Abstract
In paediatric oncology, genomics raises new ethical, legal and psychological issues, as somatic and constitutional situations intersect throughout the care pathway. The discovery of potential predisposition in this context is sometimes carried out outside the usual framework. This article focuses on the views of children, adolescents, and young adults (AYA) with cancer and their parents about their experience with genomic testing. Forty-eight semi-structured interviews were performed with children or AYAs with cancer and one of their parents, before and/or after receiving the genetic test results. The interviews were fully transcribed, coded and thematically analysed using an inductive method. This analysis revealed several themes that are key issues: perceived understanding and consenting, apprehension about the test outcomes (expectations and fears), perception and attitude towards incidental findings. The main expectation was an aetiological explanation. Children and AYAs also emphasised the altruistic meaning of genetic testing, while parents seemed to expect a therapeutic and preventive approach for their child and the rest of the family. Parents were more concerned about a family risk, while patients were more afraid of cancer relapse or transmission to their descendants. Both groups suggested possible feelings of guilt concerning family transmission and imaginary representations of what genomics may allow. Incidental findings were not understood by patients, while some parents perceived the related issues and hesitated between wanting or not to know. A multidisciplinary approach would be an interesting way to help parents and children and AYAs to better grasp the complexity of genetic and/or genomic testing.
Similar content being viewed by others
Introduction
In paediatric oncology, germline or somatic genome sequencing is proposed to characterise the cancer type, personalise therapy, obtain data on germline variants for preventive and familial implications, and for clinical research purposes. Thus, patients and their parents are confronted with situations where somatic or germline tests, targeted gene sequencing or genome sequencing intersect. Genome sequencing brings eventually information on constitutional variants linked to the current disease, but also additional data, for instance pathogenic variants not directly related to the initial indication (incidental findings or secondary findings), and variants of unknown significance apart from a usual consultation on genetic predisposition in patients [1,2,3]. Whatever the pathology, genetic testing is associated with psychological and ethical issues for the child, parents, and professionals [4,5,6,7,8]. Guidelines for genetic testing in adults have been published [9,10,11] and they should be adapted to children, adolescents, and young adults (AYAs) [5, 12, 13]. Studies on ethical and psychological issues associated with genetic and genomic testing in children and AYAs with cancer are limited. In a narrative review of 18 articles, we explored the perspectives of parents and to a lesser extent of children and AYAs with cancer and highlighted areas of ambivalence concerning the subjective implications of those tests (desire for treatment, desire for knowledge, uncertainty, and guilt) [14]. The aim of this qualitative study was, thanks to a suitable methodology, to understand how parents and also children and AYAs perceive genetic or genomic testing and to analyse their psychological implications, expectations, and representations before and after the result announcement, whatever the type of test proposed.
Methods
Context
GeneInfoKids is a national project financed by the French National Cancer Institute with three axes: ethical, legal and psychological issues raised by next generation sequencing for children and AYAs with cancer. The main objective of the psychological axis is to describe the psychological implications in families of patients undergoing genome sequencing. It includes a qualitative study (described in this article) and a quantitative study based on the themes defined by this qualitative study.
Participant recruitment
The inclusion criteria were children, adolescents and young adults (AYAs), also referred to as patients in the text, with cancer or past history of cancer age between 10 and 25 year at the time of inclusion in the study, having undergone somatic or constitutional testing in a clinical or research context (i.e. MAPPYACT) at one partner centre (Gustave Roussy, Hôpital Robert-Debré, Hôpital Armand-Trousseau, Institut Curie), and French speaking [15, 16]. Children and AYAs who met the inclusion criteria and at least one of their parents received an information leaflet by the clinical team. Then, a research psychologist contacted by phone the parents (for minors) or the patients directly (for adults), explained again the study, and validated their consent to participate.
