Abstract
Purpose
To examine the feasibility and utility of dark-adapted two-color fundus-controlled perimetry (FCP) in patients with cuticular, reticular, and soft drusen, and to compare FCP data to microstructural spectral-domain optical coherence tomography (SD-OCT) data.
Methods
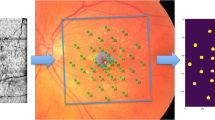
Forty-four eyes (24 eyes of 24 patients with drusen, age 69.4 ± 12.6 years; 20 normal eyes of 16 subjects, 61.7 ± 12.4 years) underwent duplicate mesopic, dark-adapted cyan and dark-adapted red FCP within 14° of the central retina (total of 12 936 threshold tests) using the Scotopic Macular Integrity Assessment (S-MAIA, CenterVue, Padova, Italy) device. FCP data were registered to SD-OCT data to obtain outer nuclear layer, inner and outer photoreceptor segment, and retinal pigment epithelium drusen complex (RPEDC) thickness data spatially corresponding to the stimulus location and area (0.43°). Structure-function correlations were assessed using mixed-effects models.
Results
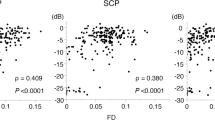
Mean deviation values for eyes with cuticular, soft, and reticular drusen were similar for mesopic (−2.1, −3.4, and −3.6 dB) and dark-adapted red (−1.4, −2.6, and −3.3 dB) FCP. For the dark-adapted cyan FCP (0.1, −1.9, and −5.0 dB) and for the cyan–red sensitivity difference (+1.0, +0.5, and −2.4 dB), the mean deviation values differed significantly in dependence of the predominant drusen type (one-way ANOVA; p < 0.05). RPEDC thickness was associated with reduction of mesopic sensitivity (−0.34 dB/10 µm RPEDC thickening; p < 0.001), dark-adapted cyan sensitivity (−0.11 dB/10 µm RPEDC thickening; p = 0.003), and dark-adapted red sensitivity (−0.26 dB/10 µm RPEDC thickening; p < 0.001).
Conclusions
In contrast to mesopic FCP, dark-adapted two-color FCP allowed for meaningful differential testing of rod and cone function in patients with drusen indicating predominant cone dysfunction in eyes with cuticular drusen and predominant rod dysfunction in eyes with reticular drusen. RPEDC thickness was the strongest predictor of the evaluated SD-OCT biomarkers for point-wise sensitivity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cheung LK, Eaton A. Age-related macular degeneration. Pharmacother J Hum Pharmacol Drug Ther. 2013;33:838–55.
Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124:1430–8.
Rasmussen A, Sander B. Long-term longitudinal study of patients treated with ranibizumab for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2014;25:158–63.
Schaal KB, Rosenfeld PJ, Gregori G, Yehoshua Z, Feuer WJ. Anatomic clinical trial endpoints for nonexudative age-related macular degeneration. Ophthalmology. 2016;123:1060–79.
Dimitrov PN, Robman LD, Varsamidis M, Aung KZ, Makeyeva GA, Guymer RH, et al. Visual function tests as potential biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:9457–69.
Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–49.
Owsley C, Jackson GR, Cideciyan AV, Huang Y, Fine SL, Ho AC, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–73.
Steinberg JS, Fitzke FW, Fimmers R, Fleckenstein M, Holz FG, Schmitz-Valckenberg S. Scotopic and photopic microperimetry in patients with reticular drusen and age-related macular degeneration. JAMA Ophthalmol. 2015;133:690–7.
Fraser RG, Tan R, Ayton LN, Caruso E, Guymer RH, Luu CD. Assessment of retinotopic rod photoreceptor function using a dark-adapted chromatic perimeter in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:5436–42.
Owsley C, Jackson GR, White M, Feist R, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001;108:1196–202.
Sevilla MB, McGwin G, Lad EM, Clark M, Yuan EL, Farsiu S, et al. Relating retinal morphology and function in aging and early to intermediate age-related macular degeneration subjects. Am J Ophthalmol. 2016;165:65–77.
Flamendorf J, Agrón E, Wong W, Thompson D, Wiley H, Doss E, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122:2053–62.
Owsley C, McGwin G, Clark ME, Jackson GR, Callahan MA, Kline LB, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123:344–51.
Steinberg JS, Saßmannshausen M, Fleckenstein M, Fimmers R, Oishi A, Holz FG, et al. Correlation of partial outer retinal thickness with scotopic and mesopic fundus-controlled perimetry in patients with reticular drusen. Am J Ophthalmol. 2016;168:52–61.
