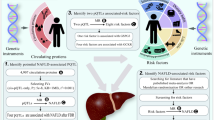
Abstract
Background
Observational studies have associated nonalcoholic fatty liver disease (NAFLD) with adverse pregnancy events, but findings show heterogeneity, leaving the causal direction and mediating pathways unclear. We aimed to investigate the causal relation between NAFLD and various pregnancy events, and to elucidate the underlying mediating pathways while determining the proportion of this correlation that is mediated through these pathways.
Methods
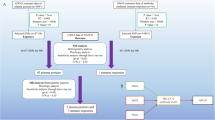
A genome-wide association study involving over 6 million participants employing Mendelian randomization (MR) and mediation analysis was performed. The study used genetically predicted NAFLD as exposures and cardiometabolic traits as mediators, with various adverse pregnancy events as outcomes. The main analysis was performed using the inverse variance weighted (IVW) approach, while sensitivity analyses included the weighted median, weighted mode, MR-Egger, and MR-PRESSO methods. Mediation analyses were performed using a two-step MR framework.
Results
In this MR cohort study, NAFLD was found to be strongly associated with elevated risks of GDM (P = 0.019 for the discovery dataset, P < 0.001 for the discovery dataset) and HDPs, including any HDP (P < 0.001 for the both datasets), gestational hypertension (P = 0.007 for the discovery dataset, P < 0.001 for the discovery dataset), and pre-eclampsia or eclampsia (P = 0.040 for the discovery dataset, P < 0.001 for the discovery dataset). However, no significant associations were found with hemorrhage in early pregnancy, postpartum hemorrhage, preterm birth, or offspring birthweight for both datasets. Cardiometabolic traits played a significant mediating role in these associations, rather than solely acting as confounding factors.
Conclusions
This study provided evidence supporting a correlation between NAFLD and a higher risk of adverse pregnancy events and introduces some new insights. These findings may inform preventions and interventions for remediating adverse pregnancy outcomes attributable to NAFLD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the GWAS datasets in this study were from primary published articles and publicly accessible summary data online, including FinnGen and UK Biobank et al. Further details and other data that support the findings of this study are available from the corresponding author upon request.
Code availability
The codes are available from the corresponding author upon request.
References
Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60–78.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61.
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21:619–29.e7.
Le MH, Yeo YH, Zou B, Barnet S, Henry L, Cheung R, et al. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical Bayesian approach. Clin Mol Hepatol. 2022;28:841–50.
Arshad T, Golabi P, Paik J, Mishra A, Younossi ZM. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatol Commun. 2019;3:74–83.
Sarkar M, Grab J, Dodge JL, Gunderson EP, Rubin J, Irani RA, et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73:516–22.
Hagstrom H, Hoijer J, Ludvigsson JF, Bottai M, Ekbom A, Hultcrantz R, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016;36:268–74.
Qian Y, Zhang Y, Fan X, Yan H, Li X, Fan Y, et al. Nonalcoholic fatty liver disease and adverse pregnancy events in women with normal prepregnant weight. J Clin Endocrinol Metab. 2023;108:463–71.
Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–36.
Creanga AA, Catalano PM, Bateman BT. Obesity in pregnancy. N Engl J Med. 2022;387:248–59.
Park SH, Kim DJ, Plank LD. Association of grip strength with non-alcoholic fatty liver disease: investigation of the roles of insulin resistance and inflammation as mediators. Eur J Clin Nutr. 2020;74:1401–9.
Mnatzaganian G, Woodward M, McIntyre HD, Ma L, Yuen N, He F, et al. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: an eight-year population-based panel study. BMC Pregnancy Childbirth. 2022;22:95.
Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health. 2010;100:1047–52.
Gross A, Lange M, Rosenbluth E, Carroll C, Sperling R, Juliano C, et al. Evaluation of 2-year outcomes in infants born to mothers with and without NAFLD in pregnancy. Eur J Pediatr. 2023;182:3765–74.
Chai TY, Byth K, George J, Pasupathy D, Cheung NW. Elevated hepatic steatosis index is associated with the development of adverse maternal, but not adverse neonatal, outcomes: a retrospective cohort study. Int J Women’s Health. 2023;15:589–98.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
Vujkovic M, Ramdas S, Lorenz KM, Guo X, Darlay R, Cordell HJ, et al. A multiancestry genome-wide association study of unexplained chronic ALT elevation as a proxy for nonalcoholic fatty liver disease with histological and radiological validation. Nat Genet. 2022;54:761–71.
Zeng L, Li Y, Hong C, Wang J, Zhu H, Li Q, et al. Association between fatty liver index and controlled attenuation parameters as markers of metabolic dysfunction-associated fatty liver disease and bone mineral density: observational and two-sample Mendelian randomization studies. Osteoporos Int. 2024;35:679–89.
Khan SS, Brewer LC, Canobbio MM, Cipolla MJ, Grobman WA, Lewey J, et al. Optimizing prepregnancy cardiovascular health to improve outcomes in pregnant and postpartum individuals and offspring: a scientific statement from the American Heart Association. Circulation. 2023;147:e76–91.
Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2018;28:166–74.
Mbatchou J, Barnard L, Backman J, Marcketta A, Kosmicki JA, Ziyatdinov A, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. 2021;53:1097–103.
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–13.
Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600:675–9.
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53:1415–24.
