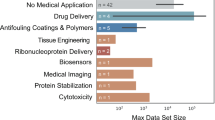
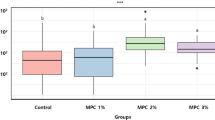
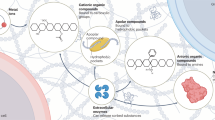
Abstract
Medical devices are indispensable in modern medicine for saving patients with injuries or illnesses and improving their quality of life. The development of polymers that elicit minimal biological reactions upon contact with tissues or biological components is critical for the safe and effective use of these devices. 2-Methacryloyloxyethyl phosphorylcholine (MPC) polymers, invented in Japan, represent a milestone in bioinspired materials science because they have been designed to mimic the phosphorylcholine groups naturally present on cell membranes, endowing them with excellent biocompatibility and resistance to nonspecific protein adsorption. Diverse MPC polymer architectures have been synthesized to optimize functional expression and tailor surface properties. Moreover, molecular designs have enabled the modification of conventional polymers, metals, and ceramics, thereby expanding their utility in diverse biomedical applications. This review highlights the molecular design concept, interfacial properties, and current status of MPC polymers as highly reliable surface treatment materials and summarizes their broad implementation in medical devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83.
Zhao J, Ruan J, Lv G, Shan Q, Fan Z, Wang H, et al. Cell membrane-based biomimetic nanosystems for advanced drug delivery in cancer therapy: a comprehensive review. Colloids Surf B Biointerfaces. 2022;215:112503.
Miyata Y, Segawa K. Protocol to analyze lipid asymmetry in the plasma membrane. STAR Protoc. 2022;3:101870.
Weinstein JN, Leserman LD. Liposomes as drug carriers in cancer chemotherapy. Pharm Ther. 1984;24:207–33.
Yamaguchi T, Mizushima Y. Lipid microspheres for drug delivery from the pharmaceutical viewpoint. Crit Rev Ther Drug Carr Syst. 1994;11:215–29.
Hub HH, Hupfer B, Koch H, Ringsdorf H. Polymerizable phospholipid analogues—New stable biomembrane and cell models. Angew Chem Int Ed Engl. 1980;19:938–40.
Hayward JA, Johnston DS, Chapman D. Polymeric phospholipids as new biomaterials. Ann N Y Acad Sci. 1985;446:267–81.
Ishihara K, Ueda T, Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym J. 1990;23:355–60.
Bonte F, Hsu MJ, Papp A, Wu K, Regen SL, Juliano RL. Interactions of polymerizable phosphatidylcholine vesicles with blood components: relevance to biocompatibility. Biochim Biophys Acta. 1987;900:1–9.
Ishihara K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J Biomed Mater Res A. 2019;107:933–43.
Ueda T, Oshida H, Kurita K, Ishihara K, Nakabayashi N. Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym J. 2002;24:1259–69.
Lobb EJ, Ma I, Billingham NC, Armes SP, Lewis AL. Facile synthesis of well-defined, biocompatible phosphorylcholine-based methacrylate copolymers via atom transfer radical polymerization at 20 °C. J Am Chem Soc. 2001;123:7913–14.
Ma I, Lobb EJ, Billingham NC, Armes SP, Lewis AL, Lloyd AW, et al. Synthesis of biocompatible polymers. 1. Homopolymerization of 2-methacryloyloxyethyl phosphorylcholine via ATRP in protic solvents: an optimization study. Macromolecules. 2002;35:9306–14.
Adibnia V, Olszewski M, De Crescenzo G, Matyjaszewski K, Banquy X. Superlubricity of zwitterionic bottlebrush polymers in the presence of multivalent ions. J Am Chem Soc. 2020;142:14843–47.
Chantasirichot S, Inoue Y, Ishihara K. Photoinduced atom transfer radical polymerization in a polar solvent to synthesize a water-soluble poly(2-methacryloyloxyethyl phosphorylcholine) and its block-type copolymers. Polymer. 2015;61:55–60.
