Abstract
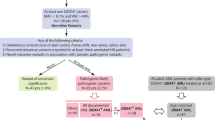
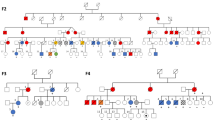
Monoallelic germline DDX41 pathogenic variants (PV) are responsible for the most frequent monogenic predisposition to myeloid neoplasms (MN), i.e., to myelodysplastic syndrome and acute myeloid leukemia. MN are rare clonal diseases affecting hematopoietic tissues, and curative approaches frequently implicate hematopoietic stem cell transplantation, requesting testing for relatives of DDX41-mutated MN patients. Establishing the penetrance of germline DDX41 PV is crucial to determine how to monitor the unaffected relatives who carry the familial DDX41 PV. Our study aims to assess the risk of MN in relatives of affected DDX41 carriers using the Genotype-Restricted-Likelihood (GRL) approach. We identified 63 families with probands carrying a germline DDX41 PV (ACMG class 4 or class 5 variant) affected with MN, from 11 French centers. One hundred and sixty relatives, including 73 males (46%), were genotyped at the median age of 51 [range: 15–84] years, 80 of them (50%) being carriers of the familial DDX41 PV. The cumulative risk of MN at 70 years for DDX41 PV carriers was estimated at 19.5% [95% CI: 5.8%–62.5%], corresponding to a relative risk compared to the general population of 55 [95% CI: 16.4–176.5]. This risk appeared higher in males, although the difference did not reach statistical significance. Based on these findings, we suggest yearly complete blood count from the age of 50 for DDX41 PV carriers. Of note, we observed cases before the age of 50 in females, which could suggest sex-differentiated monitoring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
All calculations were performed using R version 4.4.1 [13] and the open-source generisk package version 0.1.1 available at https://github.com/youenndrouet/generisk.
References
Rotter LK, Shimony S, Ling K, Chen E, Shallis RM, Zeidan AM, et al. Epidemiology and Pathogenesis of myelodysplastic syndrome. Cancer J. 2023;29:111–21. https://doi.org/10.1097/PPO.0000000000000665.
Venugopal S, Sekeres MA. Contemporary management of acute myeloid leukemia: a review. JAMA Oncol. 2024;10:1417–25. https://doi.org/10.1001/jamaoncol.2024.2662.
Bernard E, Hasserjian RP, Greenberg PL, Arango Ossa JE, Creignou M, Tuechler H, et al. Molecular taxonomy of myelodysplastic syndromes and its clinical implications. Blood. 2024;144:1617–32. https://doi.org/10.1182/blood.2023023727.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. https://doi.org/10.1038/s41375-022-01613-1.
Gurnari C, Robin M, Godley LA, Drozd-Sokołowska J, Włodarski MW, Raj K, et al. Germline predisposition traits in allogeneic hematopoietic stem-cell transplantation for myelodysplastic syndromes: a survey-based study and position paper on behalf of the Chronic Malignancies Working Party of the EBMT. Lancet Haematol. 2023;10:e994–e1005. https://doi.org/10.1016/S2352-3026(23)00265-X.
Hamilton KV, Maese L, Marron JM, Pulsipher MA, Porter CC, Nichols KE. Stopping leukemia in its tracks: should preemptive hematopoietic stem-cell transplantation be offered to patients at increased genetic risk for acute myeloid leukemia?. J Clin Oncol. 2019;37:2098–104. https://doi.org/10.1200/JCO.19.00181.
Sébert M, Passet M, Raimbault A, Rahmé R, Raffoux E, Sicre De Fontbrune F, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134:1441–4. https://doi.org/10.1182/blood.2019000909.
Duployez N, Largeaud L, Duchmann M, Kim R, Rieunier J, Lambert J, et al. Prognostic impact of DDX41 germline mutations in intensively treated acute myeloid leukemia patients: an ALFA-FILO study. Blood. 2022;140:756–68. https://doi.org/10.1182/blood.2021015328.
Sébert M, Freiman L, Chaffaut C, Guerci A, Peterlin P, Thépot S, et al. Clinical impact of genetic alterations including germline DDX41 mutations in MDS/low-blast count AML patients treated with azacitidine-based regimens. Leukemia. 2024;38:918–22. https://doi.org/10.1038/s41375-024-02180-3.
Makishima H, Saiki R, Nannya Y, Korotev S, Gurnari C, Takeda J, et al. Germ line DDX41 mutations define a unique subtype of myeloid neoplasms. Blood. 2023;141:534–49. https://doi.org/10.1182/blood.2022018221.
Cheloor Kovilakam S, Gu M, Dunn WG, Marando L, Barcena C, Nik-Zainal S, et al. Prevalence and significance of DDX41 gene variants in the general population. Blood. 2023;142:1185–92. https://doi.org/10.1182/blood.2023020209.
