, Ward BD, et al. Simple Zn(ii) complexes for the production and degradation of polyesters. RSC Adv. 2022;12:1416–24.
Kim JG. Chemical recycling of poly(bisphenol A carbonate). Polym Chem. 2020;11:4830–49.
Esquer R, García JJ. Metal-catalysed Poly(Ethylene) terephthalate and polyurethane degradations by glycolysis. J Organomet Chem. 2019;902:120972.
Nederberg F, Connor EF, Möller M, Glauser T, Hedrick JL. New paradigms for organic catalysts: the first organocatalytic living polymerization. Angew Chem Int Ed. 2001;40:2712–5.
Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL. Organocatalytic ring-opening polymerization. Chem Rev. 2007;107:5813–40.
Fukushima K, Nozaki K. Organocatalysis: a paradigm shift in the synthesis of aliphatic polyesters and polycarbonates. Macromolecules. 2020;53:5018–22.
Pratt RC, Lohmeijer BGG, Long DA, Waymouth RM, Hedrick JL. Triazabicyclodecene: a simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J Am Chem Soc. 2006;128:4556–7.
Nyce GW, Glauser T, Connor EF, Möck A, Waymouth RM, Hedrick JL. In situ generation of carbenes: a general and versatile platform for organocatalytic living polymerization. J Am Chem Soc. 2003;125:3046–56.
Kamber NE, Tsujii Y, Keets K, Waymouth RM, Pratt RC, Nyce GW, et al. The depolymerization of poly(ethylene terephthalate) (PET) using N-heterocyclic carbenes from ionic liquids. J Chem Educ. 2010;87:519–21.
Shapiro AJ, Brigandi PJ, Moubarak M, Sengupta SS, Epps TH III. Cross-linked polyolefins: opportunities for fostering circularity throughout the materials lifecycle. ACS Appl Polym Mater. 2024;6:11859–76.
Hermens JGH, Jensma A, Feringa BL. Highly efficient biobased synthesis of acrylic acid. Angew Chem Int Ed. 2022;61:202112618.
Stadler BM, Wulf C, Werner T, Tin S, de Vries JG. Catalytic approaches to monomers for polymers based on renewables. ACS Catal. 2019;9:8012–67.
Fukushima K, Kimura Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym Int. 2006;55:626–42.
Becker G, Wurm FR. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem Soc Rev. 2018;47:7739–82.
Fukushima K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater Sci. 2016;4:9–24.
Fukushima K. Biodegradable functional biomaterials exploiting substituted trimethylene carbonates and organocatalytic transesterification. Polym J. 2016;48:1103–14.
List B, Lerner RA, Barbas CF. Proline-catalyzed direct asymmetric aldol reactions. J Am Chem Soc. 2000;122:2395–6.
Ahrendt KA, Borths CJ, MacMillan DWC. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels-Alder reaction. J Am Chem Soc. 2000;122:4243–4.
Choi J, Hermans TM, Lohmeijer BGG, Pratt RC, Dubois G, Frommer J, et al. Monolayered organosilicate toroids and related structures: a phase diagram for templating from block copolymers. Nano Lett. 2006;6:1761–4.
Saito T, Aizawa Y, Tajima K, Isono T, Satoh T. Organophosphate-catalyzed bulk ring-opening polymerization as an environmentally benign route leading to block copolyesters, end-functionalized polyesters, and polyester-based polyurethane. Polym Chem. 2015;6:4374–84.
Bourissou D, Martin-Vaca B, Dumitrescu A, Graullier M, Lacombe F. Controlled cationic polymerization of lactide. Macromolecules. 2005;38:9993–8.
Coady DJ, Fukushima K, Horn HW, Rice JE, Hedrick JL. Catalytic insights into acid/base conjugates: highly selective bifunctional catalysts for the ring-opening polymerization of lactide. Chem Commun. 2011;47:3105–7.
Basterretxea A, Jehanno C, Mecerreyes D, Sardon H. Dual organocatalysts based on ionic mixtures of acids and bases: a step toward high temperature polymerizations. ACS Macro Lett. 2019;8:1055–62.