Yusa SI, Fukuda K, Yamamoto T, Ishihara K, Morishima Y. Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules. 2005;6:663–70.
Inoue Y, Watanabe J, Takai M, Yusa SI, Ishihara K. Synthesis of sequence-controlled copolymers from extremely polar and apolar monomers by living radical polymerization and their phase-separated structures. J Polym Sci Part A: Polym Chem. 2005;43:6073–83.
Bhuchar N, Deng Z, Ishihara K, Narain R. Detailed study of the reversible addition-fragmentation chain transfer polymerization and co-polymerization of 2-methacryloyloxyethyl phosphorylcholine. Polym Chem. 2011;2:632–39.
Ishihara K, Iwasaki Y, Nakabayashi N. Polymeric lipid nanosphere consisting of water soluble poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate). Polym J. 1999;31:1231–6.
Monge S, Canniccioni B, Graillot A, Robin JJ. Phosphorus-containing polymers: a great opportunity for the biomedical field. Biomacromolecules. 2011;12:1973–82.
Iwasaki Y. Photoassisted Surface modification with zwitterionic phosphorylcholine polymers for the fabrication of ideal biointerfaces. Langmuir. 2023;39:15417–30.
Seetasang S, Xu Y. Recent progress and perspectives in applications of 2-methacryloyloxyethyl phosphorylcholine polymers in biodevices at small scales. J Mater Chem B. 2022;10:2323–37.
Matsuno R, Takami K, Ishihara K. Simple synthesis of a library of zwitterionic surfactants via Michael-type addition of methacrylate and alkane thiol compounds. Langmuir. 2010;26:13028–32.
Takami K, Matsuno R, Ishihara K. Synthesis of polyurethanes by polyaddition using diol compounds with methacrylate-derived functional groups. Polymer. 2011;52:5445–51.
Ye SH, Jang YS, Yun YH, Shankarraman V, Woolley JR, Hong Y, et al. Surface modification of a biodegradable magnesium alloy with phosphorylcholine (PC) and sulfobetaine (SB) functional macromolecules for reduced thrombogenicity and acute corrosion resistance. Langmuir. 2013;29:8320–7.
Kadoma Y, Nakabayashi N, Masuhara E, Yamauchi J. Synthesis and hemolysis test of the polymer containing phosphorylcholine groups. Kobunshi Ronbunshu. 1978;35:423 (Japanese Journal of Polymer Science and Technology)(in Japanese).
Fukushima S, Kadoma Y, Nakabayashi N. Interaction between polymer containing phosphorylcholine group and cells. Kobunshi Ronbunshu. 1983;40:785–93. (Japanese Journal of Polymer Science and Technology)(in Japanese).
Nakaya T, Toyoda H, Imoto M. Polymeric phospholipid analogues XIII. Synthesis and properties of vinyl polymers containing phosphatidyl choline groups. Polym J. 1986;18:881–5.
Yang H, Zheng Q, Cheng R. New insight into “polyelectrolyte effect. Colloids Surf A Physicochem Eng Asp. 2012;407:1–8.
Ratner BD. Surface modification of polymers: chemical, biological and surface analytical challenges. Biosens Bioelectron. 1995;10:797–804.
Amoako K, Ukita R, Cook KE. Antifouling zwitterionic polymer coatings for blood-bearing medical devices. Langmuir. 2025;41:2994–3006.
Iwashita H, Itokawa T, Suzuki T, Okajima Y, Kakisu K, Hori Y. Evaluation of in vitro wettability of soft contact lenses using tear supplements. Eye Contact Lens. 2021;47:244–8.
Fujiwara N, Yumoto H, Miyamoto K, Hirota K, Nakae H, Tanaka S, et al. 2-Methacryloyloxyethyl phosphorylcholine(MPC)-polymer suppresses an increase of oral bacteria: a single-blind, crossover clinical trial. Clin Oral Investig. 2019;23:739–6.