Pfau M, Lindner M, Fleckenstein M, Finger RP, Rubin GS, Harmening WM, et al. Test-retest reliability of scotopic and mesopic fundus-controlled perimetry using a modified MAIA (Macular Integrity Assessment) in normal eyes. Ophthalmologica. 2017;237:42–54.
Pfau M, Lindner M, Muller PL, Birtel J, Finger RP, Harmening WM, et al. Effective dynamic range and retest reliability of dark-adapted two-color fundus-controlled perimetry in patients with macular diseases. Invest Ophthalmol Vis Sci. 2017;58:BIO158–67.
Steinberg JS, Sassmannshausen M, Pfau M, Fleckenstein M, Finger RP, Holz FG, et al. Evaluation of two systems for fundus-controlled scotopic and mesopic perimetry in eye with age-related macular degeneration. Transl Vis Sci Technol. 2017;6:7.
Pfau M, Lindner M, Steinberg JS, Thiele S, Brinkmann CK, Fleckenstein M. et al. Visual field indices and patterns of visual field deficits in mesopic and dark-adapted two-colour fundus-controlled perimetry in macular diseases. Br J Ophthalmol. 2018;102:1054-1059.
Wu Z, Ayton LN, Makeyeva G, Guymer RH, Luu CD. Impact of reticular pseudodrusen on microperimetry and multifocal electroretinography in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:2100–6.
Wu Z, Ayton LN, Luu CD, Guymer RH. Microperimetry of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:115–21.
Wu Z, Cunefare D, Chiu E, Luu CD, Ayton LN, Toth CA, et al. Longitudinal associations between microstructural changes and microperimetry in the early stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:3714.
Hartmann KI, Bartsch DU, Cheng L, Kim JS, Gomez ML, Klein H, et al. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–31.
Acton JH, Theodore Smith R, Hood DC, Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:7618–24.
Midena E, Vujosevic S, Convento E, Manfre’ A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–503.
Vujosevic S, Pucci P, Casciano M, Longhin E, Convento E, Bini S. Long-term longitudinal modifications in mesopic microperimetry in early and intermediate age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255:301–9.
Boon CJF, van de Ven JPH, Hoyng CB, den Hollander AI, Klevering BJ. Cuticular drusen: stars in the sky. Prog Retin Eye Res. 2013;37:90–113.
Cohen SY, Meunier I, Soubrane G, Glacet-Bernard A, Coscas GJ. Visual function and course of basal laminar drusen combined with vitelliform macular detachment. Br J Ophthalmol. 1994;78:437–40.
Finger RP, Charbel Issa P, Kellner U, Schmitz-Valckenberg S, Fleckenstein M, Scholl HP, et al. Spectral domain optical coherence tomography in adult-onset vitelliform macular dystrophy with cuticular drusen. Retina. 2010;30:1455–64.
Querques G, Massamba N, Srour M, Boulanger E, Georges A, Souied EH. Impact of reticular pseudodrusen on macular function. Retina. 2014;34:321–9.
Alten F, Heiduschka P, Clemens CR, Eter N. Multifocal electroretinography in eyes with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012;53:6263–70.
Schmitz-Valckenberg S, Steinberg JS, Fleckenstein M, Visvalingam S, Brinkmann CK, Holz FG. Combined confocal scanning laser opthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology. 2010;117:1169–76.
Auge J, Steinberg JS, Fleckenstein M, Holz FG, Schmitz-Valckenberg S. Retikuläre Drusen über die Zeit mittels SD-OCT. Ophthalmologe. 2014;111:765–71.
Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51.
Cnaan A, NANM Laird, Slasor, Using P. the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–80.
Staurenghi G, Sadda S, Frcophth UC, Spaide RF, Nomenclature I, Tomography C, et al. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography the IN OCT consensus. Ophthalmology. 2014;121:1572–8.
Sadigh S, Cideciyan AV, Sumaroka A, Huang WC, Luo X, Swider M, et al. Abnormal thickening as well as thinning of the photoreceptor layer in intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:1603–12.
Chiu SJ, Izatt JA, Connell RVO, Winter KP. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci. 2017;53:53–61.
Gliem M, Müller PL, Finger RP, McGuinness MB, Holz FG, Charbel Issa P. Quantitative fundus autofluorescence in early and intermediate age-related macular degeneration. JAMA Ophthalmol. 2016;134:817–24.
Pinheiro J, Bates D, DebRoy S, Sarkar D and R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-137, (2018). https://CRAN.R-project.org/package=nlme.
Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96.