Fukushima K, Coulembier O, Lecuyer JM, Almegren HA, Alabdulrahman AM, Alsewailem FD, et al. Organocatalytic depolymerization of poly(ethylene terephthalate). J Polym Sci Part A Polym Chem. 2011;49:1273–81.
Horn HW, Jones GO, Wei DS, Fukushim K, Coady DJ, Lecuyer JM, et al. Mechanisms of organocatalytic amidation and trans-esterification of aromatic esters as a model for the depolymerization of poly(ethylene) terephthalate. J Phys Chem A. 2012;116:12389–98.
Fukushima K, Coady DJ, Jones GO, Almegren HA, Alabdulrahman AM, Alsewailem FD, et al. Unexpected efficiency of cyclic amidine catalysts in depolymerizing poly(ethylene terephthalate) under specific conditions. J Polym Sci Part A Polym Chem. 2013;51:1606–11.
Olazabal I, Gabirondo E, Jehanno C, Fukushima K, Sardon H. Chapter 9: Upcycling of polyesters. In: Greener J, Cakmak M, editors. Polyester films: materials, processes and applications. John Wiley & Sons, Inc.; 2023. p. 343–63.
Fukushima K, Lecuyer JM, Wei DS, Horn HW, Jones GO, Al-Megren HA, et al. Advanced chemical recycling of poly(ethylene terephthalate) through organocatalytic aminolysis. Polym Chem. 2013;4:1610–6.
Shukla SR, Harad AM. Aminolysis of polyethylene terephthalate waste. Polym Degrad Stab. 2006;91:1850–4.
Fukushima K, Jones GO, Horn HW, Rice JE, Kato T, Hedric JL. Formation of bis-benzoxazole and bis-benzimidazole through organocatalytic depolymerization of poly(ethylene terephthalate) and its mechanism. Polym Chem. 2020;11:4904–13.
Yu C, Guo X, Shen B, Xi Z, Li Q, Yin Z, et al. One-pot formic acid dehydrogenation and synthesis of benzene-fused heterocycles over reusable AgPd/WO2.72 nanocatalyst. J Mater Chem A. 2018;6:23766–72.
Fukushima K, Tan JPK, Korevaar PA, Yang YY, Pitera J, Nelson A, et al. Broad spectrum antimicrobial supramolecular assemblies with distinctive size and shape. ACS Nano. 2012;6:9191–9.
Fukushima K, Liu S, Engler AC, Coady DJ, Maune H, Pitera J, et al. Supramolecular high aspect ratio assemblies with strong antifungal activity. Nat Commun. 2013;4:2861.
Fukushima K, Matsuzaki K, Oji M, Higuchi Y, Watanabe G, Suzuki Y, et al. Anisotropic, degradable polymer assemblies driven by a rigid hydrogen-bonding motif that induce shape-specific cell responses. Macromolecules. 2022;55:15–25.
Jehanno C, Flores I, Dove AP, Müller AJ, Ruipérez F, Sardon H. Organocatalysed depolymerisation of PET in a fully sustainable cycle using thermally stable protic ionic salt. Green Chem. 2018;20:1205–12.
Quaranta E, Sgherza D, Tartaro G. Depolymerization of poly(bisphenol A carbonate) under mild conditions by solvent-free alcoholysis catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene as a recyclable organocatalyst: a route to chemical recycling of waste polycarbonate. Green Chem. 2017;19:5422–34.
Sardon H, Pascual A, Mecerreyes D, Taton D, Cramail H, Hedrick JL. Synthesis of polyurethanes using organocatalysis: a perspective. Macromolecules. 2015;48:3153–65.
Olazabal I, González A, Vallejos S, Rivilla I, Jehanno C, Sardon H. Upgrading polyurethanes into functional ureas through the asymmetric chemical deconstruction of carbamates. ACS Sustain Chem Eng. 2023;11:332–42.
Saito K, Jehanno C, Meabe L, Olmedo J, Mecerreyes D, Fukushima K, et al. From plastic waste to polymer electrolytes for batteries through chemical upcycling of polycarbonate. J Mater Chem A. 2020;8:13921–6.