Ayaki M, Iwasawa A, Niwano Y. Cytotoxicity assays of new artificial tears containing 2-methacryloyloxyethyl phosphorylcholine polymer for ocular surface cells. Jpn J Ophthalmol. 2011;55:541–6.
Kanekura T, Nagata Y, Miyoshi H, Ishihara K, Nakabayashi N, Kanzaki T. Beneficial effects of synthetic phospholipid polymer, poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate), on stratum corneum function. Clin Exp Dermatol. 2002;27:230–4.
Cho Lee AR, Moon H, Ishihara K. Stabilization of lipid lamellar bilayer structure of stratum corneum modulated by poly(2-methacryloyloxyethyl phosphorylcholine) in relation to skin hydration and skin protection. Tissue Eng Regen Med. 2021;18:953–62.
Kihara S, Yamazaki K, Litwak KN, Litwak P, Kameneva MV, Ushiyama H, et al. In vivo evaluation of a MPC polymer coated continuous flow left ventricular assist system. Artif Organs. 2003;27:188–92.
Fujii K, Matsumoto HN, Koyama Y, Iwasaki Y, Ishihara K, Takakuda K. Prevention of biofilm formation with a coating of 2-methacryloyloxyethyl phosphorylcholine polymer. J Vet Med Sci. 2008;70:167–73.
Kaneko T, Saito T, Shobuike T, Miyamoto H, Matsuda J, Fukazawa K, et al. 2-Methacryloyloxyethyl phosphorylcholine polymer coating inhibits bacterial adhesion and biofilm formation on a suture: An in vitro and in vivo study. Biomed Res Int. 2020;2020:5639651.
Pappalardo F, Della Valle P, Crescenzi G, Corno C, Franco A, Torracca L, et al. Phosphorylcholine coating may limit thrombin formation during high-risk cardiac surgery: a randomized controlled trial. Ann Thorac Surg. 2006;81:886–91.
Iida Y, Hongo K, Onoda T, Kita Y, Ishihara Y, Takabayashi N, et al. Use of catheter with 2-methacryloyloxyethyl phosphorylcholine polymer coating is associated with long-term availability of central venous port. Sci Rep. 2021;11:5385.
Nishida K, Sakakida M, Ichinose K, Uemura T, Uehara M, Kajiwara K, et al. Development of a ferrocene-mediated needle-type glucose sensor covered with newly designed biocompatible membrane, 2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate. Med Prog Technol. 1995;21:91–103.
Ranucci M, Isgrò G, Soro G, Canziani A, Menicanti L, Frigiola A. Reduced systemic heparin dose with phosphorylcholine coated closed circuit in coronary operations. Int J Artif Organs. 2004;27:311–9.
Marguerite S, Levy F, Quessard A, Dupeyron JP, Gros C, Steib A. Impact of a phosphorylcholine-coated cardiac bypass circuit on blood loss and platelet function: a prospective, randomized study. J Extra Corpor Technol. 2012;44:5–9.
Ishihara K, Fukumoto K, Miyazaki H, Nakabayashi N. Improvement of hemocompatibility on a cellulose dialysis membrane with a novel biomedical polymer having a phospholipid polar group. Artif Organs. 1994;18:559–64.
Whelan DM, van der Giessen WJ, Krabbendam SC, van Vliet EA, Verdouw PD, Serruys PW, et al. Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart. 2000;83:338–45.
Collingwood R, Gibson L, Sedlik S, Virmani R, Carter AJ. Stent-based delivery of ABT-578 via a phosphorylcholine surface coating reduces neointimal formation in the porcine coronary model. Catheter Cardiovasc Inter. 2005;65:227–32.
Hagen MW, Girdhar G, Wainwright J, Hinds MT. Thrombogenicity of flow diverters in an ex vivo shunt model: effect of phosphorylcholine surface modification. J Neurointerv Surg. 2017;9:1006–11.