Heijl A, Lindgren G, Olsson J. A package for the statistical analysis of visual fields. In: Greve EL, Heijl A, editors. Seventh international visual field symposium, Amsterdam, September 1986. Documenta Ophthalmologica Proceedings Series, vol 49. Springer, Dordrecht 1987. pp. 153-68.
Bebie H, Flammer J, Bebie T. The cumulative defect curve: Separation of local and diffuse components of visual field damage. Graefes Arch Clin Exp Ophthalmol. 1989;227:9–12.
Sloan LL. The threshold gradients of the rods and the cones in the dark-adapted and in the partially light-adapted eye. Am J Ophthalmol. 1950;33:1077–89.
Sloan LL. The Tubinger perimeter of Harms and Aulhorn. Recommended procedures and supplementary equipment. Arch Ophthalmol. 1971;86:612–22.
Simunovic MP, Moore AT, MacLaren RE. Selective automated perimetry under photopic, mesopic, and scotopic conditions: detection mechanisms and testing strategies. Transl Vis Sci Technol. 2016;5:10.
Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–76.
Kubota R, Al-Fayoumi S, Mallikaarjun S, Patil S, Bavik C, Chandler JW. Phase 1, dose-ranging study of emixustat hydrochloride (Acu-4429), a novel visual cycle modulator, in healthy volunteers. Retina. 2014;34:603–9.
Zeavin BH, Wald G. Rod and cone vision in retinitis pigmentosa. Am J Ophthalmol. 1956;42:253–69.
Jacobson SG, Voigt WJ, Parel J-M, Apathy PP, Nghiem-Phu L, Myers SW, et al. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986;93:1604–11.
Lujan BJ, Roorda A, Knighton RW, Carroll J. Revealing Henle’s fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:1486–92.
Sadigh S, Luo X, Cideciyan AV, Sumaroka A, Boxley SL, Hall LM, et al. Drusen and photoreceptor abnormalities in African-Americans with intermediate non-neovascular age-related macular degeneration. Curr Eye Res. 2015;40:398–406.
Greenberg JP, Sherman J, Zweifel SA, Chen RWS, Duncker T, Kohl S, et al. Spectral-domain optical coherence tomography staging and autofluorescence imaging in achromatopsia. JAMA Ophthalmol. 2014;132:437–45.
Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33:1800–8.
Wu Z, Luu CD, Ayton LN, Goh JK, Lucci LM, Hubbard WC, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121:2415–22.
Weleber RG, Smith TB, Peters D, Chegarnov EN, Gillespie SP, Francis PJ, et al. VFMA: topographic analysis of sensitivity data from full-field static perimetry. Transl Vis Sci Technol. 2015;4:14.
Charng J, Tan R, Luu CD, Sadigh S, Stambolian D, Guymer RH, et al. Imaging lenticular autofluorescence in older subjects. Invest Ophthalmol Vis Sci. 2017;58:4940–7.
Acknowledgements
We are grateful for the technical support of Jan Dechent (Heidelberg Engineering GmbH, Heidelberg, Germany) and Carlo Pellizzari (CenterVue SpA, Padova, Italy).
Funding
This study was supported by the BONFOR GEROK Program of the Faculty of Medicine, University of Bonn, Grant No O-137.0022 to MP and Grant No O-137.0020 to ML, and by the German Research Foundation (DFG), Grant No 658/4-1 and 658/4-2 to MF, and Grant No LI2846/1-1 to ML.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CenterVue SpA, Padova, Italy has provided research material (S-MAIA) for the conduct of this study. CenterVue had no role in the design or conduct of the experiments.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pfau, M., Lindner, M., Gliem, M. et al. Mesopic and dark-adapted two-color fundus-controlled perimetry in patients with cuticular, reticular, and soft drusen. Eye 32, 1819–1830 (2018). https://doi.org/10.1038/s41433-018-0183-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-018-0183-3
This article is cited by
-
Einsatz von künstlicher Intelligenz zur Erkennung von Biomarkern bei der intermediären altersabhängigen Makuladegeneration
Die Ophthalmologie (2024)
-
Comparison of colour contrast sensitivity in eyes at high risk of neovascular age‐related macular degeneration with and without subsequent choroidal neovascular membrane development
Eye (2023)
-
Point-to-point associations of drusen and hyperreflective foci volumes with retinal sensitivity in non-exudative age-related macular degeneration
Eye (2023)
-
Evaluation of the microperimetry in eyes with cuticular drusen
Scientific Reports (2022)
-
Association of complement C3 inhibitor pegcetacoplan with reduced photoreceptor degeneration beyond areas of geographic atrophy
Scientific Reports (2022)