Olazabal I, Luna E, De Meester S, Jehanno C, Sardon H. Upcycling of BPA-PC into trimethylene carbonate by solvent assisted organocatalyzed depolymerization. Polym Chem. 2023;14:2299–307.
Fukushima K. Chapter 7: ROP of cyclic carbonates. In: Dove A, Sardon H, Naumann S, editors. Organic catalysis for polymerization. Royal Society of Chemistry; 2019. p. 274–327.
Zhang Z, Kuijer R, Bulstra SK, Grijpm DW, Feijen J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials. 2006;27:1741–8.
Hou Z, Chen S, Li Z, Chen Z, Hu J, Guo J, et al. Controllable degradation of poly (trimethylene carbonate) via self-blending with different molecular weights. Polym Degrad Stab. 2021;189:109596.
Haramiishi Y, Chanthaset N, Kan K, Akashi M, Ajiro H. Contrast effect on hydrolysis of poly(trimethylene carbonate) depending on accelerated species due to the hydrophilic oligo(ethylene glycol) units at side groups. Polym Degrad Stab. 2016;130:78–82.
Fukushima K, Watanabe Y, Ueda T, Nakai S, Kato T. Organocatalytic depolymerization of poly(trimethylene carbonate). J Polym Sci. 2022;60:3489–500.
Wu W, Zhai H, Wu K, Wang X, Rao W, Ding J, et al. Cheap organocatalyst diphenyl phosphate for efficient chemical recycling of poly(lactic acid), other polyesters and polycarbonates. Chem Eng J. 2024;480:148131.
Jiang X, Nie X, Guo X, Song C, Chen JG. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem Rev. 2020;120:7984–8034.
Do T, Baral ER, Kim JG. Chemical recycling of poly(bisphenol A carbonate): 1,5,7-Triazabicyclo[4.4.0]-dec-5-ene catalyzed alcoholysis for highly efficient bisphenol A and organic carbonate recovery. Polymer. 2018;143:106–14.
Fukushima K, Inoue Y, Haga Y, Ota T, Honda K, Sato C, et al. Monoether-tagged biodegradable polycarbonate preventing platelet adhesion and demonstrating vascular cell adhesion: a promising material for resorbable vascular grafts and stents. Biomacromolecules. 2017;18:3834–43.
Ajiro H, Haramiishi Y, Chanthaset N, Akashi M. Polymer design using trimethylene carbonate with ethylene glycol units for biomedical applications. Polym J. 2016;48:751–60.
Watanabe Y, Kato R, Fukushima K, Kato T. Degradable and nanosegregated elastomers with multiblock sequences of biobased aromatic mesogens and biofunctional aliphatic oligocarbonates. Macromolecules. 2022;55:10285–93.
Watanabe Y, Fukushima K, Kato T. Degradation of a wholly aromatic main-chain thermotropic liquid-crystalline polymer mediated by superbases. JACS Au. 2024;4:2944–56.
Minami Y, Inagaki Y, Tsuyuki T, Sato K, Nakajima Y. Hydroxylation-depolymerization of oxyphenylene-based super engineering plastics to regenerate arenols. JACS Au. 2023;3:2323–32.
Minami Y, Honobe R, Inagaki Y, Sato K, Yoshida M. Alcoholysis of oxyphenylene-based super engineering plastics mediated by readily available bases. Polym J. 2024;56:369–77.
McNeeley A, Liu YA. Assessment of PET depolymerization processes for circular economy. 2. process design options and process modeling evaluation for methanolysis, glycolysis, and hydrolysis. Ind Eng Chem Res. 2024;63:3400–24.
Pham DD, Cho J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2021;23:511–25.
Fukushima K, Fujiwara T. Chapter 6: New routes to tailor-made polyesters. In: Scholz C, editor. Polymers for biomedicine: synthesis, characterization, and applications. John Wiley & Sons, Inc; 2017. p. 149–189.
Yu W, Maynard E, Chiaradia V, Arno MC, Dove AP. Aliphatic polycarbonates from cyclic carbonate monomers and their application as biomaterials. Chem Rev. 2021;121:10865–907.
Dove AP. Organic catalysis for ring-opening polymerization. ACS Macro Lett. 2012;1:1409–12.