Ikeya K, Iwasa F, Inoue Y, Fukunishi M, Takahashi N, Ishihara K, et al. Inhibition of denture plaque deposition on complete dentures by 2-methacryloyloxyethyl phosphorylcholine polymer coating: A clinical study. J Prosthet Dent. 2018;119:67–74.
Moro T, Takatori Y, Ishihara K, Konno T, Takigawa Y, Matsushita T, et al. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mater. 2004;3:829–36.
Ishihara K, Nakabayashi N, Fukumoto K, Aoki J. Improvement of blood compatibility on cellulose dialysis membrane. I. Grafting of 2-methacryloyloxyethyl phosphorylcholine on to a cellulose membrane surface. Biomaterials. 1992;13:145–9.
Kyomoto M, Ishihara K. Self-initiated surface graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on poly(ether ether ketone) by photoirradiation. ACS Appl Mater Interfaces. 2009;1:537–42.
Shi X, Cantu-Crouch D, Sharma V, Pruitt J, Yao G, Fukazawa K, et al. Surface characterization of a silicone hydrogel contact lens having bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer layer in hydrated state. Colloids Surf B Biointerfaces. 2021;199:111539.
Yoneyama T, Ishihara K, Nakabayashi N, Ito M, Mishima Y. Short-term in vivo evaluation of small-diameter vascular prosthesis composed of segmented poly(etherurethane)/2-methacryloyloxyethyl phosphorylcholine polymer blend. J Biomed Mater Res. 1998;43:15–20.
Hasegawa T, Iwasaki Y, Ishihara K. Preparation and performance of protein-adsorption-resistant asymmetric porous membrane composed of polysulfone/phospholipid polymer blend. Biomaterials. 2001;22:243–51.
Ueda H, Watanabe J, Konno T, Takai M, Saito A, Ishihara K. Asymmetrically functional surface properties on biocompatible phospholipid polymer membrane for bioartificial kidney. J Biomed Mater Res A. 2006;77:19–27.
Ishihara K, Nishiuchi D, Watanabe J, Iwasaki Y. Polyethylene/phospholipid polymer alloy as an alternative to poly(vinylchloride)-based materials. Biomaterials. 2004;25:1115–22.
Iwasaki Y, Nakabayashi N, Ishihara K. In vitro and ex vivo blood compatibility study of 2-methacryloyloxyethyl phosphorylcholine (MPC) copolymer-coated hemodialysis hollow fibers. J Artif Organs. 2003;6:260–6.
Ye SH, Watanabe J, Takai M, Iwasaki Y, Ishihara K. In situ Modification on Cellulose Acetate Hollow Fiber Membrane Modified Phospholipid Polymer for Biomedical Application. J Membr Sci. 2005;249:133–45.
Konno T, Ito T, Takai M, Ishihara K. Enzymatic photochemical sensing using luciferase-immobilized polymer nanoparticles covered with artificial cell membrane. J Biomater Sci Polym Ed. 2006;17:1347–57.
Shimizu T, Goda T, Minoura N, Takai M, Ishihara K. Super-hydrophilic silicone hydrogels with interpenetrating poly(2-methacryloyloxyethyl phosphorylcholine) networks. Biomaterials. 2010;31:3274–80.
Iwasaki Y, Aiba Y, Morimoto N, Nakabayashi N, Ishihara K. Semi-interpenetrating polymer networks composed of biocompatible phospholipid polymer and segmented polyurethane. J Biomed Mater Res. 2000;52:701–8.
Ishihara K. Highly lubricated polymer interfaces for advanced artificial hip joint through biomimetic design. Polym J. 2015;47:585–97.
Iwata R, Suk-In P, Hoven VP, Takahara A, Akiyoshi K, Iwasaki Y. Control of nanobiointerfaces generated from well-defined biomimetic polymer brushes for protein and cell manipulations. Biomacromolecules. 2004;5:2308–14.
Feng W, Zhu S, Ishihara K, Brash JL. Adsorption of fibrinogen and lysozyme on silicon grafted with poly(2-methacryloyloxyethyl phosphorylcholine) via surface-initiated atom transfer radical polymerization. Langmuir. 2005;21:5980–7.