Interviews
Interview guides were developed in a vocabulary and style suitable for parents and children and AYAs, based on literature data and on the clinical experience of the study investigators involved in genetic testing for childhood cancer [17] (supplementary materials). Two semi-structured interviews were planned: one after the genetic test proposition and one after the genetic test result announcement. In the first interview, the main discussion topics were: family and disease context, interest in genetic or genomic testing, knowledge of the possible result types (e.g. primary result, incidental findings), expectations and fears, consent decision-making modalities. In the second interview, the main topics were: knowledge about the possible results, associated emotions, match between expectations and results, consequences and implications of the results, temporality of genetic testing in the care pathway, need of a dedicated psychologist consultation. Only parents were asked to give their opinion on their preparation, from information to announcement. Information on the pathology and type of test performed were also collected.
Data analysis
All interviews were audio-recorded, transcribed and pseudonymized. They were analysed using MAXQDA 2020 with a systematic coding and thematic analysis using an inductive method [18]. Eight interviews were double coded to define the themes, the others were coded by one researcher and discussed with the other one. The final thematic analysis plan was discussed by three researchers. The number of occurrences corresponds to the number of patients or parents who stated these ideas which were questioned or not according to the clinical context and understanding.
Results
Description of the semi-structured interviews
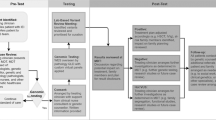
Interviews (n = 48) took place between July 2020 and May 2021. They lasted 15–90 min (mean: 35 min): 29 interviews (60%) were face-to-face in the hospital and 19 (40%) were by videoconference, due to the COVID-19 pandemic. In total, 21 families (19 children and AYAs and 18 parents) were interviewed before and after, only before, or only after the test (Fig. 1 and Table 1). Both parents had the opportunity to participate, but in only one family both parents were interviewed due to their availability. Among the families who agreed to be contacted for this study, five declined to participate (refusal by one parent) mainly because they felt overwhelmed or insufficiently informed on the genetic approach (confirmation rate: 80.8%). Therefore, we interviewed 14 patients with cancer and 13 parents before the test disclosure and 10 patients and 11 parents afterwards. The thematic analysis of the interviews led to the construction of four categories: children and AYAs, parents, before, and after the genetic results (Tables 2 and 3).
Patients’ description and genetic or genomic testing context
The GeneInfoKid interview took place within a year of the cancer diagnosis for the vast majority of patients, with the remainder, now young adults, having been diagnosed up to 10 years. The patients’ mean age was 14.4 years (10–24); 7 patients had a haematological malignancy and 14 a solid tumour. Children and AYAs underwent somatic testing (n = 5), germline and somatic testing (n = 7), and germline testing (n = 9) including also for research purposes in four families (Table 4). How genetic testing was proposed varied in function of the hospital and the indication: one or more genetic consultations dedicated to genetic/genomic testing (n = 7); genetic information given during a standard oncology consultation preceded by genetic counselling (n = 7); genetic information given during an oncology consultation (n = 6); and no genetic consultation (did not attend or did not remember attending it (n = 1).
Factors influencing genetic testing consent decision-making
The families’ feelings about consenting to genetic testing seemed to be influenced by how the test was proposed (i.e. consultation type, clinical and psychological context, perception of the type of test and medical indications).
Antagonistic feelings between genetic testing and cancer
Eight families described antagonistic feelings concerning genetic testing in the context of cancer. Cancer was described as a time of urgency and suspended present, due to the disease traumatic intrusion and death anxiety. It left little room to decide, “it leaves no choice to decide, whatever it is” [mother]. Some families explained their indifference and distance concerning genetic testing by its perceived lack of immediate usefulness for cancer treatment. Four families facing a therapeutic impasse initially showed few explicit expectations and distanced themselves from genetic testing. However, as the interview progressed, they expressed a form of last therapeutic hope concerning genetic testing. Four families would have liked to have more time to think about genetic testing to better understand what was done. Three parents would have preferred to wait until the treatment end.
Psychological availability and understanding
Most families showed only a relative psychological availability to genetic testing that influenced their understanding. As a protection when faced with too much information in a difficult context, some interviewees used some defence mechanisms. Four children and AYAs described this psychic unavailability: “I was so down at that moment that even if I had been informed, it would have been useless. I wouldn’t have understood…” [patient, 15–25 years]. This influenced the families’ capacity to consent. Some did not remember consenting to genetic testing, others vaguely, with a feeling of accumulating information “without really digesting it” [mother].