Pastusiak M, Jaworska J, Kawalec M, Kasperczyk J, Dobrzynski P. Obtaining aliphatic branched polycarbonates via simple copolymerization of trimethylene carbonate with cyclic carbonate containing pendant ester groups. J Polym Sci, Part A Polym Chem. 2017;55:808–19.
Pratt RC, Nederberg F, Waymouth RM, Hedrick JL. Tagging alcohols with cyclic carbonate: a versatile equivalent of (meth)acrylate for ring-opening polymerization. Chem Commun. 2008;114–6.
Sanders DP, Fukushima K, Coady DJ, Nelson A, Fujiwara M, Yasumoto M, et al. A simple and efficient synthesis of functionalized cyclic carbonate monomers using a versatile pentafluorophenyl ester intermediate. J Am Chem Soc. 2010;132:14724–6.
Watanabe Y, Takaoka S, Haga Y, Kishi K, Hakozaki S, Narumi A, et al. Organic carboxylate salt-enabled alternative synthetic routes for bio-functional cyclic carbonates and aliphatic polycarbonates. Polym Chem. 2022;13:5193–9.
Tanaka M, Kobayashi S, Murakami D, Aratsu F, Kashiwazaki A, Hoshiba T, et al. Design of polymeric biomaterials: the “intermediate water concept. Bull Chem Soc Jpn. 2019;92:2043–57.
Nishida K, Anada T, Tanaka M. Roles of interfacial water states on advanced biomedical material design. Adv Drug Deliv Rev. 2022;186:114310.
Tanaka M, Motomura T, Kawada M, Anzai T, Yuu K, Shiroya T, et al. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)–relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials. 2000;21:1471–81.
Montagna V, Takahashi J, Tsai MY, Ota T, Zivic N, Kawaguchi S, et al. Methoxy-functionalized glycerol-based aliphatic polycarbonate: organocatalytic synthesis, blood compatibility, and hydrolytic property. ACS Biomater Sci Eng. 2021;7:472–81.
Fukushima K, Ota Y, Kato T. Polydioxanone derivative bearing methoxy groups toward bio-functional degradable polymers exhibiting hydration-driven biocompatibility. Macromol Chem Phys. 2022;223:2200192.
Fukushima K, Hakozaki S, Lang R, Haga Y, Nakai S, Narumi A, et al. Hydrolyzable and biocompatible aliphatic polycarbonates with ether-functionalized side chains attached via amide linker. Polym J. 2024;56:431–42.
Van Guyse JFR, Leiske MN, Verjans J, Bernhard Y, Hoogenboom R. Accelerated post-polymerization amidation of polymers with side-chain ester groups by intramolecular activation. Angew Chem Int Ed. 2022;61:e202201781.
Suzuki M, Tachibana Y, Kasuya K. Biodegradability of poly(3-hydroxyalkanoate) and poly(ε-caprolactone) via biological carbon cycles in marine environments. Polym J. 2025;53:47–66.
Wang G-X, Huang D, Ji J-H, Völker C, Wurm FR. Seawater-degradable polymers—fighting the marine plastic pollution. Adv Sci. 2021;8:2001121.
Wen L, Zhang S, Xiao Y, He J, Zhu S, Zhang J, et al. Organocatalytic ring-opening polymerization toward poly(γ-amide-ε-caprolactone)s with tunable lower critical solution temperatures. Macromolecules. 2020;53:5096–104.
Ota T, Montagna V, Higuchi Y, Kato T, Tanaka M, Sardon H, et al. Organocatalyzed ring-opening reactions of γ-carbonyl-substituted ε-caprolactones. RSC Adv. 2023;13:27764–71.
Hong M, Chen EY-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat Chem. 2016;8:42–9.
Acknowledgements
This study was supported by JST PRESTO (JPMJPR21N7), JST-Mirai Program (JPMJMI21EH), and JSPS KAKENHI (JP19H05716 and JP23H02019). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fukushima, K. Degradation technologies for condensation polymers mediated by organic catalysts. Polym J 57, 1083–1094 (2025). https://doi.org/10.1038/s41428-025-01069-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41428-025-01069-x