Zhang Z, Morse AJ, Armes SP, Lewis AL, Geoghegan M, Leggett GJ. Effect of brush thickness and solvent composition on the friction force response of poly(2-(methacryloyloxy)ethylphosphorylcholine) brushes. Langmuir. 2011;27:2514–21.
Jiang Y, Su Y, Zhao L, Meng F, Wang Q, Ding C, et al. Preparation and antifouling properties of 2-(meth-acryloyloxy)ethyl cholinephosphate based polymers modified surface with different molecular architectures by ATRP. Colloids Surf B Biointerfaces. 2017;156:87–94.
Futamura K, Matsuno R, Konno T, Takai M, Ishihara K. Rapid development of hydrophilicity and protein adsorption resistance by polymer surfaces bearing phosphorylcholine and naphthalene groups. Langmuir. 2008;24:10340–4. 16.
Kobayashi M, Terayama Y, Hosaka N, Kaido M, Suzuki A, Yamada N, et al. Friction behavior of high-density poly(2-methacryloyloxyethyl phosphorylcholine) brush in aqueous media. Soft Matter. 2007;3:740–6.
Ishihara K, Mu M, Konno T, Inoue Y, Fukazawa K. The unique hydration state of poly(2-methacryloyloxyethyl phosphorylcholine). J Biomater Sci Polym Ed. 2017;28:884–99.
Kitano H, Imai M, Mori T, Gemmei-Ide M, Yokoyama Y, Ishihara K. Structure of water in the vicinity of phospholipid analogue copolymers as studied by vibrational spectroscopy. Langmuir. 2003;19:10260–6.
Kitano H. Characterization of polymer materials based on structure analyses of vicinal water. Polym J. 2016;48:15–24.
Shiomoto S, Inoue K, Higuchi H, Nishimura SN, Takaba H, Tanaka M, et al. Characterization of hydration water bound to choline phosphate-containing polymers. Biomacromolecules. 2022;23:2999–3008.
Ishihara K. Biomimetic polymers with phosphorylcholine groups as biomaterials for medical devices. Proc Jpn Acad, Ser B. 2024;100:579–606.
Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir. 2001;17:2841–50.
Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A survey of structure−property relationships of surfaces that resist the adsorption of protein. Langmuir. 2001;17:5605–20.
Ishihara K. Biomimetic materials based on zwitterionic polymers toward human-friendly medical devices. Sci Technol Adv Mater. 2022;23:498–524.
Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption?. J Biomed Mater Res. 1998;39:323–30.
Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM. Protein adsorption from human plasma is reduced on phospholipid polymers. J Biomed Mater Res. 1991;25:1397–407.
Ishihara K. Blood-compatible surfaces with phosphorylcholine-based polymers for cardiovascular medical devices. Langmuir. 2019;35:1778–87.
Çelebioğlu EC, Blevrakis E, Yilmaz M, Doğan İ, Erkent FD, Kortun Ş, et al. Efficacy and performance of the new pipeline vantage flow diverter stent with shield technology: Short-term results of a single-center experience. Sci Prog. 2025;108:368504251349714.
Campbell EJ, O’Byrne V, Stratford PW, Quirk I, Vick TA, Wiles MC, et al. Biocompatible surfaces using methacryloylphosphorylcholine laurylmethacrylate copolymer. ASAIO J. 1994;40:M853–7.
Snyder TA, Tsukui H, Kihara S, Akimoto T, Litwak KN, Kameneva MV, et al. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: coating comparison and platelet activation. J Biomed Mater Res A. 2007;81:85–92.