During the first interview before result, children and AYAs expressed their relative (6/14) or complete (5/14) lack of understanding of genetic testing. Four did not even know the word genetic. Two had forgotten they had a dedicated consultation (confirmed by the teams and parents). In the second interview after genetic test result, most children and AYAs (6/10) reported a lack of knowledge about genetic testing. One patient said that the adults had not told her about it. Five patients forgot about the result or most of the consultation or did not really understand the results and their implications. Only one patient (15–25 years) seemed to have generally understood the genetic results, but not the more complex information.
Before result one parent (1/13) reported a good understanding of genetic testing, four parents had a partial understanding, and two parents partially or completely forgot about it. After the results, most parents (7/11) described a form of psychological unavailability that may have hindered their understanding; two parents understood very little of the results, and two were completely unaware of the results. Two parents felt that they had understood the results and their implications. One of them said that the preparatory work (drawing the family tree) before the genetic consultation helped him to better understand the results. Two parents relied on their trust in medical professionals, “I assume that they too are doing their best to find a cure for her. […] I don’t want to see it any other way […] it risks traumatizing my conscience (laughs)” [mother]. The degree of understanding was also related to the initial expectations concerning genetic testing. When expectations were low (e.g. therapeutic impasse), families felt not implicated and did not try to understand.
Influence of the consultation type
In function of the genetic testing indication and hospital, information and/or the results were given in different contexts. The patients’ discourses suggested that these differences influenced their understanding and feelings about the received information.
In the first interview, parents who discussed about genetic testing (indication and results) during one or more dedicated onco-genetic consultation, better understood the involved issues and started to think about incidental findings (9/13). Conversely, parents who received genetic information during an oncology consultation (4/13) said that their attention was focused on their child’s cancer rather than on genetic investigations. Similarly, in the second interview, attention was more on the cancer than on the results when the genetic results were transmitted during an oncological consultation (4/11), as testified by a mother: “in the end it almost… erased all this questioning about genetic predisposition”. Genetic counselling helped some patients to better understand (3/14), but not others (4/14) due to concerns about their recovery, cognitive side effects, and young age. Children and AYAs who attended a onco-genetic consultation were more familiar with the meaning of the word genetics. Moreover, most parents and patients remembered having met a psychologist or psychiatrist during the cancer care pathway, but only two for a discussion on genetics.
Somatic/germline testing, a blurred perception by families
The understanding of the different test types by families (Table 1) was variable and some could not distinguish them (11/37). One parent who understood the difference between somatic and germline tests did not know exactly which test type was offered to his child. Three parents who understood that a tumour analysis was done to find therapeutic adjustments, expressed a sense of urgency that left little time to think about the other potential implications of results. Concerning testing done for research purposes (n = 4), one parent wondered about its temporality and the possible updating of his child’s consent when adult. One child felt that by entering a research protocol, he gave some sort of consent for germline testing. Two patients said that it was essential to participate in research but did not remember what the objective was.
Is there room for an informed decision?
Some parents justified their consent by the healthcare professional’s explanation: search of the cancer cause (4/13), tumour characterisation and treatment optimisation (2/13), research purposes (2/13), and possible family prevention (2/13). A mother explained that the rarity of her child’s cancer led the healthcare professionals to propose genetic testing. Most children and AYAs (7/14) said that they were influenced by the adults, “it was my parents who wanted to do it” [patient, 10–14 years]. They remembered particularly the adults’ explanations on aetiological research and treatment. Before the test, decision-making varied. Among children and AYAs, some accepted without hesitation (2/14), while others (2/14) felt that they had not really decided, and they needed more explanations or time to form a more precise opinion (1/14). Some parents (3/13) accepted without hesitation and with a sense of urgency. Two parents expressed a sense of responsibility, or even a moral duty, to undergo genetic testing for the family, and thus the absence of a real choice, “we don’t want anyone in our family to go through what we went through […] I say to myself, it’s our responsibility […] for our children, and our nephews and nieces” [mother]. Two parents felt that they had not really consented, two forgot that they had given their consent, and another was uneasy about the test purpose.
