Abstract
Purpose
To describe treatment outcomes in a cohort of Caucasian patients with polypoidal choroidal vasculopathy (PCV).
Methods
Clinical charts from 48 eyes of 45 Caucasian patients with PCV were retrospectively reviewed. All cases were diagnosed with indocyanine green angiography. Best corrected visual acuity (BCVA) and optical coherence tomography (OCT) imaging were analyzed at baseline and final follow-up.
Results
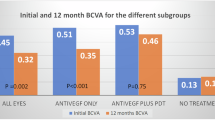
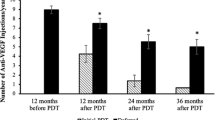
Eyes were treated with a combination of verteporfin photodynamic therapy (PDT) and anti-vascular endothelial growth factor (VEGF) (n = 24), or PDT monotherapy (n = 9), or anti-VEGF monotherapy (n = 8), or no treatment (n = 7). Aflibercept was the anti-VEGF agent in 30 out of 32 eyes. Sixteen out of 24 eyes in the combination treatment group received initial PDT at diagnosis. All treatments led to stabilization of BCVA at final visit with a trend for better visual acuity in the anti-VEGF monotherapy group. There was a substantial reduction in central retinal thickness associated with resolution of subfoveal fluid and improvement in retinal pigment epithelial detachment in all treatment groups. BCVA and OCT findings remained stable in eyes which received no treatment. The use of PDT was associated with 0.5 fewer intravitreal injections per annum, which was not statistically significant.
Conclusions
In the largest series of Caucasian patients with PCV presented to date, anti-VEGF monotherapy, PDT, or their combination preserved visual acuity and improved subfoveal exudative changes. Combination treatment was not superior to anti-VEGF monotherapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8.
Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol. 2010;55:501–15.
Yannuzzi LA, Wong DW, Sforzolini BS, Goldbaum M, Tang KC, Spaide RF, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol. 1999;117:1503–10.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10.
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I, Wong TY, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107–39.
Scassellati-Sforzolini B, Mariotti C, Bryan R, Yannuzzi LA, Giuliani M, Giovannini A. Polypoidal choroidal vasculopathy in Italy. Retina. 2001;21:121–5.
Yadav S, Parry DG, Beare NA, Pearce IA. Polypoidal choroidal vasculopathy: a common type of neovascular age-related macular degeneration in Caucasians. Br J Ophthalmol. 2017;101:1377–80.
Ladas ID, Rouvas AA, Moschos MM, Synodinos EE, Karagiannis DA, Koutsandrea CN. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye. 2004;18:455–9.
Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752–9.
Davis SJ, Lauer AK, Flaxel CJ. Polypoidal choroidal vasculopathy in white patients. Retina. 2014;34:2185–91.
Hatz K, Prünte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol. 2014;98:188–94.
Lorentzen TD, Subhi Y, Sørensen TL. Prevalence of polypoidal choroidal vasculopathy in white patients with exudative age-related macular degeneration. Systematic review and meta-analysis. Retina. 2017; https://doi.org/10.1097/IAE.0000000000001872
Nowak-Sliwinska P, van den Bergh H, Sickenberg M, Koh AH. Photodynamic therapy for polypoidal choroidal vasculopathy. Prog Retin Eye Res. 2013;37:182–99.
Wong CW, Cheung CM, Mathur R, Li X, Chan CM, Yeo I, et al. Three-year results of polypoidal choroidal vasculopathy treated with photodynamic therapy: retrospective study and systematic review. Retina. 2015;35:1577–93.
Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002;22:529–35.
Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye. 2012;26:426–33.
Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94:297–301.
Koh A, Lee WK, Chen LJ, Tan NW, Lim TH, EVEREST Study Group. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
Koh AH, Expert PCV Panel, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013;33:686–716.
Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan study. Retina. 2015;35:1569–76.
Hara C, Sawa M, Sayanagi K, Nishida K. One-year results of intravitreal aflibercept for polypoidal choroidal vasculopathy. Retina. 2016;36:37–45.
Maruyama-Inoue M, Sato S, Yamane S, Kadonosono K. Intravitreal injection of aflibercept in patients with polypoidal choroidal vasculopathy: a 3-year follow-up. Retina. 2017; https://doi.org/10.1097/IAE.0000000000001818
Inoue M, Yamane S, Taoka R, Arakawa A, Kadonosono K. Aflibercept for polypoidal choroidal vasculopathy: as needed versus fixed interval dosing. Retina. 2016;36:1527–34.
Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014;34:2192–201.
Azuma K, Obata R, Nomura Y, Tan X, Takahashi H, Yanagi Y. Angiographic findings of ranibizumab-resistant polypoidal choroidal vasculopathy after switching to a treat-and-extend regimen with intravitreal aflibercept. Retina. 2016;36:2158–65.
Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K, Nagai Y, et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol. 1999;117:1035–42.
Ma L, Li Z, Liu K, Rong SS, Brelen ME, Young AL, et al. Association of genetic variants with polypoidal choroidal vasculopathy: a systematic review and updated meta-analysis. Ophthalmology. 2015;122:1854–65.
Rouvas AA, Papakostas TD, Ntouraki A, Douvali M, Vergados I, Ladas ID. Photodynamic therapy, ranibizumab, and ranibizumab with photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Retina. 2011;31:464–74.
Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH, EVEREST Study Group. EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99:624–8.
Gharehbagh SS, Subhi Y, Sørensen TL. Efficacy of aflibercept for polypoidal choroidal vasculopathy in Caucasians. Acta Ophthalmol. 2018;96:e94–5.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Koh A, Lai TYY, Takahashi K, Wong TY, Chen LJ, Ruamviboonsuk P, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1206–13.
Lee WK, Iida T, Ogura Y, Chen SJ, Wong TY, Mitchell P et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018; https://doi.org/10.1001/jamaophthalmol.2018.1804
Acknowledgements
We are indebted to Dr Gabriela Czanner for her advice on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ian A. Pearce has received consultancy fees from Bayer and Novartis and travel grants from Bayer. Nicholas A.V. Beare is a member of the NICE AMD Clinical Guidelines Development Committee, has participated in advisory boards for Santen Pharmaceuticals and AbbVie on uveitis, and has received a travel grant from Bayer.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Agorogiannis, E.I., Pearce, I.A., Yadav, S. et al. Clinical outcomes in Caucasian patients with polypoidal choroidal vasculopathy. Eye 32, 1731–1739 (2018). https://doi.org/10.1038/s41433-018-0168-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0168-2
This article is cited by
-
Real world treatment outcomes in polypoidal choroidal vasculopathy in a Caucasian population of British ethnicity
Eye (2024)
-
Real-world outcomes of combined therapy of photodynamic therapy with anti-vascular endothelial growth factor for polypoidal choroidal vasculopathy
Eye (2022)
-
The spectrum of polypoidal choroidal vasculopathy in Caucasians: clinical characteristics and proposal of a classification
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Comment on: Clinical outcomes in Caucasian patients with polypoidal choroidal vasculopathy
Eye (2019)
-
Indocyanine green angiography findings in patients with neovascular age-related macular degeneration refractory to ranibizumab switched to aflibercept
International Ophthalmology (2019)