Yamabe T. Artificial organs with nano-technology and development of the new diagnosis tool. Ann NanoBME. 2009;2:1–10.
https://www.evaheart-usa.com/clinical-trial?utm_source=chatgpt.com
Chen HB, Wang XQ, Du J, Shi J, Ji BY, Shi L, et al. Long-term outcome of EVAHEART I implantable ventricular assist device for the treatment of end stage heart failure: clinical 3-year follow-up results of 15 cases. Zhonghua Xin Xue Guan Bing Za Zhi. Chin J Cardiovascular Dis). 2023;51:393–99. (in Chinese).
Lewis AL, Stratford PW. A review on phosphorylcholine-coated stents. J Long Term Eff Med Implants. 2017;27:233–52.
Song PS, Hahn JY, Kim DI, Song YB, Choi SH, Choi JH, et al. Duration of clopidogrel-based dual antiplatelet therapy and clinical outcomes after endeavor sprint zotarolimus eluting stent implantation in patients presenting with acute coronary syndrome. Eur J Intern Med. 2015;26:521–7.
Caroff J, Tamura T, King RM. Phosphorylcholine surface modified flow diverter associated with reduced intimal hyperplasia. J Neurointerv Surg. 2018;10:1097–101.
Ishihara K, Iwasaki Y, Ebihara S, Shindo Y, Nakabayashi N. Photoinduced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on polyethylene membrane surface for obtaining blood cell adhesion resistance. Colloids Surf B Biointerfaces. 2000;18:325–35.
Kyomoto M, Moro T, Saiga K, Hashimoto M, Ito H, Kawaguchi H, et al. Biomimetic hydration lubrication with various polyelectrolyte layers on cross-linked polyethylene orthopedic bearing materials. Biomaterials. 2012;33:4451–9.
Moro T, Takatori Y, Kyomoto M, Ishihara K, Hashimoto M, Ito H, et al. Long-term hip simulator testing of the artificial hip joint bearing surface grafted with biocompatible phospholipid polymer. J Orthop Res. 2014;32:369–76.
Moro T, Takatori Y, Tanaka S, Ishihara K, Oda H, Kim YT, et al. Clinical safety and wear resistance of the phospholipid polymer-grafted highly cross-linked polyethylene liner. J Orthop Res. 2017;35:2007–16.
Kyomoto M, Moro T, Yamane S, Watanabe K, Hashimoto M, Takatori Y, et al. Poly(2-methacryloyloxyethyl phosphorylcholine) grafting and vitamin E blending for high wear resistance and oxidative stability of orthopedic bearings. Biomaterials. 2014;35:6677–86.
Kyomoto M, Moro T, Yamane S, Takatori Y, Tanaka S, Ishihara K. A hydrated phospholipid polymer-grafted layer prevents lipid-related oxidative degradation of cross-linked polyethylene. Biomaterials. 2017;112:122–32.
Kyomoto M, Moro T, Yamane S, Watanabe K, Hashimoto M, Tanaka S, et al. A phospholipid polymer graft layer affords high resistance for wear and oxidation under load bearing conditions. J Mech Behav Biomed Mater. 2018;79:203–12.
Goda T, Ishihara K. Soft contact lens biomaterials from bioinspired phospholipid polymers. Expert Rev Med Devices. 2006;3:167–74.
Walsh K, Jones LW, Morgan P, Papas EB, Sulley A. Topical review: Twenty-five years of silicone hydrogel soft contact lenses. Optom Vis Sci. 2025;102:361–74.
Ishihara K, Shi X, Fukazawa K, Yamaoka T, Yao G, Wu JY. Biomimetic-engineered silicone hydrogel contact lens materials. ACS Appl Bio Mater. 2023;6:3600–16.
Capote-Puente R, Sánchez-González JM, Sánchez-González MC, Bautista-Llamas MJ. Evaluation of Celligent® biomimetic water gradient contact lens effects on ocular surface and subjective symptoms. Diagnostics (Basel). 2023;13:1258.
Shi X, Sharma V, Cantu-Crouch D, Yao G, Fukazawa K, Ishihara K, et al. Nanoscaled morphology and mechanical properties of a biomimetic polymer surface on a silicone hydrogel contact lens. Langmuir. 2021;37:13961–7.