What place for children and AYAs?
When genetic testing was discussed and when the results were given, children and AYAs were sometimes not present. Some did not want or could not participate, but others did not know about it. A 16-year-old patient said, after some hesitation, that she would have liked to have been informed and present to talk about genetic testing. For others, discussion with their parents helped to better understand the issues.
In the first interview, most parents (7/13) said that they had discussed about genetic testing with their child to ensure that they were well informed and agreed to participate. Two parents did not discuss about genetic testing with their children and AYAs because they felt that they were psychologically unavailable. Four parents remembered that the healthcare professionals spoke directly to the children and AYAs and one parent emphasised the adjusted speech used: “ she addressed him in very simple words. And… she let him talk a lot and rephrase what she was saying to make sure he understood” [mother].
Concerning their child’s role during the result consultation, some parents remembered an active (2/11) or a more withdrawn presence (3/11). One parent noted that their child was not present at this consultation. Two parents said that they could not talk about genetic testing with their child because they were too preoccupied with the ongoing cancer treatment. One parent seemed to regret this: “we quickly moved on to other things while, in fact, for her I think it was important and… I realize that we didn’t discuss it again”. Two mothers remembered their child’s presence at the result consultation, whereas their children thought they were not there.
Thus, the children and AYAs’s place varied according to their age, their supposed maturity, the clinical and family situation, the parent-child relationship, and the place given by the healthcare professionals to them in this consultation.
Anticipating and reacting to the genetic test outcome
Anticipating the results: expectations and fears
In the first interview, patients and parents expressed expectations and fears. For some children and AYAs, the lack of knowledge about genetic testing made it difficult to anticipate the results. They listed as expectations: aetiological explanation (4/14), altruistic participation in research (4/14), possible prevention (3/14), and therapy adjustment (3/14).
The parents’ expectations were: characterisation of their child’s disease (4/13), explanation of their child’s cancer aetiology (8/13), targeted treatment (8/13) (in line with the test aim), and hope of a last chance (2/13) in case of therapeutic impasse, and possible prevention (8/13) for their child and family. Five parents did not really know what to expect, and five expressed ambivalent expectations: anxiety about the possible impact of the results and reassurance about the possible prevention.
The fears expressed by the children and AYAs were: risk of relapse (3/14), risk of transmission to their future children (3/14), not knowing what to expect in terms of results (3/14), imaginary and erroneous representations of what genetics would allow (3/14) (e.g. to find an environmental cause of the cancer, to give access to one’s whole identity). Two patients feared that a non-response concerning their cancer aetiology with genetic testing would leave them with a void of meaning about the disease. A young patient evoked and denied the guilt her parents could feel, “I’m not going to blame dad or mum, because they didn’t do it on purpose” [patient, 10–14 years].
Parents feared the discovery of a familial risk (5/13), not understanding the results and their implications (2/14) and feeling some guilt (2/14) if the genetic result was positive. Two expressed concerns, about the future uses of their sample.
Reactions to the genetic testing outcome
In the second interview, patients and parents described different emotions and reactions to the result announcement. Some children and AYAs distanced themselves from the results (5/10) because they did not affect their daily life, unlike cancer. Three expressed relief when the results validated the therapeutic strategy, confirmed that the disease would not be transmitted to their future children, or confirmed the links of filiation. Two expressed disappointment or ambivalent feelings because the results did not elucidate the cancer aetiology.
Most parents experienced relief (9/11) because: the results validated the therapy or allowed a therapeutic adjustment (5/11), there was no family risk (3/11). Five parents were disappointed because: the results did not provide an aetiological explanation (4/11), lacked details and explanations (3/11) unlike their initial expectations, and did not allow treatment adjustment. Others expressed uncertainty (3/11), distance (3/11), or surprise (2/11) at the results. Two parents reported ambivalence between the non-hereditary transmission and the lack of an aetiological explanation.