Wu Y, Zhong X, Lin Y, Zhao Z, Chen J, Zheng B, et al. Estimating genetic nurture with summary statistics of multigenerational genome-wide association studies. Proc Natl Acad Sci USA 2021;118:e2023184118.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–18.
Ye CJ, Kong LJ, Wang YY, Dou C, Zheng J, Xu M, et al. Mendelian randomization evidence for the causal effects of socio-economic inequality on human longevity among Europeans. Nat Hum Behav. 2023;7:1357–70.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9.
Lee SM, Kwak SH, Koo JN, Oh IH, Kwon JE, Kim BJ, et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62:238–48.
Lee SM, Jung YM, Choi ES, Kwak SH, Koo JN, Oh IH, et al. Metabolic dysfunction-associated fatty liver disease and subsequent development of adverse pregnancy events. Clin Gastroenterol Hepatol. 2022;20:2542–50.e8.
Hershman M, Mei R, Kushner T. Implications of Nonalcoholic Fatty Liver Disease on Pregnancy and Maternal and Child Outcomes. Gastroenterol Hepatol (N Y). 2019;15:221–8.
Lavery J, Friedman A, Keyes K, Wright J, Ananth C. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124:804–13.
McCance DR, Cassidy L. Diabetes in pregnancy. In: Textbook of diabetes. John Wiley & Sons Ltd. 2024. pp. 1034–71.
El Jamaly H, Eslick GD, Weltman M. Systematic review with meta-analysis: non-alcoholic fatty liver disease and the association with pregnancy events. Clin Mol Hepatol. 2022;28:52–66.
Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289.
Eslam M, El-Serag HB, Francque S, Sarin SK, Wei L, Bugianesi E, et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat Rev Gastroenterol Hepatol. 2022;19:638–51.
Zhang Z, Li L, Hu Z, Zhou L, Zhang Z, Xiong Y, et al. The causal associations of non-alcoholic fatty liver disease with blood pressure and the mediating effects of cardiometabolic risk factors: a Mendelian randomization study. Nutr Metab Cardiovasc Dis. 2023;33:2151–9.
Liu Z, Zhang Y, Graham S, Wang X, Cai D, Huang M, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 2020;73:263–76.
Xie J, Huang H, Liu Z, Li Y, Yu C, Xu L, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology. 2023;77:949–64.
Song X, Wang C, Wang T, Zhang S, Qin J. Obesity and risk of gestational diabetes mellitus: a two-sample Mendelian randomization study. Diabetes Res Clin Pract. 2023;197:110561.
Rogne T, Gill D, Liew Z, Shi X, Stensrud VH, Nilsen TIL, et al. Mediating factors in the association of maternal educational level with pregnancy events: a Mendelian Randomization Study. JAMA Netw Open. 2024;7:e2351166.
Ardissino M, Slob EAW, Millar O, Reddy RK, Lazzari L, Patel KHK, et al. Maternal hypertension increases risk of preeclampsia and low fetal birthweight: genetic evidence from a Mendelian Randomization Study. Hypertension. 2022;79:588–98.
Hosier H, Lipkind HS, Rasheed H, DeWan AT, Rogne T. Dyslipidemia and risk of preeclampsia: a multiancestry Mendelian Randomization Study. Hypertension. 2023;80:1067–76.
Chen Y, Mu L. Non-alcoholic fatty liver disease, pregnancy loss and preterm birth: a Mendelian Randomization study. Fertil Steril. 2023;120:e102.
Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131–41.
DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227:429–36.
Huang J, Meng X, Li J, Gong X, Wu T, Shi H, et al. Serum lipid reference values recommended during a twin pregnancy and evaluating its association with perinatal outcomes. BMC Pregnancy Childbirth. 2024;24:18.
Hu J, Gillies CL, Lin S, Stewart ZA, Melford SE, Abrams KR, et al. Association of maternal lipid profile and gestational diabetes mellitus: a systematic review and meta-analysis of 292 studies and 97,880 women. EClinicalMedicine. 2021;34:100830.
Steinthorsdottir V, McGinnis R, Williams NO, Stefansdottir L, Thorleifsson G, Shooter S, et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun. 2020;11:5976.
Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12:721–37.
Kiiskinen T, Mars NJ, Palviainen T, Koskela J, Rämö JT, Ripatti P, et al. Genomic prediction of alcohol-related morbidity and mortality. Transl Psychiatry. 2020;10:23.
Author information
Authors and Affiliations
Contributions
Cun Li had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Qiuyan Luo, Cun Li, and Yukui Huang conceived and designed the research methods. Qiuyan Luo, Guoting Liu, Qiulan Li, Jinghong Lu, and Wenjing Zheng collected and analyzed the data. Qiuyan Luo and Cun Li wrote the original draft. Yukui Huang reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All the GWAS datasets in this study were from publicly accessible summary data online, including FinnGen and UK Biobank et al. The approval for utilizing FinnGen data was granted by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District under reference number HUS/990/2017. Additionally, the ethical approvals for individual studies included in the analysis were detailed elsewhere [53]. For the UK Biobank data, ethical approval was obtained from the North West Multi-centre Research Ethics Committee (MREC). The study’s procedures were conducted in accordance with publicly accessible summary data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Q., Liu, G., Li, Q. et al. Novel insights into causal effects of maternal nonalcoholic fatty liver disease on adverse pregnancy outcomes: evidence from Human Genetics and Mendelian Randomization Study. Eur J Clin Nutr 78, 1041–1050 (2024). https://doi.org/10.1038/s41430-024-01489-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01489-7