Sharma V, Shi XC, Yao G, Zheng Y, Spencer ND, Wu JY. Fluid confinement within a branched polymer structure enhances tribological performance of a poly(2-methacryloyloxyethyl phosphorylcholine)-surface-modified contact lens. R Soc Open Sci. 2024;11:240957.
Liang S, Zheng Y, Sharma V, Shows A, Dunbar DC, Shi X, et al. Surface and antifouling properties of a biomimetic reusable contact lens material. ACS Omega. 2025;10:19697–704.
Ishihara K, Fukazawa K, Sharma V, Liang S, Shows A, Dunbar DC, et al. Antifouling silicone hydrogel contact lenses with a bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer surface. ACS Omega. 2021;6:7058–67.
Harris V, Pifer R, Shannon P, Crary M. Comparative evaluation of pseudomonas aeruginosa adhesion to a poly-(2-methacryloyloxyethyl phosphorylcholine)-modified silicone hydrogel contact lens. Vis (Basel). 2023;7:27.
Mimura T, Nakagomi R. Comparison of non-water proof mascara adhesion on the surface of different two-week frequent replacement silicone hydrogel contact lenses. Clin Optom (Auckl). 2025;17:73–82.
Mimura T, Nakagomi R, Fujishima H. Comparison of asian dust adhesion on the urface of different reusable silicone hydrogel contact lenses. Int Ophthalmol. 2025;45:226.
Sin MC, Chen SH, Chang Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym J. 2014;46:436–43.
Hiranphinyophat S, Iwasaki Y. Controlled biointerfaces with biomimetic phosphorus-containing polymers. Sci Technol Adv Mater. 2021;22:301–16.
Moayedi S, Xia W, Lundergan L, Yuan H, Xu J. Zwitterionic polymers for biomedical applications: Antimicrobial and antifouling strategies toward implantable medical devices and drug delivery. Langmuir. 2024;40:23125–45.
Lv W, Wang Y, Fu H, Liang Z, Huang B, Jiang R, et al. Recent advances of multifunctional zwitterionic polymers for biomedical application. Acta Biomater. 2024;181:19–45.
Ahmed ST, Madinya JJ, Leckband DE. Ionic strength dependent forces between end-grafted Poly (sulfobetaine) films and mica. J Colloid Interface Sci. 2022;606:298–306.
Lien C-C, Chen P-J, Venault A, Tang S-H, Fu Y, Dizon GV, et al. A zwitterionic interpenetrating network for improving the blood compatibility of polypropylene membranes applied to leukodepletion. J Membr Sci. 2019;584:148–60.
Ye SH, Orizondo RA, De BN, Kim S, Frankowski BJ, Federspiel WJ, et al. Epoxy silane sulfobetaine block copolymers for simple, aqueous thromboresistant coating on ambulatory assist lung devices. J Biomed Mater Res A. 2024;112:99–109.
Xiang Y, Xu RG, Leng Y. Molecular understanding of ion effect on polyzwitterion conformation in an aqueous environment. Langmuir. 2020;36:7648–57.
Venault A, Ye CC, Lin YC, Tsai CW, Jhong JF, Ruaan RC, et al. Zwitterionic fibrous polypropylene assembled with amphiphatic carboxybetaine copolymers for hemocompatible blood filtration. Acta Biomater. 2016;40:130–41.
Lin X, Wu K, Zhou Q, Jain P, Boit MO, Li B, et al. Photoreactive carboxybetaine copolymers impart biocompatibility and inhibit plasticizer leaching on polyvinyl chloride. ACS Appl Mater Interfaces. 2020;12:41026–37.
Ryujin T, Shimizu T, Miyahara R, Asai D, Shimazui R, Yoshikawa T, et al. Blood retention and antigenicity of polycarboxybetaine-modified liposomes. Int J Pharm. 2020;586:119521.
Hu G, Emrick T. Functional choline phosphate polymers. J Am Chem Soc. 2020;138:1828–31.