For some children and AYAs and parents, genetic testing led to updating their personal history, the nature of the family ties, and the search of a disease explanation, “I think that it is really the genetic test that has renewed um… the question… of the origin of the disease” [patient, 15–25 years]. Some participants started to rethink about their initial cancer theories, especially because the negative test result left a void. After the genetic result consultation, three parents (3/11) and one child (1/10) remembered the cancer diagnosis announcement and compared their meaning and intensity. For one patient and her mother, the results gave a sense of possible empowerment, with cancer prevention measures to be put in place.
Perception of incidental findings
The question “Did you expect other possible outcomes?” was not often asked, particularly to parents or patients with little knowledge about genetic testing. The concept of incidental findings was understood by one child (included in a research protocol), and by ten parents. For example, one mother (trio-based exome sequencing) heard that other discoveries were possible (predisposition to other cancers or diseases), but that such results would be given only if prevention was possible, which was acceptable for her. Three parents theoretically imagined the existence of incidental findings but did not relate them to their child. Six parents considered the discovery of incidental findings, which they called “predisposition” to other pathologies, for their child and/or the rest of the family. According to the information at our disposal, 14 children and AYAs (n = 7 germline testing and n = 7 mixed analysis) could have been concerned by a genomic technology with potential incidental data.
Participants expressed the desire to know about incidental findings to be better prepared, but also their fear. One mother said: “Well, my fear is that, through the illness she already had, more serious things will be discovered behind it”. After the genetic test result announcement, two parents expressed relief at the lack of incidental findings and one of them questioned the limit of his initial desire to know, “Although I said last time: we want to know… Yeah, up to a certain point, maybe”.
Discussion
This study identified some subjective representations and perceptions of children and AYAs with cancer and their parents before and after genetic testing. The main emerging issues were the diversity of feelings, of understanding and consenting, their apprehensions and perceptions of the results, and finally their ambivalent attitude towards incidental findings.
Children and AYAs were more concerned about the risk of cancer relapse and transmission to their offspring, and lack of answer on their cancer aetiology. Conversely, parents feared the family risk. Patients and parents talked about the possible feeling of guilt of the parents about family transmission. Healthcare teams should consider these representations because they influence the families’ listening and psychological availability [19, 20].
The perception of consenting was sometimes blurred. Some families expressed the fact that they did not feel they had had the choice of whether to undergo genetic testing, but that they trusted the health professionals in any case. This raises questions about the genetic testing consent validity, which is required by the French law (article 16 of the French Civil Code). Consent may have different value, such a symbolic one, and may be expected as a process for patients and their parents. We previously proposed that the delivery of results which does not concern the child’s treatment and direct care could entail a renewed consent to preserve the right not to know, but also to change one’s mind [20]. Discussions at a distance from the genetic proposal and urgent care, or even at the time of the child’s transition to adulthood, also should be considered [20, 21]. Therefore, it might be important to create spaces to talk again about what the family has agreed to and its implications during cancer treatment, remission, at the child’s age of majority, and even after the child’s death.
The understanding of the genetic proposition varied among parents and children and AYAs and according to the context, raising questions on how to better deliver information. The traditional onco-genetic consultation increases understanding [17, 22, 23]. For genomic proposition, other strategies have been experienced such as the two-visit consent model [24] or a systematic consultation with a genetic counsellor at the cancer treatment initiation [25]. For example, the construction of the family tree allows a first representation and understanding of the personal and family issues linked to a future genetic test. The timeframe of information transmission also could be improved. One parent suggested to give a document summarising the ongoing genetic testing process. Patients’ understanding was much poorer, and not only because of their age. This suggests that information for children and AYAs should be adapted to what they can and wish to hear, in a dynamic interchange, and with suitable supports [21, 26]. As psychological unavailability and forgetfulness could be protective mechanisms in children and AYAs with cancer and their parents, it may be necessary to give professionals the means to identify them. A psychologist consultation could be systematically proposed to identify the patients’ psychological readiness and to adapt the genomic pathway. Psychologists also can play an essential role in supporting families by discussing with them their representations, expectations, and emotions before and after the results [22, 23].