Carayol J, Bonaïti-Pellié C. Estimating penetrance from family data using a retrospective likelihood when ascertainment depends on genotype and age of onset. Genet Epidemiol. 2004;27:109–17. https://doi.org/10.1002/gepi.20007.
Le Guyader-Peyrou S, Defossez G, Dantony E, Mounier M, Cornet E, Uhry Z, et al. Estimations Nationales de L’incidence et de la Mortalité par Cancer en France Métropolitaine Entre 1990 et 2018; Volume 2—Hémopathies malignes; Étude à partir des registres des cancers du réseau Francim. Santé Publique: Paris, France. 2019. https://www.santepubliquefrance.fr/docs/estimations-nationales-de-l-incidence-et-de-la-mortalite-par-cancer-en-france-metropolitaine-entre-1990-et-2018-volume-2-hemopathies-malignes.
Efron B. Bootstrap methods: another look at the jackknife. Annals Stat. 1979;7:1–26.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. https://www.R-project.org.
Rowlands CF, Allen S, Balmaña J, Domchek SM, Evans DG, Hanson H, et al. Population-based germline breast cancer gene association studies and meta-analysis to inform wider mainstream testing. Ann Oncol. 2024;35:892–901. https://doi.org/10.1016/j.annonc.2024.07.244.
Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies [published correction appears in Am J Hum Genet. 2003 Sep;73(3):709]. Am J Hum Genet. 2003;72:1117–30. https://doi.org/10.1086/375033.
Metcalfe K, Lubinski J, Lynch HT, Ghadirian P, Foulkes WD, Kim-Sing C, et al. Family history of cancer and cancer risks in women with BRCA1 or BRCA2 mutations. J Natl Cancer Inst. 2010;102:1874–8. https://doi.org/10.1093/jnci/djq443.
Jackson L, Weedon MN, Green HD, Mallabar-Rimmer B, Harrison JW, Wood AR, et al. Influence of family history on penetrance of hereditary cancers in a population setting. EClinicalMedicine. 2023;64. 102159. https://doi.org/10.1016/j.eclinm.2023.102159.
Brayne C, Moffitt TE. The limitations of large-scale volunteer databases to address inequalities and global challenges in health and aging. Nat Aging. 2022;2:775–83. https://doi.org/10.1038/s43587-022-00277-x.
Gong G, Hannon N, Whittemore AS. Estimating gene penetrance from family data. Genet Epidemiol. 2010;34:373–81. https://doi.org/10.1002/gepi.20493.
Alarcon F, Bourgain C, Gauthier-Villars M, Planté-Bordeneuve V, Stoppa-Lyonnet D, Bonaïti-Pellié C. PEL: an unbiased method for estimating age-dependent genetic disease risk from pedigree data unselected for family history. Genet Epidemiol. 2009;33:379–85. https://doi.org/10.1002/gepi.20390.
Deuitch N, Broadbridge E, Cunningham L, Liu P. RUNX1 familial platelet disorder with associated myeloid malignancies. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 2021.
Baliakas P, Tesi B, Cammenga J, Stray-Pedersen A, Jahnukainen K, Andersen MK, et al. How to manage patients with germline DDX41 variants: recommendations from the Nordic working group on germline predisposition for myeloid neoplasms. Hemasphere. 2024;8. e145. https://doi.org/10.1002/hem3.145.
Lewinsohn M, Brown AL, Weinel LM, Phung C, Rafidi G, Lee MK, et al. Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood. 2016;127:1017–23. https://doi.org/10.1182/blood-2015-10-676098.
Kusne Y, Badar T, Lasho T, Marando L, Mangaonkar A, Finke C, et al. Prevalence and clinical features of non-myeloid neoplasms in patients with DDX41 mutant germline predisposition syndrome [abstract #440]. Abstract from the 2024 American Society of Hematology Annual Meeting. https://ash.confex.com/ash/2024/webprogram/Paper203879.html.
Acknowledgements
The authors would like to thank the patients and their families.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MCV, CC, DSL, and MS designed the project. MCV, BD, LFr, MMA, LFe, SN, MW, EL, DL, ML, OI, NG, TC, ML, JS, PFG, PT, LV, LP, and BB collected the clinical, genetic and familial data. YV, LL, ND, and EC analyzed the molecular genetic data. YD and MCV performed the statistical analyses. MCV, YD, and MS wrote the manuscript and designed the figures and tables. All authors revised the manuscript critically and approved the version of the manuscript to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was not sought for the present study because all data were obtained as part of routine care. All probands and relatives gave their written consent for genetic testing and for use of their data for research purposes. The data used for this study comes from a database approved by a National Review Board, in accordance with the Declaration of Helsinki and French ethics regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Villy, MC., Drouet, Y., Larcher, L. et al. Myeloid neoplasms risks for germline DDX41 pathogenic variants carriers. Eur J Hum Genet (2025). https://doi.org/10.1038/s41431-025-01952-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41431-025-01952-4