Mukai M, Ihara D, Chu CW, Cheng CH, Takahara A. Synthesis and hydration behavior of a hydrolysis-resistant quasi-choline phosphate zwitterionic polymer. Biomacromolecules. 2020;21:2125–31.
Yu X, Yang X, Horte S, Kizhakkedathu JN, Brooks DE. A thermoreversible poly(choline phosphate) based universal biomembrane adhesive. Macromol Biosci. 2014;14:334–9.
Yao Y, Dang X, Qiao X, Li R, Chen J, Huang Z, et al. Crosslinked biomimetic coating modified stainless-steel-mesh enables completely self-cleaning separation of crude oil/water mixtures. Water Res. 2022;224:119052.
He K, Duan H, Chen GY. Cleaning of oil fouling with water enabled by zwitterionic polyelectrolyte coatings: Overcoming the imperative challenge of oil-water separation membranes. ACS Nano. 2015;9:9188–98.
Niu J, Wang H, Chen J. Bio-inspired zwitterionic copolymers for antifouling surface and oil-water separation. Colloids Surf A Physicochemical Eng Asp. 2021;626:127016.
Liu Q, Locklin J. Transparent grafted zwitterionic copolymer coatings that exhibit both antifogging and self-cleaning properties. ACS Omega. 2018;3:17743–50.
Ma MQ, Zhang C, Chen TT, Yang J, Wang JJ, Ji J, et al. Bioinspired polydopamine/polyzwitterion coatings for underwater anti-oil and -freezing surfaces. Langmuir. 2019;35:1895–901.
Taylor ME, Panzer MJ. Fully-zwitterionic polymer-supported ionogel electrolytes featuring a hydrophobic ionic liquid. J Phys Chem. 2018;122:8469–76.
Yoshizawa-Fujita M, Ohno H. Applications of zwitterions and zwitterionic polymers for Li-ion batteries. Chem Rec. 2023;23:e202200287.
Tadesse MY, Zhang Z, Marioni N, Zofchak ES, Duncan TJ, Ganesan V. Mechanisms of ion transport in lithium salt-doped zwitterionic polymer-supported ionic liquid electrolytes. J Chem Phys. 2024;160:024905.
Alsaedi MK, Tadesse MY, Ganesan V. Zwitterionic polymer ionogel electrolytes supported by coulombic cross-links: Impacts of alkali metal cation identity. J Phys Chem B. 2024;128:3273–81.
Kim H, Hight-Huf N, Kang JH, Bisnoff P, Sundararajan S, Thompson T, et al. Polymer zwitterions for stabilization of CsPbBr3 perovskite nanoparticles and nanocomposite films. Angew Chem Int Ed Engl. 2020;59:10802–6.
Zhang L, Gao J, You Z. A multifunctional phosphorylcholine-based polymer reduces energy loss for efficient perovskite solar cells. J Mater Chem C. 2022;10:16781–8.
Acknowledgements
The author would like to thank the Guest Editor of this special issue, Prof. Chie Kojima, Institute of Science Tokyo, for the invitation to contribute to the manuscript. The author deeply appreciates Dr. Kyoko Fukazawa of Alcon Research, LLC, for reviewing the draft manuscript and providing significant suggestions. Additionally, the author thanks Prof. Yasuhiko Iwasaki, Kansai University; Dr. Kenji Yamazaki, Hokkaido Cardiovascular Hospital; Prof. Toru Moro, The University of Tokyo; and Dr. Masayuki Kyomoto, Kyocera Co., for providing important information used in this review manuscript. The author would also like to thank all the staff, students, and collaborators who supported the execution of this research.
Author information
Authors and Affiliations
Contributions
KI wrote the manuscript alone.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ishihara, K. Invention and global impact of bioinspired 2-methacryloyloxyethyl phosphorylcholine polymers: molecular design, functions, and implementation in medical devices. Polym J (2025). https://doi.org/10.1038/s41428-025-01109-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41428-025-01109-6