Some emotions and reactions to the result announcement were similar in children and AYAs and parents: relief, disappointment (due to the lack of aetiological explanation), distancing, low emotional distress, ambivalence. Conversely, others were specific to parents, such as uncertainty and surprise. The low distress level associated with the results could be linked to the cancer experience that overshadowed all other fears, as previously reported [3, 26]. These differences between children and AYAs and parents were previously described [3, 27] and highlight different expectations (altruism is more emphasised by adolescents, while hope for a cure is stronger among parents).
Lastly, it was often difficult to discuss about incidental findings because of the interviewees’ limited understanding of genetics. Parents with a relatively accurate understanding said that they wanted to know but were also afraid [3]. A recent study highlighted the parents’ strong expectations regarding updating the genomic results for their children when new information becomes available, but not after the child’s death for some of them [28].
Study limitations
As the study population was heterogeneous in terms of disease, treatment, prognosis and genomic test type (somatic or constitutional), the psychological issues experienced by children, AYAs and parents were different. Moreover, professionals involved were not the same in the different teams, with or without geneticist, genetic counsellor, or psychologist. We can consider that we have reached data saturation for most of the topics covered. However, we have not yet reached this criterion for some of them, particularly those relating to children and AYAs’ perspectives. As this study was entirely exploratory, we did not collect information on the parents’ socio-educational level, nor on the patient’s medical history, and this may perhaps limit certain interpretations of the responses.
Conclusion
Nevertheless, the qualitative approach enriched the understanding of the various and complex situations in real-life clinical situations. In conclusion, the interviewed families expressed varying levels of understanding and different apprehensions concerning genetic testing. Some families would have liked to have more time to think about the test meaning or at a distance from the acute phase. Therefore, it should be important to develop an extended time and step-by-step approach to explain genetic or genomic testing during dedicated multidisciplinary consultations or during the paediatric oncologic consultations with other healthcare professionals (genetic counsellor, psychologist).
The themes identified by the qualitative analysis will allow us to design specific questionnaires to be tested in larger populations in the quantitative study of the GeneInfoKid project. This will provide more information to understand the families’ expectations and needs, particularly on incidental findings, and to promote shared decision-making by healthcare professionals, parents, and children and AYAs.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
ABM Agence de Biomédecine. [Draft recommendations for good professional practice concerning the management of the results of a genome-wide sequencing examination not directly related to the initial indication in the context of care]. 2020. https://www.agence-biomedecine.fr/Conseil-d-orientation-126.
Kratz CP, Jongmans MC, Cavé H, Wimmer K, Behjati S, Guerrini-Rousseau L, et al. Predisposition to cancer in children and AYAs and adolescents. Lancet Child Adolesc Health. 2021;5:142–54.
Mandrell BN, Gattuso JS, Pritchard M, Caples M, Howard Sharp KM, Harrison L, et al. Knowledge is power: benefits, risks, hopes, and decision-making reported by parents consenting to next-generation sequencing for children and adolescents with cancer. Semin Oncol Nurs. 2021;37:151–67.
Bertier G, Sénécal K, Borry P, Vears DF. Unsolved challenges in pediatric whole-exome sequencing: a literature analysis. Crit Rev Clin Lab Sci. 2017;54:134–42.
Botkin JR, Belmont JW, Berg JS, Berkman BE, Bombard Y, Holm IA, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 2015;97:6–21.
Chassagne A, Pélissier A, Houdayer F, Cretin E, Gautier E, Salvi D, et al. Exome sequencing in clinical settings: preferences and experiences of parents of children with rare diseases (SEQUAPRE study). Eur J Hum Genet. 2019;27:701–10.
Houdayer F, Putois O, Babonneau ML, Chaumet H, Joly L, Juif C, et al. Secondary findings from next generation sequencing: psychological and ethical issues. Family and patient perspectives. Eur J Med Genet. 2019;62:103711.
Wade CH, Tarini BA, Wilfond BS. Growing up in the genomic era: implications of whole-genome sequencing for children, families, and pediatric practice. Annu Rev Genomics Hum Genet. 2013;14:535–55.
Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19:249–55.
Frey MK, Lee SS, Gerber D, Schwartz ZP, Martineau J, Lutz K, et al. Facilitated referral pathway for genetic testing at the time of ovarian cancer diagnosis: uptake of genetic counseling and testing and impact on patient-reported stress, anxiety and depression. Gynecol Oncol. 2020;157:280–6.
Forbes C, Fayter D, de Kock S, Quek RGW. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer. Cancer Manag Res. 2019;11:2321–37.
Matthijs G, Souche E, Alders M, Corveleyn A, Eck S, Feenstra I, et al. Guidelines for diagnostic next-generation sequencing. Eur J Hum Genet. 2016;24:2–5.
van El CG, Cornel MC, Borry P, Hastings RJ, Fellmann F, Hodgson SV, et al. Whole-genome sequencing in health care. Eur J Hum Genet. 2013;21:580–4.
Droin-Mollard M, Hervouet L, Lahlou-Laforet K, de Montgolfier S. Narrative review on ethical and psychological issues raised by genetic and genomic testing in pediatric oncology care. J Genet Couns. 2024 (under review)
Ferrari A, Stark D, Peccatori FA, Fern L, Laurence V, Gaspar N, et al. Adolescents and young adults (AYA) with cancer: a position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open. 2021;6. https://doi.org/10.1016/j.esmoop.2021.100096.
Berlanga P, Pierron G, Lacroix L, Chicard M, Adam de Beaumais T, Marchais A, et al. The European MAPPYACTS trial: precision medicine program in pediatric and adolescent patients with recurrent malignancies. Cancer Discov. 2022;12:1266–81.
Lahlou-Laforêt K, Consoli SM, Jeunemaitre X, Gimenez-Roqueplo AP. Presymptomatic genetic testing in minors at risk of paraganglioma and pheochromocytoma: our experience of oncogenetic multidisciplinary consultation. Horm Metab Res. 2012;44:354–8.
Paillé P, Mucchielli A. Qualitative analysis in the humanities and social sciences. Armand Colin publisher, Paris, France. 2012. https://doi.org/10.3917/arco.paill.2012.01.
de Montgolfier S, Hervouet L. [Imagination as a methodological lever for mobilizing ethical questioning: how can children with cancer and their parents be encouraged to think about the issues involved in consenting to genomic research?]. Rev Fr Ethique Appl. 2022;12:37–52.
Droin-Mollard M, Hervouet L, Lahlou-Laforêt K, de Montgolfier S. [Genomic propositions in oncopediatry: disruption of temporalities and ethical reference points—patients’, parents’ and professionals’ perspectives. Dolbeault]. S, Seigneur E, éditeurs. Psycho-Oncol. 2021;15:152–7.
de Montgolfier S, Hervouet L, Le Tirant S, Rial-Sebbag E. [Integrating the child’s opinion in care decisions: the case of consent to genetic investigations in oncopediatrics]. Anthropol Santé. 2021. https://doi.org/10.4000/anthropologiesante.9269.
Claret B, Brugières L, Guerrini-Rousseau L, Dauchy S, Gargiulo M. [Paediatric oncogenetic consultations: what place should be given to the child? How should we communicate with the child and his or her parents?]. Psycho-Oncol. 2018;12:46–9.
Vibert R, Lahlou-Laforêt K, Samadi M, Krivosic V, Blanc T, Amar L, et al. Minors at risk of von Hippel-Lindau disease: 10 years’ experience of predictive genetic testing and follow-up adherence. Eur J Hum Genet. 2022;30:1171–7.
Johnson LM, Sykes AD, Lu Z, Valdez JM, Gattuso J, Gerhardt E, et al. Speaking genomics to parents offered germline testing for cancer predisposition: use of a 2-visit consent model. Cancer. 2019;125:2455–64.
Simaga F, Bourdeaut F, Aerts I, Bouchoucha Y, Cordero C, Delattre O, et al. [Assessment of one year’s activity of systematic genetic information consultations in paediatric oncology in the era of very high throughput sequencing]. [Internet]. Rennes, France: 11ème Assises de la génétique humaines; 2022. https://assises2022.mycongressonline.net/Doc-Agenda_pdf.html.
Weber E, Shuman C, Wasserman JD, Barrera M, Patenaude AF, Fung K, et al. “A change in perspective”: exploring the experiences of adolescents with hereditary tumor predisposition. Pediatr Blood Cancer. 2019;66:e27445.
Waldman L, Hancock K, Gallinger B, Johnstone B, Brunga L, Malkin D, et al. Perspectives and Experiences of Parents and Adolescents Who Participate in a Pediatric Precision Oncology Program: “When You Feel Helpless, This Kind of Thing Is Very Helpful”. JCO Precision Oncology 2022;6:e2100444.
Johnson LM, Mandrell BN, Li C, Lu Z, Gattuso J, Harrison LW, et al. Managing Pandora’s box: familial expectations around the return of (future) germline results. AJOB Empir Bioeth. 2022;13:152–65.
Acknowledgements
We are deeply grateful to the parents, the children and the AYAs who agreed to take part in this study. We thank Elisabetta Andermarcher for translation and improvement of the manuscripts.
Funding
This study is part of the “GeneInfoKid” multidisciplinary research project, which uses a range of tools from the humanities and social sciences (HSS) to study the social, ethical, psychological, and legal issues posed by the use of sequencing in medical care in paediatric oncology. The study was funded by French National Cancer Institute (INCa) grant no. 2018-127 and led by the Cancéropôle Île de France. Sandrine de Montgolfier holds the DemoCan research chair funded by the French National Cancer Institute (INCa) grant no. 16048 (2022-2026). Open access funding provided by Université Paris-Est Créteil.
Author information
Authors and Affiliations
Contributions
SDM elaborated the GeneInfoKid project and funding application. KLL directed the Gene-InfoKid psychological sub-study. SDM, FB, SJ, ERS, IC, LB, HC, APGR, and LH as members of the GeneInfokid scientific committee participated in the discussion concerning the methodology (design and adaptation). KLL, APGR, and LH prepared the interview guides. LH prepared the project submission to the ethical committee. FB, LB, HC, AP, FS, LGR, BC, and MS participated actively in the clinical field study. MDM realised the interviews, their transcription, pseudonymization and analysed the first round of data with KLL. CF gave methodological support to the thematic analysis of the interviews. MDM, KLL, and SDM discussed the final thematic analysis. The first version of the paper was written by MDM, reviewed actively by SDM and KLL, before reviewing by all authors. The authors confirm that they had full access to all the data in the study and take responsibility for the data integrity and the accuracy of the data analysis. All authors gave their final approval to this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was reviewed and approved by the Human Research Ethics Committee of the Société française de pédiatrie (No. CER_SFP_2019_104_2, 2019/09/19). Informed consent was obtained from all parents and oral approval was obtained from each child to confirm their willingness to participate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Droin-Mollard, M., de Montgolfier, S., Gimenez-Roqueplo, AP. et al. Psychological and ethical issues raised by genomic in paediatric care pathway, a qualitative analysis with parents and childhood cancer patients. Eur J Hum Genet 32, 1446–1455 (2024). https://doi.org/10.1038/s41431-024-01653-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41431-024-01653-4
This article is cited by
-
Beyond the Diagnosis: Valuing Genome-Wide Sequencing for Rare Disease Diagnosis Using Contingent Valuation
Applied Health Economics and Health Policy (2025)
-
Fear of cancer recurrence among adolescent and young adult cancer survivors: a mixed-methods systematic review
Journal of Cancer Survivorship (2025)
-
November in EJHG: looking at genetic counsellor training in Europe, novel clinical guidelines and ancestral impact on variant interpretation
European Journal of Human Genetics (2024)